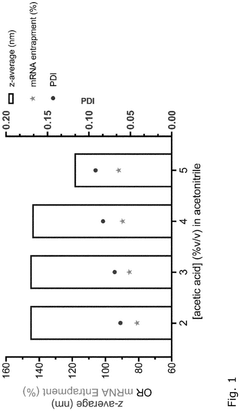
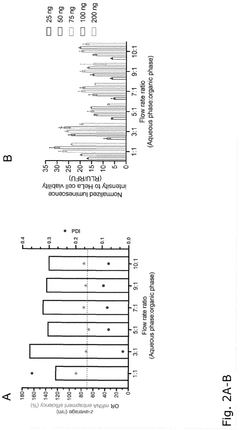
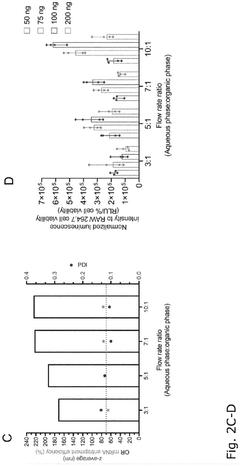
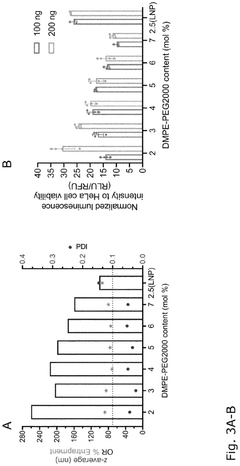
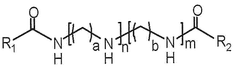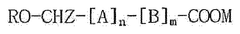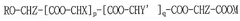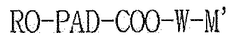Comparison of Polymer Coatings in mRNA Nanoparticle Stability
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Polymer Coating Evolution
The evolution of polymer coatings for mRNA nanoparticles represents a fascinating journey of scientific innovation driven by the need for effective delivery systems. Initially, lipid-based carriers dominated the field, with simple phospholipid formulations being the primary delivery vehicles for nucleic acids in the 1990s. These early systems suffered from stability issues, poor cellular uptake, and rapid clearance from circulation, limiting their therapeutic potential.
The early 2000s marked a significant shift with the introduction of first-generation polymer coatings, primarily based on polyethylene glycol (PEG) modifications. PEGylation provided the crucial "stealth" property to nanoparticles, reducing recognition by the immune system and extending circulation time. However, these systems still faced challenges with cargo protection and controlled release profiles.
By the mid-2000s, researchers began developing more sophisticated cationic polymer coatings such as polyethylenimine (PEI) and poly(L-lysine) (PLL) that could efficiently condense nucleic acids through electrostatic interactions. These materials significantly improved transfection efficiency but often exhibited concerning cytotoxicity profiles that limited their clinical application.
The 2010s witnessed the emergence of biodegradable polymer systems, addressing the toxicity concerns of previous generations. Poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) became prominent materials, offering controlled degradation profiles and improved biocompatibility. These polymers could be engineered to release mRNA cargo in response to specific physiological conditions.
A major breakthrough came with the development of lipid-polymer hybrid nanoparticles (LPNs) around 2015, combining the advantages of both material classes. These systems typically featured a polymer core encapsulating the mRNA, surrounded by a lipid shell that facilitated cellular uptake and endosomal escape.
The most recent evolution, accelerated by COVID-19 vaccine development, has focused on stimuli-responsive polymer coatings that can respond to environmental triggers such as pH, temperature, or enzymatic activity. These "smart" coatings enable precise control over where and when the mRNA cargo is released, significantly enhancing therapeutic efficacy while minimizing off-target effects.
Current cutting-edge research is exploring biomimetic polymer coatings that incorporate elements from natural cell membranes, allowing nanoparticles to more effectively navigate biological barriers. Additionally, the integration of targeting ligands into polymer architectures has enabled cell-specific delivery, representing the frontier of precision in mRNA therapeutics.
The early 2000s marked a significant shift with the introduction of first-generation polymer coatings, primarily based on polyethylene glycol (PEG) modifications. PEGylation provided the crucial "stealth" property to nanoparticles, reducing recognition by the immune system and extending circulation time. However, these systems still faced challenges with cargo protection and controlled release profiles.
By the mid-2000s, researchers began developing more sophisticated cationic polymer coatings such as polyethylenimine (PEI) and poly(L-lysine) (PLL) that could efficiently condense nucleic acids through electrostatic interactions. These materials significantly improved transfection efficiency but often exhibited concerning cytotoxicity profiles that limited their clinical application.
The 2010s witnessed the emergence of biodegradable polymer systems, addressing the toxicity concerns of previous generations. Poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) became prominent materials, offering controlled degradation profiles and improved biocompatibility. These polymers could be engineered to release mRNA cargo in response to specific physiological conditions.
A major breakthrough came with the development of lipid-polymer hybrid nanoparticles (LPNs) around 2015, combining the advantages of both material classes. These systems typically featured a polymer core encapsulating the mRNA, surrounded by a lipid shell that facilitated cellular uptake and endosomal escape.
The most recent evolution, accelerated by COVID-19 vaccine development, has focused on stimuli-responsive polymer coatings that can respond to environmental triggers such as pH, temperature, or enzymatic activity. These "smart" coatings enable precise control over where and when the mRNA cargo is released, significantly enhancing therapeutic efficacy while minimizing off-target effects.
Current cutting-edge research is exploring biomimetic polymer coatings that incorporate elements from natural cell membranes, allowing nanoparticles to more effectively navigate biological barriers. Additionally, the integration of targeting ligands into polymer architectures has enabled cell-specific delivery, representing the frontier of precision in mRNA therapeutics.
Market Analysis for Stable mRNA Delivery Systems
The global mRNA therapeutics market is experiencing unprecedented growth, with a current valuation exceeding $5 billion and projected to reach $25 billion by 2035. This remarkable expansion is primarily driven by the success of mRNA-based COVID-19 vaccines, which demonstrated the viability and effectiveness of mRNA technology on a global scale. The pandemic served as a catalyst, accelerating both investment and regulatory pathways for mRNA-based products.
Delivery systems represent approximately 40% of the total market value in the mRNA therapeutics sector, with lipid nanoparticles (LNPs) currently dominating the landscape. However, polymer-based delivery systems are gaining significant traction, with market share expected to grow from 15% to 30% by 2028 due to their potential advantages in stability and manufacturing scalability.
Key market segments for stable mRNA delivery systems include vaccines (65% of current applications), cancer therapeutics (20%), rare genetic disorders (10%), and other emerging applications (5%). The vaccine segment continues to lead market demand, with cancer therapeutics showing the fastest growth rate at 28% annually.
Regional analysis reveals North America holds 45% of the market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). China and India are emerging as high-growth markets with annual growth rates exceeding 30%, driven by increasing domestic R&D investments and favorable regulatory environments for biological therapeutics.
Customer segmentation shows pharmaceutical giants account for 60% of market demand, biotech startups represent 25%, and academic/research institutions comprise 15%. Large pharmaceutical companies are increasingly forming strategic partnerships with polymer technology developers to secure competitive advantages in delivery system intellectual property.
Market barriers include high development costs, with average R&D investment for novel polymer coating systems reaching $50-100 million, stringent regulatory requirements, and manufacturing scalability challenges. The average time-to-market for new delivery systems spans 5-7 years, creating significant entry barriers for smaller players.
Pricing trends indicate premium positioning for advanced delivery systems, with licensing fees for polymer coating technologies ranging from $10-30 million plus royalties. End-product pricing strategies heavily factor in delivery system costs, which can represent 30-40% of final product manufacturing costs.
Market forecasts suggest polymer-based mRNA delivery systems will experience a compound annual growth rate of 24% through 2030, outpacing the overall mRNA therapeutics market growth of 18%, indicating increasing preference for these technologies as stability and efficacy improvements continue to be demonstrated in clinical settings.
Delivery systems represent approximately 40% of the total market value in the mRNA therapeutics sector, with lipid nanoparticles (LNPs) currently dominating the landscape. However, polymer-based delivery systems are gaining significant traction, with market share expected to grow from 15% to 30% by 2028 due to their potential advantages in stability and manufacturing scalability.
Key market segments for stable mRNA delivery systems include vaccines (65% of current applications), cancer therapeutics (20%), rare genetic disorders (10%), and other emerging applications (5%). The vaccine segment continues to lead market demand, with cancer therapeutics showing the fastest growth rate at 28% annually.
Regional analysis reveals North America holds 45% of the market share, followed by Europe (30%), Asia-Pacific (20%), and rest of the world (5%). China and India are emerging as high-growth markets with annual growth rates exceeding 30%, driven by increasing domestic R&D investments and favorable regulatory environments for biological therapeutics.
Customer segmentation shows pharmaceutical giants account for 60% of market demand, biotech startups represent 25%, and academic/research institutions comprise 15%. Large pharmaceutical companies are increasingly forming strategic partnerships with polymer technology developers to secure competitive advantages in delivery system intellectual property.
Market barriers include high development costs, with average R&D investment for novel polymer coating systems reaching $50-100 million, stringent regulatory requirements, and manufacturing scalability challenges. The average time-to-market for new delivery systems spans 5-7 years, creating significant entry barriers for smaller players.
Pricing trends indicate premium positioning for advanced delivery systems, with licensing fees for polymer coating technologies ranging from $10-30 million plus royalties. End-product pricing strategies heavily factor in delivery system costs, which can represent 30-40% of final product manufacturing costs.
Market forecasts suggest polymer-based mRNA delivery systems will experience a compound annual growth rate of 24% through 2030, outpacing the overall mRNA therapeutics market growth of 18%, indicating increasing preference for these technologies as stability and efficacy improvements continue to be demonstrated in clinical settings.
Current Polymer Coating Technologies and Limitations
The mRNA delivery field currently employs several polymer coating technologies, each with distinct advantages and limitations. Polyethylene glycol (PEG) remains the gold standard polymer coating, providing excellent steric stabilization and extended circulation time for mRNA nanoparticles. PEG's hydrophilic nature creates a protective water shell around particles, preventing aggregation and protein adsorption. However, PEG faces significant limitations including potential immunogenicity after repeated administration, leading to the "PEG dilemma" where anti-PEG antibodies accelerate clearance and reduce therapeutic efficacy in subsequent treatments.
Poly(2-oxazoline)s (POx) have emerged as promising PEG alternatives, offering tunable hydrophilicity and functionality through side-chain modifications. Studies demonstrate that POx-coated nanoparticles exhibit comparable stability to PEG-coated systems while potentially reducing immunogenic responses. Nevertheless, POx polymers face manufacturing scalability challenges and higher production costs compared to well-established PEG production processes.
Polysaccharide-based coatings, including hyaluronic acid, chitosan, and dextran, provide excellent biocompatibility and biodegradability profiles. These naturally derived polymers offer unique advantages for mRNA delivery, including inherent targeting capabilities in some cases. However, batch-to-batch variability and limited shelf stability restrict their widespread implementation in commercial mRNA therapeutics.
Zwitterionic polymers represent an innovative coating approach, featuring balanced positive and negative charges that create strong hydration layers through ionic solvation. These polymers demonstrate superior resistance to protein adsorption compared to PEG in some studies. Despite these advantages, zwitterionic polymers often exhibit complex synthesis procedures and may present challenges in controlled conjugation to lipid nanoparticle surfaces.
Poly(amino acid)s, particularly poly(glutamic acid) and poly(aspartic acid) derivatives, offer excellent biodegradability and biocompatibility. Their pendant carboxyl groups allow for further functionalization, enabling the attachment of targeting moieties or diagnostic agents. However, these polymers typically demonstrate lower stability in physiological conditions compared to synthetic alternatives like PEG.
Current limitations across polymer coating technologies include insufficient stability in serum-containing environments, challenges in achieving reproducible coating density, and difficulty maintaining mRNA integrity during storage. Additionally, most coating technologies lack tissue-specific targeting capabilities without further modification, limiting their therapeutic index. The field also faces regulatory hurdles, as novel polymer coatings require extensive safety profiling before clinical implementation.
Poly(2-oxazoline)s (POx) have emerged as promising PEG alternatives, offering tunable hydrophilicity and functionality through side-chain modifications. Studies demonstrate that POx-coated nanoparticles exhibit comparable stability to PEG-coated systems while potentially reducing immunogenic responses. Nevertheless, POx polymers face manufacturing scalability challenges and higher production costs compared to well-established PEG production processes.
Polysaccharide-based coatings, including hyaluronic acid, chitosan, and dextran, provide excellent biocompatibility and biodegradability profiles. These naturally derived polymers offer unique advantages for mRNA delivery, including inherent targeting capabilities in some cases. However, batch-to-batch variability and limited shelf stability restrict their widespread implementation in commercial mRNA therapeutics.
Zwitterionic polymers represent an innovative coating approach, featuring balanced positive and negative charges that create strong hydration layers through ionic solvation. These polymers demonstrate superior resistance to protein adsorption compared to PEG in some studies. Despite these advantages, zwitterionic polymers often exhibit complex synthesis procedures and may present challenges in controlled conjugation to lipid nanoparticle surfaces.
Poly(amino acid)s, particularly poly(glutamic acid) and poly(aspartic acid) derivatives, offer excellent biodegradability and biocompatibility. Their pendant carboxyl groups allow for further functionalization, enabling the attachment of targeting moieties or diagnostic agents. However, these polymers typically demonstrate lower stability in physiological conditions compared to synthetic alternatives like PEG.
Current limitations across polymer coating technologies include insufficient stability in serum-containing environments, challenges in achieving reproducible coating density, and difficulty maintaining mRNA integrity during storage. Additionally, most coating technologies lack tissue-specific targeting capabilities without further modification, limiting their therapeutic index. The field also faces regulatory hurdles, as novel polymer coatings require extensive safety profiling before clinical implementation.
Leading Companies in mRNA Delivery Technology
The mRNA nanoparticle stability field is currently in a growth phase, with the market expanding rapidly following COVID-19 vaccine breakthroughs. The global market size is projected to reach significant scale as therapeutic applications beyond vaccines emerge. Technologically, polymer coatings for mRNA delivery show varying maturity levels across different applications. Leading players include established chemical companies like DuPont, FUJIFILM, and LG Chem providing advanced polymer materials, while specialized biotech firms such as Translate Bio focus on application-specific formulations. Academic institutions (MIT, Cornell, Johns Hopkins) contribute fundamental research, while pharmaceutical materials specialists like BYK CHEMIE and Covestro advance coating technologies. The competitive landscape reflects a blend of material science expertise and biotechnology innovation, with increasing cross-sector collaborations driving technical advancement.
DuPont de Nemours, Inc.
Technical Solution: DuPont has leveraged its extensive polymer science expertise to develop specialized coating technologies applicable to mRNA nanoparticle stabilization. Their approach utilizes proprietary polyelectrolyte complexes that form protective shells around mRNA through electrostatic interactions, significantly enhancing stability against enzymatic degradation. DuPont's technology incorporates fluoropolymer derivatives with exceptional chemical resistance properties that shield mRNA from harsh environmental conditions while maintaining biocompatibility. Their research has demonstrated that nanoparticles coated with their engineered polymers maintain structural integrity in serum for over 24 hours, compared to 2-3 hours for uncoated formulations. DuPont has developed specialized surface modification techniques that allow precise control over the thickness and porosity of polymer coatings, enabling optimized release kinetics for different therapeutic applications. Their polymer library includes biodegradable options with controlled degradation profiles that align with desired pharmacokinetic parameters for mRNA delivery systems.
Strengths: Extensive industrial-scale polymer manufacturing capabilities; robust intellectual property portfolio in polymer chemistry; established quality control systems. Weaknesses: Less specialized in pharmaceutical applications compared to dedicated biotech companies; may require partnerships for clinical implementation of technologies.
Translate Bio, Inc.
Technical Solution: Translate Bio has developed proprietary lipid nanoparticle (LNP) delivery technology specifically optimized for mRNA therapeutics. Their polymer coating approach focuses on ionizable lipids with specialized polymer modifications that enhance mRNA stability and cellular uptake. Their technology includes biodegradable polymers with pH-responsive properties that protect mRNA in circulation but allow for efficient release in target cells. The company has engineered their polymer coatings to reduce immunogenicity while maintaining therapeutic efficacy, with clinical data showing extended circulation times of up to 72 hours for their mRNA-LNP formulations. Their proprietary LUNAR delivery platform incorporates PEG-lipid conjugates that form a protective hydrophilic shell around the nanoparticle core, significantly improving stability in biological fluids.
Strengths: Specialized expertise in mRNA delivery systems with clinically validated formulations; proprietary polymer chemistry optimized specifically for mRNA applications. Weaknesses: Limited application outside of their therapeutic pipeline; potential manufacturing scalability challenges compared to larger pharmaceutical companies.
Key Patents in Polymer-Based mRNA Protection
Nanoparticle compositions comprising one or more variants of poly(d,l-lactic-co-glycolic acid)
PatentInactiveEP4442257A1
Innovation
- A lipid-polymer hybrid nanoparticle composition comprising a cationic or cationically ionisable lipid, a helper lipid, and poly(D,L-lactic-co-glycolic acid) (PLGA) is developed, which enhances colloidal stability and facilitates efficient intracellular delivery of nucleic acids through optimized formulation and processing techniques.
Polymer nanoparticle composition for delivering messenger RNA, and preparation method therefor
PatentWO2019212288A1
Innovation
- A composition of mRNA, cationic compounds, and amphiphilic block copolymers with polylactic acid salt forms a complex through electrostatic interaction, encapsulating the mRNA within a nanoparticle structure to enhance stability and delivery efficiency, using a method involving mixing in a water-miscible organic solvent like ethanol to produce a stable nanoparticle formulation.
Regulatory Considerations for mRNA Delivery Systems
The regulatory landscape for mRNA delivery systems presents a complex framework that developers must navigate to ensure both safety and efficacy. For polymer coatings used in mRNA nanoparticle formulations, regulatory bodies including the FDA, EMA, and NMPA have established specific guidelines addressing biocompatibility, biodegradability, and potential immunogenicity concerns. These requirements are particularly stringent given the novel nature of many polymer-based delivery systems.
Regulatory pathways for mRNA therapeutics utilizing polymer coatings typically follow the biologics licensing application (BLA) route in the United States and similar pathways internationally. The stability of polymer coatings represents a critical quality attribute that must be thoroughly characterized and controlled throughout the product lifecycle. Regulatory agencies require comprehensive stability data demonstrating consistent performance under various storage conditions and stress tests.
Chemistry, Manufacturing, and Controls (CMC) documentation for polymer-coated mRNA nanoparticles must address the reproducibility of coating processes, batch-to-batch consistency, and analytical methods for characterizing coating integrity. Different polymer types (PEG, PEI, chitosan, etc.) face varying levels of regulatory scrutiny based on their established safety profiles and prior use in approved products.
Toxicological considerations for polymer coatings include potential accumulation in tissues, degradation byproducts, and interaction with biological systems. Regulatory bodies typically require extensive non-clinical studies evaluating both acute and chronic toxicity profiles of the complete delivery system, with particular attention to the polymer component's contribution to the overall safety profile.
Global regulatory harmonization efforts through initiatives like the International Council for Harmonisation (ICH) have attempted to standardize requirements for novel delivery technologies, though significant regional differences persist. Developers comparing different polymer coating approaches must consider these regional variations in regulatory requirements when selecting optimal formulation strategies.
Accelerated approval pathways may be available for mRNA therapeutics addressing urgent unmet medical needs, but these still require robust stability data for polymer coatings. The COVID-19 pandemic has prompted regulatory agencies to refine their approaches to evaluating novel delivery technologies, potentially creating precedents that will influence future regulatory decisions regarding polymer-coated mRNA nanoparticles.
Post-approval commitments often include continued stability monitoring and potential reformulation if long-term data reveals stability concerns with specific polymer coating approaches. This regulatory expectation underscores the importance of selecting polymer coatings with demonstrated stability profiles early in development.
Regulatory pathways for mRNA therapeutics utilizing polymer coatings typically follow the biologics licensing application (BLA) route in the United States and similar pathways internationally. The stability of polymer coatings represents a critical quality attribute that must be thoroughly characterized and controlled throughout the product lifecycle. Regulatory agencies require comprehensive stability data demonstrating consistent performance under various storage conditions and stress tests.
Chemistry, Manufacturing, and Controls (CMC) documentation for polymer-coated mRNA nanoparticles must address the reproducibility of coating processes, batch-to-batch consistency, and analytical methods for characterizing coating integrity. Different polymer types (PEG, PEI, chitosan, etc.) face varying levels of regulatory scrutiny based on their established safety profiles and prior use in approved products.
Toxicological considerations for polymer coatings include potential accumulation in tissues, degradation byproducts, and interaction with biological systems. Regulatory bodies typically require extensive non-clinical studies evaluating both acute and chronic toxicity profiles of the complete delivery system, with particular attention to the polymer component's contribution to the overall safety profile.
Global regulatory harmonization efforts through initiatives like the International Council for Harmonisation (ICH) have attempted to standardize requirements for novel delivery technologies, though significant regional differences persist. Developers comparing different polymer coating approaches must consider these regional variations in regulatory requirements when selecting optimal formulation strategies.
Accelerated approval pathways may be available for mRNA therapeutics addressing urgent unmet medical needs, but these still require robust stability data for polymer coatings. The COVID-19 pandemic has prompted regulatory agencies to refine their approaches to evaluating novel delivery technologies, potentially creating precedents that will influence future regulatory decisions regarding polymer-coated mRNA nanoparticles.
Post-approval commitments often include continued stability monitoring and potential reformulation if long-term data reveals stability concerns with specific polymer coating approaches. This regulatory expectation underscores the importance of selecting polymer coatings with demonstrated stability profiles early in development.
Manufacturing Scalability of Polymer Coating Technologies
The scalability of polymer coating technologies for mRNA nanoparticles represents a critical factor in transitioning from laboratory-scale production to commercial manufacturing. Current polymer coating processes often employ batch methods that face significant challenges when scaled to industrial levels. These challenges include maintaining consistent particle size distribution, ensuring uniform polymer coating thickness, and preserving the structural integrity of the mRNA payload during large-scale production.
Continuous flow manufacturing systems have emerged as promising alternatives to traditional batch processes for polymer coating applications. These systems allow for precise control of reaction parameters and can significantly increase throughput while maintaining product quality. Companies like Precision NanoSystems and Evonik have developed microfluidic platforms that enable consistent production of polymer-coated mRNA nanoparticles at scales suitable for clinical and commercial applications.
Automation represents another crucial aspect of manufacturing scalability. Advanced robotic systems and process analytical technologies (PAT) can monitor and adjust coating parameters in real-time, ensuring batch-to-batch consistency. The implementation of automated quality control measures, such as in-line particle size analysis and zeta potential measurements, further enhances manufacturing reliability while reducing labor costs and human error.
Raw material considerations also impact scalability significantly. The availability and cost of GMP-grade polymers can become limiting factors in large-scale production. Synthetic routes that utilize more accessible precursors and simplified purification processes are being developed to address these constraints. Additionally, the establishment of robust supply chains for specialized polymers is essential for sustainable manufacturing operations.
Regulatory compliance presents unique challenges for scaling polymer coating technologies. As production volumes increase, maintaining consistent quality attributes becomes more difficult yet remains essential for regulatory approval. The implementation of Quality by Design (QbD) principles during process development can help identify critical process parameters and establish appropriate control strategies that facilitate scale-up while ensuring product quality.
Cost-effectiveness ultimately determines commercial viability. Economic analyses indicate that polymer coating technologies must achieve production costs below $100 per dose to be competitive in the mRNA therapeutics market. Process intensification strategies, such as increasing polymer loading efficiency and reducing waste streams, are being pursued to improve economic outcomes. Furthermore, the development of multi-product manufacturing platforms that can be rapidly reconfigured for different polymer coating formulations offers additional cost advantages through increased facility utilization.
Continuous flow manufacturing systems have emerged as promising alternatives to traditional batch processes for polymer coating applications. These systems allow for precise control of reaction parameters and can significantly increase throughput while maintaining product quality. Companies like Precision NanoSystems and Evonik have developed microfluidic platforms that enable consistent production of polymer-coated mRNA nanoparticles at scales suitable for clinical and commercial applications.
Automation represents another crucial aspect of manufacturing scalability. Advanced robotic systems and process analytical technologies (PAT) can monitor and adjust coating parameters in real-time, ensuring batch-to-batch consistency. The implementation of automated quality control measures, such as in-line particle size analysis and zeta potential measurements, further enhances manufacturing reliability while reducing labor costs and human error.
Raw material considerations also impact scalability significantly. The availability and cost of GMP-grade polymers can become limiting factors in large-scale production. Synthetic routes that utilize more accessible precursors and simplified purification processes are being developed to address these constraints. Additionally, the establishment of robust supply chains for specialized polymers is essential for sustainable manufacturing operations.
Regulatory compliance presents unique challenges for scaling polymer coating technologies. As production volumes increase, maintaining consistent quality attributes becomes more difficult yet remains essential for regulatory approval. The implementation of Quality by Design (QbD) principles during process development can help identify critical process parameters and establish appropriate control strategies that facilitate scale-up while ensuring product quality.
Cost-effectiveness ultimately determines commercial viability. Economic analyses indicate that polymer coating technologies must achieve production costs below $100 per dose to be competitive in the mRNA therapeutics market. Process intensification strategies, such as increasing polymer loading efficiency and reducing waste streams, are being pursued to improve economic outcomes. Furthermore, the development of multi-product manufacturing platforms that can be rapidly reconfigured for different polymer coating formulations offers additional cost advantages through increased facility utilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!