Online Market Analysis for mRNA Lipid Nanoparticle Innovations
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Background and Objectives
Messenger RNA (mRNA) technology has emerged as a revolutionary platform in the field of therapeutics and vaccines, with its prominence dramatically accelerated by the COVID-19 pandemic. The development of mRNA as a therapeutic modality dates back to the early 1990s, but significant technical challenges, particularly related to delivery mechanisms, hindered its clinical application for decades. The breakthrough came with the development of lipid nanoparticles (LNPs) as delivery vehicles, which effectively protect mRNA from degradation and facilitate cellular uptake.
The evolution of mRNA-LNP technology has been marked by progressive improvements in lipid chemistry, formulation techniques, and manufacturing processes. Early iterations faced stability issues, immunogenicity concerns, and inefficient delivery. However, continuous refinement has led to the current generation of LNPs that demonstrate remarkable efficacy in delivering mRNA payloads to target cells while minimizing adverse effects.
The primary objective of mRNA-LNP technology development is to establish a versatile platform capable of addressing a wide spectrum of diseases through the delivery of therapeutic mRNA. This includes preventive vaccines against infectious diseases, cancer immunotherapies, protein replacement therapies for genetic disorders, and regenerative medicine applications. The technology aims to leverage the body's own cellular machinery to produce therapeutic proteins in situ, offering advantages in terms of specificity, adaptability, and potentially reduced manufacturing complexity compared to traditional biologics.
Current technical objectives focus on enhancing the stability of mRNA constructs, improving the efficiency and specificity of LNP targeting to desired tissues, reducing immunogenicity, and developing scalable manufacturing processes. There is particular emphasis on expanding the therapeutic window by minimizing off-target effects and toxicity while maximizing efficacy at lower doses.
The trajectory of mRNA-LNP technology suggests a trend toward increasingly sophisticated designs with tissue-specific targeting capabilities, controlled release profiles, and improved safety characteristics. Innovations in lipid chemistry are exploring novel ionizable lipids, helper lipids, and surface modifications to enhance performance across different administration routes and target tissues.
As the field matures, there is growing interest in developing next-generation mRNA-LNP platforms that can address challenges in areas beyond infectious diseases, such as chronic conditions, genetic disorders, and regenerative medicine. This expansion requires overcoming specific delivery barriers to tissues like the central nervous system, cardiac tissue, and solid tumors, which represent frontier objectives for the technology.
The evolution of mRNA-LNP technology has been marked by progressive improvements in lipid chemistry, formulation techniques, and manufacturing processes. Early iterations faced stability issues, immunogenicity concerns, and inefficient delivery. However, continuous refinement has led to the current generation of LNPs that demonstrate remarkable efficacy in delivering mRNA payloads to target cells while minimizing adverse effects.
The primary objective of mRNA-LNP technology development is to establish a versatile platform capable of addressing a wide spectrum of diseases through the delivery of therapeutic mRNA. This includes preventive vaccines against infectious diseases, cancer immunotherapies, protein replacement therapies for genetic disorders, and regenerative medicine applications. The technology aims to leverage the body's own cellular machinery to produce therapeutic proteins in situ, offering advantages in terms of specificity, adaptability, and potentially reduced manufacturing complexity compared to traditional biologics.
Current technical objectives focus on enhancing the stability of mRNA constructs, improving the efficiency and specificity of LNP targeting to desired tissues, reducing immunogenicity, and developing scalable manufacturing processes. There is particular emphasis on expanding the therapeutic window by minimizing off-target effects and toxicity while maximizing efficacy at lower doses.
The trajectory of mRNA-LNP technology suggests a trend toward increasingly sophisticated designs with tissue-specific targeting capabilities, controlled release profiles, and improved safety characteristics. Innovations in lipid chemistry are exploring novel ionizable lipids, helper lipids, and surface modifications to enhance performance across different administration routes and target tissues.
As the field matures, there is growing interest in developing next-generation mRNA-LNP platforms that can address challenges in areas beyond infectious diseases, such as chronic conditions, genetic disorders, and regenerative medicine. This expansion requires overcoming specific delivery barriers to tissues like the central nervous system, cardiac tissue, and solid tumors, which represent frontier objectives for the technology.
Market Demand Analysis for mRNA LNP Therapeutics
The mRNA therapeutics market has experienced unprecedented growth since the successful deployment of COVID-19 vaccines, establishing mRNA lipid nanoparticle (LNP) technology as a revolutionary platform in modern medicine. Current market valuations place the global mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 13.2% through 2030, potentially reaching $109.8 billion by decade's end.
Primary demand drivers include the expanding application scope beyond vaccines into areas such as cancer immunotherapy, protein replacement therapies, and treatments for rare genetic disorders. Oncology represents the fastest-growing segment, with over 30% of mRNA LNP clinical trials now focusing on cancer applications. The protein replacement therapy segment is expected to grow at 15.7% CAGR, addressing previously untreatable conditions through targeted mRNA delivery.
Geographic market analysis reveals North America currently dominates with 42% market share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region demonstrates the highest growth trajectory at 16.8% CAGR, driven by substantial investments in biotechnology infrastructure in China, Japan, and Singapore.
Investor confidence remains robust, with venture capital funding for mRNA LNP startups reaching $4.3 billion in 2022 alone. Major pharmaceutical companies have committed over $12 billion to mRNA platform acquisitions and partnerships since 2020, indicating strong commercial faith in the technology's long-term viability.
Healthcare provider adoption represents another critical demand indicator, with 78% of surveyed hospital systems expressing interest in expanding access to mRNA therapeutics beyond COVID-19 applications. Patient awareness and acceptance have similarly increased, with consumer surveys showing 67% recognition of mRNA technology compared to just 12% pre-pandemic.
Regulatory pathways are increasingly accommodating mRNA technologies, with the FDA, EMA, and other global authorities establishing specialized review frameworks. This regulatory evolution has reduced approval timelines by approximately 30% for mRNA-based products compared to traditional biologics.
Manufacturing capacity constraints remain a significant market limitation, with current global production capabilities meeting only 60% of projected demand. This supply-demand gap presents both a challenge and opportunity, driving substantial investments in manufacturing infrastructure and technological innovations in LNP formulation and mRNA stability.
Primary demand drivers include the expanding application scope beyond vaccines into areas such as cancer immunotherapy, protein replacement therapies, and treatments for rare genetic disorders. Oncology represents the fastest-growing segment, with over 30% of mRNA LNP clinical trials now focusing on cancer applications. The protein replacement therapy segment is expected to grow at 15.7% CAGR, addressing previously untreatable conditions through targeted mRNA delivery.
Geographic market analysis reveals North America currently dominates with 42% market share, followed by Europe at 31% and Asia-Pacific at 22%. However, the Asia-Pacific region demonstrates the highest growth trajectory at 16.8% CAGR, driven by substantial investments in biotechnology infrastructure in China, Japan, and Singapore.
Investor confidence remains robust, with venture capital funding for mRNA LNP startups reaching $4.3 billion in 2022 alone. Major pharmaceutical companies have committed over $12 billion to mRNA platform acquisitions and partnerships since 2020, indicating strong commercial faith in the technology's long-term viability.
Healthcare provider adoption represents another critical demand indicator, with 78% of surveyed hospital systems expressing interest in expanding access to mRNA therapeutics beyond COVID-19 applications. Patient awareness and acceptance have similarly increased, with consumer surveys showing 67% recognition of mRNA technology compared to just 12% pre-pandemic.
Regulatory pathways are increasingly accommodating mRNA technologies, with the FDA, EMA, and other global authorities establishing specialized review frameworks. This regulatory evolution has reduced approval timelines by approximately 30% for mRNA-based products compared to traditional biologics.
Manufacturing capacity constraints remain a significant market limitation, with current global production capabilities meeting only 60% of projected demand. This supply-demand gap presents both a challenge and opportunity, driving substantial investments in manufacturing infrastructure and technological innovations in LNP formulation and mRNA stability.
Current Challenges in mRNA LNP Development
Despite significant advancements in mRNA lipid nanoparticle (LNP) technology, several critical challenges continue to impede broader clinical applications and market expansion. The primary obstacle remains the stability of mRNA LNPs under various storage conditions. Current formulations typically require ultra-cold chain storage (-70°C to -20°C), significantly limiting distribution capabilities, especially in regions with underdeveloped infrastructure and increasing overall costs throughout the supply chain.
Immunogenicity presents another substantial hurdle, as LNP components can trigger unwanted immune responses, leading to reduced efficacy in repeat dosing scenarios and potential safety concerns. This challenge is particularly pronounced when targeting chronic conditions that would require multiple administrations of the therapeutic.
Delivery efficiency to specific tissues beyond the liver remains suboptimal. While current LNP formulations excel at liver targeting (making them suitable for hepatic applications), their ability to effectively reach other tissues such as lung, central nervous system, or muscle tissue is limited. This tissue-targeting limitation restricts the potential therapeutic applications of mRNA LNPs to a narrow range of diseases.
Manufacturing scalability continues to challenge widespread adoption. The production of clinical-grade LNPs requires specialized equipment and expertise, with batch-to-batch consistency remaining difficult to achieve at commercial scale. The complex manufacturing process involves multiple critical steps including microfluidic mixing under precise conditions, which presents technical challenges when scaling up.
Regulatory pathways for novel LNP formulations remain somewhat uncertain, particularly for next-generation designs incorporating novel lipids or targeting moieties. The relatively recent emergence of this technology means that regulatory frameworks are still evolving, creating uncertainty for developers.
Cost considerations further complicate market penetration. The specialized lipids used in LNP formulations, particularly ionizable lipids, remain expensive to synthesize at high purity, contributing significantly to the overall cost of goods. This economic barrier limits accessibility, particularly for applications requiring large or repeated doses.
Intellectual property landscapes surrounding LNP technology are increasingly complex, with key patents held by a limited number of companies. This concentration creates potential barriers to entry for new market participants and may slow innovation in the field through licensing requirements and potential litigation risks.
Immunogenicity presents another substantial hurdle, as LNP components can trigger unwanted immune responses, leading to reduced efficacy in repeat dosing scenarios and potential safety concerns. This challenge is particularly pronounced when targeting chronic conditions that would require multiple administrations of the therapeutic.
Delivery efficiency to specific tissues beyond the liver remains suboptimal. While current LNP formulations excel at liver targeting (making them suitable for hepatic applications), their ability to effectively reach other tissues such as lung, central nervous system, or muscle tissue is limited. This tissue-targeting limitation restricts the potential therapeutic applications of mRNA LNPs to a narrow range of diseases.
Manufacturing scalability continues to challenge widespread adoption. The production of clinical-grade LNPs requires specialized equipment and expertise, with batch-to-batch consistency remaining difficult to achieve at commercial scale. The complex manufacturing process involves multiple critical steps including microfluidic mixing under precise conditions, which presents technical challenges when scaling up.
Regulatory pathways for novel LNP formulations remain somewhat uncertain, particularly for next-generation designs incorporating novel lipids or targeting moieties. The relatively recent emergence of this technology means that regulatory frameworks are still evolving, creating uncertainty for developers.
Cost considerations further complicate market penetration. The specialized lipids used in LNP formulations, particularly ionizable lipids, remain expensive to synthesize at high purity, contributing significantly to the overall cost of goods. This economic barrier limits accessibility, particularly for applications requiring large or repeated doses.
Intellectual property landscapes surrounding LNP technology are increasingly complex, with key patents held by a limited number of companies. This concentration creates potential barriers to entry for new market participants and may slow innovation in the field through licensing requirements and potential litigation risks.
Current Delivery Solutions for mRNA Therapeutics
01 Lipid nanoparticle composition for mRNA delivery
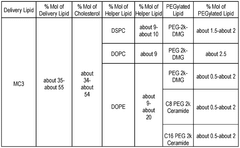
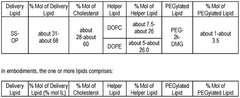
Lipid nanoparticles (LNPs) can be formulated with specific lipid compositions to effectively encapsulate and deliver mRNA to target cells. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios to enhance stability, cellular uptake, and endosomal escape of the mRNA payload. The composition of these lipids significantly impacts the efficacy of mRNA delivery and subsequent protein expression.- Lipid nanoparticle composition for mRNA delivery: Lipid nanoparticles (LNPs) can be formulated with specific lipid compositions to effectively encapsulate and deliver mRNA to target cells. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios to enhance stability, cellular uptake, and endosomal escape of the mRNA payload. The composition of these LNPs significantly impacts their efficacy in delivering therapeutic mRNA for various applications.
- mRNA-LNP manufacturing and production methods: Various manufacturing processes have been developed for the production of mRNA-loaded lipid nanoparticles. These methods include microfluidic mixing, T-junction mixing, and other controlled precipitation techniques that allow for reproducible and scalable production of LNPs with consistent size distribution and encapsulation efficiency. Process parameters such as flow rates, mixing speeds, and temperature control are critical for maintaining product quality and functionality.
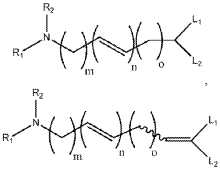
- Modified lipids for enhanced mRNA delivery: Novel lipid structures have been designed to improve the delivery efficiency of mRNA. These include ionizable lipids with optimized pKa values, biodegradable lipids with cleavable bonds, and lipids with specific structural features that enhance cellular uptake and endosomal escape. Chemical modifications to the lipid head groups, linkers, and hydrophobic tails can significantly impact the transfection efficiency and reduce the toxicity of mRNA-LNP formulations.
- Targeted delivery of mRNA-LNPs: Strategies for targeted delivery of mRNA-LNPs to specific tissues or cell types have been developed to improve therapeutic efficacy and reduce off-target effects. These approaches include surface modification of LNPs with targeting ligands such as antibodies, peptides, or aptamers that bind to specific receptors on target cells. Additionally, the incorporation of tissue-specific responsive elements in the LNP formulation can enhance the selective delivery of mRNA to desired tissues.
- Stability and storage of mRNA-LNPs: Methods to enhance the stability and shelf-life of mRNA-LNPs have been developed to address challenges in storage and distribution. These include lyophilization techniques, cryopreservation methods, and the addition of stabilizing excipients that prevent aggregation and degradation of the nanoparticles. Formulation strategies that protect the mRNA cargo from enzymatic degradation and maintain the structural integrity of the LNPs during storage at various temperatures have been implemented to improve the practical application of mRNA therapeutics.
02 Manufacturing methods for mRNA-LNP complexes
Various manufacturing techniques are employed to produce consistent and effective mRNA-loaded lipid nanoparticles. These methods include microfluidic mixing, T-junction mixing, and controlled ethanol dilution processes that allow for reproducible formation of nanoparticles with desired size distributions and encapsulation efficiencies. The manufacturing process parameters significantly influence the physical characteristics and biological performance of the final mRNA-LNP product.Expand Specific Solutions03 Surface modification of mRNA lipid nanoparticles
Surface modifications of lipid nanoparticles can enhance targeting capabilities and improve the pharmacokinetic profile of mRNA therapeutics. These modifications include the incorporation of targeting ligands, antibodies, or cell-penetrating peptides on the nanoparticle surface to facilitate tissue-specific delivery. Additionally, surface charge adjustments and PEG density optimization can reduce immune recognition and extend circulation time in the bloodstream.Expand Specific Solutions04 Stability enhancement of mRNA lipid nanoparticles
Various approaches are employed to enhance the stability of mRNA lipid nanoparticles during storage and in vivo administration. These include lyophilization techniques, cryoprotectant addition, pH optimization, and incorporation of antioxidants to prevent lipid oxidation. Improved stability formulations allow for less stringent cold chain requirements and extended shelf life of mRNA therapeutics, making them more accessible for global distribution.Expand Specific Solutions05 Therapeutic applications of mRNA-LNP technology
mRNA lipid nanoparticles have diverse therapeutic applications beyond vaccines, including protein replacement therapies, cancer immunotherapy, and genetic disease treatment. The technology enables in vivo production of therapeutic proteins, editing of genetic sequences, and modulation of immune responses. Recent advances have focused on optimizing tissue-specific delivery to organs such as the liver, lungs, and central nervous system for treating various diseases with minimal off-target effects.Expand Specific Solutions
Key Industry Players in mRNA LNP Market
The mRNA Lipid Nanoparticle (LNP) innovation market is currently in a growth phase, with an estimated global market size exceeding $5 billion and projected to expand significantly by 2030. The competitive landscape features established pharmaceutical giants like Moderna, Sanofi, and AstraZeneca alongside emerging specialists such as NanoVation Therapeutics, Translate Bio, and Abogen Biosciences. Technical maturity varies across applications, with vaccine delivery systems reaching commercial maturity while therapeutic applications remain in earlier development stages. Academic institutions including MIT, University of British Columbia, and Zhejiang University are driving fundamental research, while companies like Genevant Sciences and eTheRNA are advancing delivery technologies. Regional competition is intensifying with strong representation from North American, European, and Asian players, particularly from China's rapidly expanding biotech sector.
Sanofi
Technical Solution: Sanofi has developed an advanced mRNA LNP platform following its acquisition of Translate Bio in 2021 for $3.2 billion. Their technology incorporates proprietary ionizable amino lipids designed to enhance endosomal escape and cytoplasmic delivery of mRNA. Sanofi's LNP formulations utilize a quaternary structure comprising ionizable lipids, helper phospholipids (typically DSPC), cholesterol, and PEGylated lipids in optimized molar ratios. The company has implemented microfluidic rapid mixing technology for LNP production, achieving consistent particle sizes of 80-100nm with narrow polydispersity indices (<0.2) and high encapsulation efficiencies (>90%). A key innovation in Sanofi's platform is their development of biodegradable ionizable lipids containing ester linkages that facilitate in vivo breakdown, potentially improving safety profiles by reducing lipid accumulation in tissues after repeated dosing. Their LNP technology has demonstrated targeted delivery capabilities beyond the liver, with formulations specifically designed for pulmonary delivery for respiratory diseases like cystic fibrosis and COVID-19.
Strengths: Robust manufacturing infrastructure and global distribution capabilities; significant financial resources for continued R&D investment; integrated development approach combining mRNA expertise from Translate Bio with Sanofi's pharmaceutical development experience. Weaknesses: Relatively late market entry compared to mRNA pioneers like Moderna and BioNTech; limited clinical validation of their proprietary LNP formulations; potential challenges in differentiating their technology in an increasingly competitive landscape.
ModernaTX, Inc.
Technical Solution: ModernaTX has pioneered proprietary lipid nanoparticle (LNP) delivery systems specifically optimized for mRNA therapeutics. Their platform utilizes ionizable lipids with unique pKa properties that enable efficient endosomal escape, a critical barrier in mRNA delivery. The company has developed SM-102, a novel ionizable lipid that forms part of their LNP formulation used in their COVID-19 vaccine. Their LNP technology incorporates precise ratios of four components: ionizable lipids, helper phospholipids, cholesterol, and PEG-lipids, creating nanoparticles with controlled size distribution (typically 80-100nm) and high encapsulation efficiency (>90%). Moderna has also engineered their LNPs with tissue-targeting capabilities through surface modifications and lipid composition adjustments, allowing for preferential delivery to specific organs beyond the liver, which has traditionally been the primary target for LNP technologies.
Strengths: Industry-leading mRNA encapsulation efficiency and delivery capabilities; extensive clinical validation through COVID-19 vaccine success; proprietary ionizable lipid formulations with demonstrated safety profiles. Weaknesses: Higher manufacturing costs compared to traditional vaccine platforms; potential cold chain storage requirements limiting distribution in resource-limited settings; limited long-term safety data for novel lipid components.
Critical Patents and Innovations in LNP Formulation
Lipid nanoparticle (LNP) formulations
PatentWO2024226779A1
Innovation
- The development of lipid nanoparticle (LNP) formulations comprising specific lipids that associate with nucleic acid-based agents, including modified mRNA and plasmid DNA, to form aggregates or particles that can be delivered to the retina, utilizing a combination of cationic, anionic, and neutral lipids, along with PEGylated lipids to enhance stability and targeting.
Improved process of preparing MRNA-loaded lipid nanoparticles
PatentWO2020047061A1
Innovation
- A process involving the mixing of pre-formed lipid nanoparticles with mRNA at reduced concentrations, typically no greater than 0.5 mg/ml, to avoid aggregation and maintain encapsulation efficiency, while minimizing the use of PEG-modified lipids, and potentially involving heating or maintaining at specific temperatures.
Regulatory Framework for mRNA LNP Approval
The regulatory landscape for mRNA Lipid Nanoparticle (LNP) technologies represents a complex and evolving framework that significantly impacts market development and innovation trajectories. Currently, regulatory bodies worldwide are adapting their approval processes to accommodate this novel therapeutic modality, with the FDA, EMA, and PMDA leading efforts to establish specialized guidelines.
The FDA has implemented an accelerated pathway for mRNA LNP products, particularly evident during the COVID-19 pandemic when Emergency Use Authorization (EUA) facilitated rapid deployment of mRNA vaccines. This precedent has established a regulatory foundation that continues to evolve toward more standardized assessment protocols for safety, efficacy, and quality control parameters specific to LNP formulations.
European regulatory frameworks through the EMA emphasize additional considerations regarding biodegradability of lipid components and environmental impact assessments. Their centralized procedure for advanced therapy medicinal products (ATMPs) now includes specific provisions for mRNA LNP technologies, requiring comprehensive characterization of nanoparticle size distribution, encapsulation efficiency, and lipid composition stability.
Regulatory requirements across jurisdictions consistently emphasize several critical aspects for mRNA LNP approval: demonstration of consistent manufacturing processes, comprehensive characterization of physicochemical properties, biodistribution studies, immunogenicity assessments, and stability data under various storage conditions. These requirements create significant market entry barriers but simultaneously establish quality benchmarks that drive innovation.
Harmonization efforts between major regulatory authorities are underway through the International Council for Harmonisation (ICH), which is developing specific guidelines for lipid-based delivery systems. This initiative aims to reduce regulatory divergence and streamline global development pathways, potentially accelerating market access for innovative formulations.
Emerging markets present varying regulatory approaches, with countries like China implementing expedited review processes for mRNA technologies while maintaining stringent local clinical trial requirements. India has established a specialized committee for evaluating nucleic acid-based therapeutics, focusing on technology transfer potential and local manufacturing capabilities.
The regulatory timeline for mRNA LNP products typically spans 12-18 months for clinical trial approvals and potentially 6-10 months for marketing authorization under standard pathways, though accelerated programs can significantly compress these timeframes. Companies demonstrating robust chemistry, manufacturing, and controls (CMC) documentation for their LNP formulations typically experience fewer regulatory delays.
Future regulatory developments are likely to focus on establishing reference standards for critical quality attributes of LNPs, post-marketing surveillance requirements specific to lipid components, and refined guidelines for demonstrating bioequivalence of generic or biosimilar mRNA LNP products.
The FDA has implemented an accelerated pathway for mRNA LNP products, particularly evident during the COVID-19 pandemic when Emergency Use Authorization (EUA) facilitated rapid deployment of mRNA vaccines. This precedent has established a regulatory foundation that continues to evolve toward more standardized assessment protocols for safety, efficacy, and quality control parameters specific to LNP formulations.
European regulatory frameworks through the EMA emphasize additional considerations regarding biodegradability of lipid components and environmental impact assessments. Their centralized procedure for advanced therapy medicinal products (ATMPs) now includes specific provisions for mRNA LNP technologies, requiring comprehensive characterization of nanoparticle size distribution, encapsulation efficiency, and lipid composition stability.
Regulatory requirements across jurisdictions consistently emphasize several critical aspects for mRNA LNP approval: demonstration of consistent manufacturing processes, comprehensive characterization of physicochemical properties, biodistribution studies, immunogenicity assessments, and stability data under various storage conditions. These requirements create significant market entry barriers but simultaneously establish quality benchmarks that drive innovation.
Harmonization efforts between major regulatory authorities are underway through the International Council for Harmonisation (ICH), which is developing specific guidelines for lipid-based delivery systems. This initiative aims to reduce regulatory divergence and streamline global development pathways, potentially accelerating market access for innovative formulations.
Emerging markets present varying regulatory approaches, with countries like China implementing expedited review processes for mRNA technologies while maintaining stringent local clinical trial requirements. India has established a specialized committee for evaluating nucleic acid-based therapeutics, focusing on technology transfer potential and local manufacturing capabilities.
The regulatory timeline for mRNA LNP products typically spans 12-18 months for clinical trial approvals and potentially 6-10 months for marketing authorization under standard pathways, though accelerated programs can significantly compress these timeframes. Companies demonstrating robust chemistry, manufacturing, and controls (CMC) documentation for their LNP formulations typically experience fewer regulatory delays.
Future regulatory developments are likely to focus on establishing reference standards for critical quality attributes of LNPs, post-marketing surveillance requirements specific to lipid components, and refined guidelines for demonstrating bioequivalence of generic or biosimilar mRNA LNP products.
Manufacturing Scalability and Cost Analysis
The scalability of mRNA lipid nanoparticle (LNP) manufacturing represents a critical bottleneck in the widespread adoption of this revolutionary technology. Current manufacturing processes for mRNA-LNP formulations involve complex multi-step procedures including mRNA synthesis, lipid component preparation, and nanoparticle assembly, each requiring specialized equipment and expertise. The transition from laboratory-scale production to industrial manufacturing volumes presents significant technical challenges that directly impact production costs.
Analysis of manufacturing costs reveals that raw materials constitute approximately 30-40% of total production expenses, with specialized lipids being particularly costly components. GMP-grade ionizable lipids can cost between $50,000-100,000 per kilogram, creating a substantial cost barrier. Process inefficiencies further compound these expenses, with current manufacturing yields typically ranging from 60-80%, meaning significant material wastage occurs during production.
Energy consumption represents another substantial cost factor, particularly in the lipid extrusion and purification phases which require precise temperature control and specialized filtration systems. The cold chain requirements for both raw materials and finished products add approximately 15-20% to overall logistics expenses compared to conventional pharmaceuticals.
Equipment utilization efficiency presents additional scalability challenges. Current manufacturing setups often operate in batch processes rather than continuous manufacturing paradigms, resulting in equipment downtime between production cycles. Industry data suggests that transitioning to continuous manufacturing could potentially reduce production costs by 25-30% while increasing throughput by similar margins.
Regulatory compliance adds another layer of complexity to manufacturing scalability. Each production facility must maintain stringent quality control measures, with documentation and validation processes accounting for approximately 15% of overall manufacturing costs. These regulatory requirements become increasingly complex when scaling across multiple production sites or geographic regions.
Labor costs vary significantly by region but typically represent 10-15% of total manufacturing expenses. The specialized nature of mRNA-LNP production requires highly trained personnel, creating potential workforce bottlenecks as production scales. Automation opportunities exist but require substantial upfront investment, with ROI typically realized only at higher production volumes.
Recent technological innovations are beginning to address these scalability challenges. Microfluidic mixing technologies have demonstrated the potential to increase batch consistency while reducing material waste. Similarly, advances in lipid synthesis pathways are gradually reducing raw material costs, with several manufacturers reporting 15-20% cost reductions for next-generation ionizable lipids compared to first-generation formulations.
Analysis of manufacturing costs reveals that raw materials constitute approximately 30-40% of total production expenses, with specialized lipids being particularly costly components. GMP-grade ionizable lipids can cost between $50,000-100,000 per kilogram, creating a substantial cost barrier. Process inefficiencies further compound these expenses, with current manufacturing yields typically ranging from 60-80%, meaning significant material wastage occurs during production.
Energy consumption represents another substantial cost factor, particularly in the lipid extrusion and purification phases which require precise temperature control and specialized filtration systems. The cold chain requirements for both raw materials and finished products add approximately 15-20% to overall logistics expenses compared to conventional pharmaceuticals.
Equipment utilization efficiency presents additional scalability challenges. Current manufacturing setups often operate in batch processes rather than continuous manufacturing paradigms, resulting in equipment downtime between production cycles. Industry data suggests that transitioning to continuous manufacturing could potentially reduce production costs by 25-30% while increasing throughput by similar margins.
Regulatory compliance adds another layer of complexity to manufacturing scalability. Each production facility must maintain stringent quality control measures, with documentation and validation processes accounting for approximately 15% of overall manufacturing costs. These regulatory requirements become increasingly complex when scaling across multiple production sites or geographic regions.
Labor costs vary significantly by region but typically represent 10-15% of total manufacturing expenses. The specialized nature of mRNA-LNP production requires highly trained personnel, creating potential workforce bottlenecks as production scales. Automation opportunities exist but require substantial upfront investment, with ROI typically realized only at higher production volumes.
Recent technological innovations are beginning to address these scalability challenges. Microfluidic mixing technologies have demonstrated the potential to increase batch consistency while reducing material waste. Similarly, advances in lipid synthesis pathways are gradually reducing raw material costs, with several manufacturers reporting 15-20% cost reductions for next-generation ionizable lipids compared to first-generation formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!