What Enhances Stability in mRNA Nanoparticle Applications
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Evolution and Stability Goals
The evolution of mRNA nanoparticle technology has witnessed significant advancements since its conceptualization in the early 1990s. Initially, the primary challenge was the inherent instability of naked mRNA molecules, which are highly susceptible to degradation by ubiquitous ribonucleases. The field progressed from basic lipid encapsulation techniques to sophisticated lipid nanoparticles (LNPs) that offer enhanced protection and delivery efficiency.
The stability goals for mRNA nanoparticles have evolved considerably over time. Early research focused primarily on preventing nuclease degradation, while contemporary objectives encompass a multifaceted approach to stability. These include maintaining structural integrity during storage, preserving functionality during administration, ensuring targeted delivery to specific cells, and achieving controlled release kinetics at the target site.
A critical milestone in this evolution was the development of modified nucleosides, particularly pseudouridine and N1-methylpseudouridine, which significantly reduced immunogenicity while enhancing translation efficiency. This breakthrough, pioneered by Karikó and colleagues in the mid-2000s, fundamentally transformed the stability profile of mRNA therapeutics by addressing both physical and functional stability concerns.
The COVID-19 pandemic accelerated research into mRNA nanoparticle stability, particularly in the context of vaccine development. The stringent cold chain requirements of the first-generation mRNA vaccines highlighted the persistent challenges in achieving ambient temperature stability, prompting intensive research into novel formulation strategies and excipients.
Current stability goals focus on achieving room temperature stability for extended periods, which would revolutionize the global accessibility of mRNA therapeutics. This involves innovative approaches such as lyophilization techniques, incorporation of stabilizing excipients, and development of alternative delivery systems beyond traditional LNPs.
Another emerging stability objective is the development of stimuli-responsive nanoparticles that maintain integrity during circulation but release their cargo precisely at target sites in response to specific biological triggers. This controlled release approach aims to enhance therapeutic efficacy while minimizing off-target effects.
The field is now moving toward rational design principles for mRNA nanoparticles, leveraging computational modeling and high-throughput screening to optimize formulations for specific applications. The ultimate goal is to develop a versatile platform technology that can be readily adapted to different therapeutic contexts while maintaining consistent stability profiles across various environmental conditions.
The stability goals for mRNA nanoparticles have evolved considerably over time. Early research focused primarily on preventing nuclease degradation, while contemporary objectives encompass a multifaceted approach to stability. These include maintaining structural integrity during storage, preserving functionality during administration, ensuring targeted delivery to specific cells, and achieving controlled release kinetics at the target site.
A critical milestone in this evolution was the development of modified nucleosides, particularly pseudouridine and N1-methylpseudouridine, which significantly reduced immunogenicity while enhancing translation efficiency. This breakthrough, pioneered by Karikó and colleagues in the mid-2000s, fundamentally transformed the stability profile of mRNA therapeutics by addressing both physical and functional stability concerns.
The COVID-19 pandemic accelerated research into mRNA nanoparticle stability, particularly in the context of vaccine development. The stringent cold chain requirements of the first-generation mRNA vaccines highlighted the persistent challenges in achieving ambient temperature stability, prompting intensive research into novel formulation strategies and excipients.
Current stability goals focus on achieving room temperature stability for extended periods, which would revolutionize the global accessibility of mRNA therapeutics. This involves innovative approaches such as lyophilization techniques, incorporation of stabilizing excipients, and development of alternative delivery systems beyond traditional LNPs.
Another emerging stability objective is the development of stimuli-responsive nanoparticles that maintain integrity during circulation but release their cargo precisely at target sites in response to specific biological triggers. This controlled release approach aims to enhance therapeutic efficacy while minimizing off-target effects.
The field is now moving toward rational design principles for mRNA nanoparticles, leveraging computational modeling and high-throughput screening to optimize formulations for specific applications. The ultimate goal is to develop a versatile platform technology that can be readily adapted to different therapeutic contexts while maintaining consistent stability profiles across various environmental conditions.
Market Analysis for Stable mRNA Delivery Systems
The global market for stable mRNA delivery systems is experiencing unprecedented growth, driven by the success of mRNA-based COVID-19 vaccines and expanding therapeutic applications. Current market valuations indicate that the mRNA therapeutics market reached approximately $40 billion in 2022, with projections suggesting a compound annual growth rate of 13-15% through 2030, potentially reaching $100 billion by the end of the decade.
Lipid nanoparticle (LNP) delivery systems currently dominate the market, accounting for over 80% of commercial mRNA delivery applications. This dominance stems from their proven efficacy in vaccine delivery and relatively established manufacturing processes. However, polymer-based nanoparticles are gaining traction, with market share expected to increase from current 10% to potentially 25% by 2027 due to their enhanced stability profiles and customization capabilities.
Regional analysis reveals North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. China and India are emerging as high-growth markets with annual growth rates exceeding 20%, driven by substantial government investments in biotechnology infrastructure and favorable regulatory environments for mRNA therapeutics.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech controlling nearly 70% of the current market. However, specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and Genevant Sciences are rapidly expanding their market presence through strategic partnerships and proprietary stability-enhancing technologies.
Demand segmentation shows vaccines currently represent 65% of market applications, while therapeutic applications account for 30% and diagnostic uses comprise the remaining 5%. However, therapeutic applications are projected to grow at twice the rate of vaccines over the next five years as stability improvements enable more diverse clinical applications.
Key market drivers include increasing prevalence of chronic diseases, growing cancer burden, rising investment in personalized medicine, and expanding applications beyond infectious diseases. The COVID-19 pandemic has accelerated market adoption by demonstrating the viability of mRNA technology at scale and establishing manufacturing and distribution infrastructure.
Market challenges primarily revolve around stability issues, with end-users citing storage requirements (38%), shelf-life limitations (27%), and in vivo degradation concerns (22%) as primary barriers to broader adoption. This creates significant market opportunity for innovations specifically addressing mRNA nanoparticle stability, with potential premium pricing for solutions that extend shelf-life beyond current standards.
Lipid nanoparticle (LNP) delivery systems currently dominate the market, accounting for over 80% of commercial mRNA delivery applications. This dominance stems from their proven efficacy in vaccine delivery and relatively established manufacturing processes. However, polymer-based nanoparticles are gaining traction, with market share expected to increase from current 10% to potentially 25% by 2027 due to their enhanced stability profiles and customization capabilities.
Regional analysis reveals North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. China and India are emerging as high-growth markets with annual growth rates exceeding 20%, driven by substantial government investments in biotechnology infrastructure and favorable regulatory environments for mRNA therapeutics.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech controlling nearly 70% of the current market. However, specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and Genevant Sciences are rapidly expanding their market presence through strategic partnerships and proprietary stability-enhancing technologies.
Demand segmentation shows vaccines currently represent 65% of market applications, while therapeutic applications account for 30% and diagnostic uses comprise the remaining 5%. However, therapeutic applications are projected to grow at twice the rate of vaccines over the next five years as stability improvements enable more diverse clinical applications.
Key market drivers include increasing prevalence of chronic diseases, growing cancer burden, rising investment in personalized medicine, and expanding applications beyond infectious diseases. The COVID-19 pandemic has accelerated market adoption by demonstrating the viability of mRNA technology at scale and establishing manufacturing and distribution infrastructure.
Market challenges primarily revolve around stability issues, with end-users citing storage requirements (38%), shelf-life limitations (27%), and in vivo degradation concerns (22%) as primary barriers to broader adoption. This creates significant market opportunity for innovations specifically addressing mRNA nanoparticle stability, with potential premium pricing for solutions that extend shelf-life beyond current standards.
Current Challenges in mRNA Nanoparticle Stability
Despite significant advancements in mRNA nanoparticle technology, several critical challenges persist in maintaining stability throughout manufacturing, storage, and delivery processes. The inherent instability of mRNA molecules represents the foremost obstacle, as these nucleic acids are highly susceptible to enzymatic degradation by ubiquitous ribonucleases. This vulnerability necessitates sophisticated protection strategies to preserve therapeutic efficacy.
Temperature sensitivity poses another significant challenge, with mRNA nanoparticles requiring stringent cold chain management. Current formulations typically demand ultra-cold storage conditions (-70°C to -20°C), creating substantial logistical hurdles for global distribution, particularly in resource-limited regions lacking adequate cold chain infrastructure.
The colloidal stability of mRNA nanoparticles presents complex physicochemical challenges. These delivery systems tend to aggregate under physiological conditions, leading to reduced circulation time, altered biodistribution profiles, and potentially diminished therapeutic outcomes. Maintaining consistent particle size distribution and surface charge characteristics remains difficult across production batches.
Immunogenicity concerns further complicate stability considerations. Certain lipid components and the mRNA itself can trigger innate immune responses, leading to accelerated clearance and reduced efficacy. This immunological recognition can vary significantly between individuals, creating unpredictable stability profiles in clinical applications.
Chemical degradation pathways, including hydrolysis, oxidation, and deamination of nucleotides, compromise mRNA integrity even within protective nanoparticle formulations. These reactions accelerate at elevated temperatures and in the presence of reactive oxygen species, necessitating effective antioxidant strategies and pH control systems.
The interface between mRNA and delivery materials presents additional stability challenges. Suboptimal encapsulation efficiency leads to exposed mRNA segments vulnerable to degradation. Furthermore, premature release of cargo before reaching target cells significantly reduces therapeutic potential, while overly stable formulations may impede intracellular release.
Scale-up manufacturing introduces further stability complications. Transitioning from laboratory-scale to industrial production often results in altered physicochemical properties affecting stability profiles. Maintaining consistent quality attributes across large-scale batches remains technically challenging, with minor process variations potentially causing significant stability deviations.
Regulatory frameworks for stability assessment of these complex biologics remain evolving, with limited standardized protocols for accelerated stability testing and shelf-life determination. This regulatory uncertainty complicates development timelines and increases investment risks for commercial applications.
Temperature sensitivity poses another significant challenge, with mRNA nanoparticles requiring stringent cold chain management. Current formulations typically demand ultra-cold storage conditions (-70°C to -20°C), creating substantial logistical hurdles for global distribution, particularly in resource-limited regions lacking adequate cold chain infrastructure.
The colloidal stability of mRNA nanoparticles presents complex physicochemical challenges. These delivery systems tend to aggregate under physiological conditions, leading to reduced circulation time, altered biodistribution profiles, and potentially diminished therapeutic outcomes. Maintaining consistent particle size distribution and surface charge characteristics remains difficult across production batches.
Immunogenicity concerns further complicate stability considerations. Certain lipid components and the mRNA itself can trigger innate immune responses, leading to accelerated clearance and reduced efficacy. This immunological recognition can vary significantly between individuals, creating unpredictable stability profiles in clinical applications.
Chemical degradation pathways, including hydrolysis, oxidation, and deamination of nucleotides, compromise mRNA integrity even within protective nanoparticle formulations. These reactions accelerate at elevated temperatures and in the presence of reactive oxygen species, necessitating effective antioxidant strategies and pH control systems.
The interface between mRNA and delivery materials presents additional stability challenges. Suboptimal encapsulation efficiency leads to exposed mRNA segments vulnerable to degradation. Furthermore, premature release of cargo before reaching target cells significantly reduces therapeutic potential, while overly stable formulations may impede intracellular release.
Scale-up manufacturing introduces further stability complications. Transitioning from laboratory-scale to industrial production often results in altered physicochemical properties affecting stability profiles. Maintaining consistent quality attributes across large-scale batches remains technically challenging, with minor process variations potentially causing significant stability deviations.
Regulatory frameworks for stability assessment of these complex biologics remain evolving, with limited standardized protocols for accelerated stability testing and shelf-life determination. This regulatory uncertainty complicates development timelines and increases investment risks for commercial applications.
Established Approaches for Enhancing mRNA Stability
01 Lipid nanoparticle formulations for mRNA stability
Lipid nanoparticles (LNPs) are widely used to encapsulate mRNA molecules, providing protection against degradation and enhancing cellular uptake. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize stability. The composition and structure of these lipids significantly impact the stability of the mRNA cargo, with modifications in the lipid components leading to improved shelf-life and resistance to environmental stressors.- Lipid nanoparticle formulations for mRNA stability: Lipid nanoparticles (LNPs) are widely used to encapsulate mRNA molecules, providing protection against degradation and enhancing stability. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize mRNA protection. The composition of the lipid components significantly affects the stability of the mRNA payload, with optimized formulations showing improved shelf-life and resistance to temperature fluctuations.
- pH-responsive delivery systems for mRNA: pH-responsive nanoparticle systems enhance mRNA stability by protecting the nucleic acid cargo in physiological conditions and facilitating controlled release at target sites. These systems utilize materials that undergo conformational changes in response to pH variations, allowing for efficient endosomal escape and delivery of intact mRNA to the cytoplasm. The pH-responsive elements help maintain mRNA integrity during storage and administration while enabling effective cellular uptake and translation.
- Freeze-drying and lyophilization techniques: Freeze-drying and lyophilization processes significantly improve the long-term stability of mRNA nanoparticles by removing water content that can contribute to degradation. These techniques, when combined with appropriate cryoprotectants and lyoprotectants such as trehalose or sucrose, help maintain the structural integrity of the nanoparticles during storage. The resulting dry formulations show enhanced resistance to temperature variations and extended shelf-life compared to liquid formulations.
- Surface modification strategies: Surface modification of mRNA nanoparticles with polymers, peptides, or other functional groups can significantly enhance stability. These modifications create protective shells that shield the mRNA from enzymatic degradation and recognition by the immune system. Additionally, surface engineering can improve colloidal stability, prevent aggregation, and reduce non-specific interactions with biological components, resulting in improved pharmacokinetic profiles and extended circulation time.
- Buffer composition and ionic strength optimization: The composition of buffers and optimization of ionic strength play crucial roles in maintaining mRNA nanoparticle stability. Carefully selected buffer systems with appropriate pH values and ionic strengths can minimize electrostatic interactions that lead to aggregation. Addition of stabilizing agents such as antioxidants, chelating agents, and specific salts can further protect mRNA from degradation by preventing oxidation and inhibiting nuclease activity, thereby extending the shelf-life of mRNA nanoparticle formulations.
02 Temperature control strategies for mRNA nanoparticle preservation
Temperature management is critical for maintaining mRNA nanoparticle stability during storage and transportation. Research has focused on developing formulations that remain stable at various temperature ranges, from ultra-cold storage to refrigerated and room temperature conditions. Cryoprotectants and lyophilization techniques have been employed to prevent degradation during freeze-thaw cycles, while specific buffer systems help maintain structural integrity across temperature fluctuations.Expand Specific Solutions03 pH-responsive elements for enhanced stability
pH-responsive components in mRNA nanoparticle formulations can significantly improve stability by protecting the cargo in different physiological environments. These elements allow for structural changes in response to pH variations, maintaining integrity during storage and transportation while facilitating efficient release of mRNA at target sites. Buffering agents and pH-sensitive polymers are incorporated to prevent degradation caused by pH fluctuations and to optimize the performance of the delivery system.Expand Specific Solutions04 Surface modification techniques for improved stability
Surface modifications of mRNA nanoparticles can enhance stability by reducing aggregation, preventing protein adsorption, and improving circulation time. Techniques include PEGylation, coating with hydrophilic polymers, and functionalization with specific ligands. These modifications create a protective shell around the nanoparticles, shielding the mRNA cargo from enzymatic degradation and immune recognition while maintaining the structural integrity of the delivery system.Expand Specific Solutions05 Stabilizing excipients and buffer systems
Various excipients and buffer systems are employed to enhance the stability of mRNA nanoparticles. These include antioxidants to prevent oxidative damage, chelating agents to sequester destabilizing metal ions, and specific sugars that act as stabilizers. Carefully designed buffer compositions help maintain optimal pH and ionic strength, while cryoprotectants and lyoprotectants protect the nanoparticles during freeze-drying processes and long-term storage, resulting in extended shelf-life and preserved therapeutic efficacy.Expand Specific Solutions
Leading Organizations in mRNA Nanoparticle Development
The mRNA nanoparticle stability landscape is evolving rapidly, with the market transitioning from early-stage development to commercial applications following COVID-19 vaccine successes. Key players include established pharmaceutical companies like Moderna, BMS, and Sanofi alongside specialized firms such as SiSaf, Translate Bio, and GreenLight Biosciences. The technology is approaching maturity in vaccine applications while still developing for therapeutic uses, with academic institutions (Peking University, Zhejiang University, KAIST) collaborating with industry to overcome stability challenges. Research focuses on lipid nanoparticle formulations, silicon-stabilized hybrid systems, and novel delivery mechanisms, with market growth projected to accelerate as stability innovations enable broader applications beyond vaccines into therapeutics and diagnostics.
SiSaf Ltd.
Technical Solution: SiSaf has developed the Bio-Courier® technology platform, a novel approach to enhancing mRNA stability using silicon-based nanoparticles rather than conventional lipid-based systems. Their proprietary technology incorporates bioabsorbable silicon into nanoparticle formulations, creating hybrid carriers that offer superior protection for mRNA molecules. The silicon component provides structural rigidity and resistance to enzymatic degradation, while specialized surface modifications enable targeted delivery to specific cell types. SiSaf's nanoparticles feature a core-shell architecture where the silicon core is surrounded by a customizable lipid layer, allowing for optimization of surface properties based on the target tissue. The technology includes proprietary manufacturing processes that ensure uniform particle size distribution and high encapsulation efficiency of mRNA. SiSaf has demonstrated extended stability profiles for their formulations, with maintained activity after storage at 2-8°C for over 12 months, addressing a critical challenge in mRNA therapeutics deployment.
Strengths: Unique silicon-based technology differentiates from conventional LNPs; enhanced thermal stability properties; customizable targeting capabilities; potential for reduced cold chain requirements. Weaknesses: Less extensive clinical validation compared to lipid-based systems; potential regulatory hurdles for novel delivery material; more limited manufacturing scale-up experience.
ModernaTX, Inc.
Technical Solution: Moderna has pioneered lipid nanoparticle (LNP) delivery systems for mRNA therapeutics with significant focus on enhancing stability. Their proprietary LNP formulation incorporates ionizable lipids that protect mRNA from degradation while facilitating cellular uptake. The company has developed specialized lipid compositions that maintain a neutral charge at physiological pH but become positively charged in endosomes, enabling efficient endosomal escape. Moderna's technology includes modified nucleosides (pseudouridine and 5-methylcytidine) to reduce immunogenicity and increase translation efficiency. Their LNPs also incorporate PEGylated lipids that extend circulation time and prevent aggregation, along with cholesterol and helper phospholipids that optimize membrane fusion and release. This comprehensive approach has resulted in mRNA products with enhanced thermal stability, allowing storage at refrigerator temperatures rather than ultra-cold conditions.
Strengths: Industry-leading expertise in LNP formulation; extensive clinical validation through COVID-19 vaccines; proprietary ionizable lipid technology; advanced manufacturing capabilities. Weaknesses: Higher cost of production compared to traditional vaccines; some formulations still require cold chain logistics; potential for rare inflammatory responses in some patients.
Critical Patents and Innovations in Stability Enhancement
RNA stabilizing nanoparticles
PatentPendingUS20240307552A1
Innovation
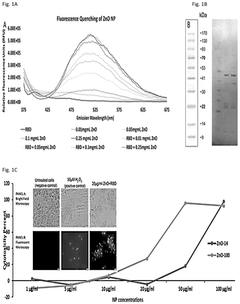
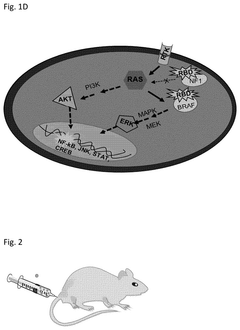
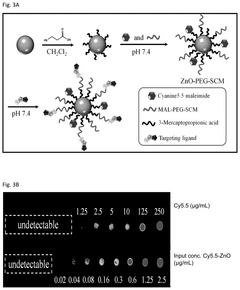
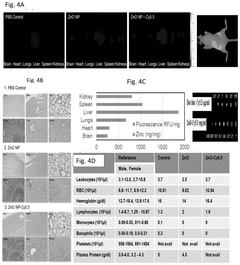
- Zinc oxide nanoparticles (ZnO NPs) are used to stabilize RNA by enhancing its temperature stability through interaction, forming complexes that retain structural and functional integrity even at elevated temperatures, and are administered via various routes to ensure effective delivery and immune response.
Nanoparticle compositions comprising one or more variants of poly(d,l-lactic- co-glycolic acid)
PatentWO2024209011A1
Innovation
- A nanoparticle composition comprising a cationic or cationically ionisable lipid, a helper lipid, a lipopolymer, and one or more variants of poly(D,L-lactic-co-glycolic acid) (PLGA) is developed, which enhances colloidal stability and facilitates efficient intracellular delivery of nucleic acids, thereby improving the handling and administration of mRNA vaccines.
Regulatory Framework for mRNA-Based Therapeutics
The regulatory landscape for mRNA-based therapeutics is evolving rapidly as these innovative technologies advance toward widespread clinical applications. Regulatory bodies worldwide, including the FDA, EMA, and NMPA, have established specific frameworks to address the unique characteristics of mRNA nanoparticle formulations, with particular emphasis on stability considerations. These frameworks typically require comprehensive stability data across various environmental conditions, storage temperatures, and timeframes to ensure product efficacy and safety throughout the shelf life.
Current regulatory requirements mandate extensive characterization of mRNA nanoparticle stability profiles, including assessment of structural integrity, encapsulation efficiency, and particle size distribution over time. Manufacturers must demonstrate that their formulations maintain critical quality attributes under anticipated storage and handling conditions, with accelerated stability studies often required to predict long-term performance.
The FDA's guidance specifically addresses lipid nanoparticle (LNP) formulations for mRNA delivery, requiring detailed information on lipid composition stability and the potential formation of degradation products that could impact safety or efficacy. Similarly, the EMA has published guidelines emphasizing the importance of demonstrating consistent manufacturing processes that yield stable mRNA nanoparticle products with predictable in vivo performance.
Regulatory submissions must include data on freeze-thaw stability, which is particularly relevant for mRNA nanoparticles that often require ultra-cold storage conditions. The impact of excipients on stability must be thoroughly documented, with justification for each component's inclusion in the final formulation. This includes demonstrating how specific buffer systems, cryoprotectants, and antioxidants contribute to enhanced stability profiles.
Batch-to-batch consistency represents another critical regulatory consideration, with authorities requiring manufacturers to establish robust analytical methods capable of detecting stability-related changes across production lots. These methods must be validated to ensure they can accurately identify potential degradation pathways that might compromise product performance.
Looking forward, regulatory agencies are increasingly adopting risk-based approaches that consider the intended clinical application when evaluating stability requirements. For example, mRNA vaccines may face different stability expectations compared to therapeutic mRNA products intended for chronic administration. This evolving regulatory landscape encourages ongoing dialogue between developers and authorities to establish appropriate stability specifications that balance innovation with patient safety.
As the field advances, harmonization efforts between international regulatory bodies are underway to standardize stability testing requirements for mRNA nanoparticle applications, potentially streamlining global development pathways while maintaining rigorous quality standards that ensure these promising therapeutics can be safely and effectively deployed worldwide.
Current regulatory requirements mandate extensive characterization of mRNA nanoparticle stability profiles, including assessment of structural integrity, encapsulation efficiency, and particle size distribution over time. Manufacturers must demonstrate that their formulations maintain critical quality attributes under anticipated storage and handling conditions, with accelerated stability studies often required to predict long-term performance.
The FDA's guidance specifically addresses lipid nanoparticle (LNP) formulations for mRNA delivery, requiring detailed information on lipid composition stability and the potential formation of degradation products that could impact safety or efficacy. Similarly, the EMA has published guidelines emphasizing the importance of demonstrating consistent manufacturing processes that yield stable mRNA nanoparticle products with predictable in vivo performance.
Regulatory submissions must include data on freeze-thaw stability, which is particularly relevant for mRNA nanoparticles that often require ultra-cold storage conditions. The impact of excipients on stability must be thoroughly documented, with justification for each component's inclusion in the final formulation. This includes demonstrating how specific buffer systems, cryoprotectants, and antioxidants contribute to enhanced stability profiles.
Batch-to-batch consistency represents another critical regulatory consideration, with authorities requiring manufacturers to establish robust analytical methods capable of detecting stability-related changes across production lots. These methods must be validated to ensure they can accurately identify potential degradation pathways that might compromise product performance.
Looking forward, regulatory agencies are increasingly adopting risk-based approaches that consider the intended clinical application when evaluating stability requirements. For example, mRNA vaccines may face different stability expectations compared to therapeutic mRNA products intended for chronic administration. This evolving regulatory landscape encourages ongoing dialogue between developers and authorities to establish appropriate stability specifications that balance innovation with patient safety.
As the field advances, harmonization efforts between international regulatory bodies are underway to standardize stability testing requirements for mRNA nanoparticle applications, potentially streamlining global development pathways while maintaining rigorous quality standards that ensure these promising therapeutics can be safely and effectively deployed worldwide.
Manufacturing Scalability and Quality Control Considerations
The scalability of mRNA nanoparticle manufacturing represents a critical challenge for widespread clinical applications. Current laboratory-scale production methods often employ microfluidic mixing devices that achieve high encapsulation efficiency but face significant hurdles when scaled to industrial production volumes. The transition from milliliter to liter-scale batches introduces variables that can compromise nanoparticle homogeneity, size distribution, and ultimately mRNA stability.
Quality control considerations must evolve in parallel with manufacturing scale-up. Analytical methods for characterizing lipid nanoparticle (LNP) formulations require standardization across the industry. Key parameters including particle size distribution, polydispersity index, zeta potential, and encapsulation efficiency demand robust measurement protocols that can be validated across different manufacturing sites and equipment configurations.
Process analytical technology (PAT) implementation offers promising solutions for real-time monitoring of critical quality attributes during manufacturing. Inline sensors capable of detecting subtle changes in nanoparticle characteristics can enable adaptive process control, reducing batch-to-batch variability that often compromises mRNA stability. However, the sensitivity and reliability of current PAT tools for LNP production remain insufficient for comprehensive quality assurance.
Regulatory frameworks present additional complexity for manufacturing scale-up. Current good manufacturing practice (cGMP) guidelines for mRNA-LNP products continue to evolve, creating uncertainty for manufacturers. Establishing clear acceptance criteria for critical quality attributes that directly impact mRNA stability is essential for consistent regulatory compliance and product performance.
Cold chain management during manufacturing represents another significant challenge. Temperature excursions during production, filling, and transfer operations can trigger mRNA degradation through hydrolysis or enzymatic pathways. Implementing controlled environmental conditions throughout the manufacturing process requires substantial infrastructure investment but remains essential for maintaining product stability.
Automation technologies offer promising solutions for enhancing manufacturing consistency. Robotic systems for precise reagent handling, continuous processing equipment, and automated quality testing can minimize human error while increasing throughput. These technologies can help maintain the narrow processing parameters required for optimal mRNA nanoparticle stability, though their implementation requires significant capital investment and specialized expertise.
Quality control considerations must evolve in parallel with manufacturing scale-up. Analytical methods for characterizing lipid nanoparticle (LNP) formulations require standardization across the industry. Key parameters including particle size distribution, polydispersity index, zeta potential, and encapsulation efficiency demand robust measurement protocols that can be validated across different manufacturing sites and equipment configurations.
Process analytical technology (PAT) implementation offers promising solutions for real-time monitoring of critical quality attributes during manufacturing. Inline sensors capable of detecting subtle changes in nanoparticle characteristics can enable adaptive process control, reducing batch-to-batch variability that often compromises mRNA stability. However, the sensitivity and reliability of current PAT tools for LNP production remain insufficient for comprehensive quality assurance.
Regulatory frameworks present additional complexity for manufacturing scale-up. Current good manufacturing practice (cGMP) guidelines for mRNA-LNP products continue to evolve, creating uncertainty for manufacturers. Establishing clear acceptance criteria for critical quality attributes that directly impact mRNA stability is essential for consistent regulatory compliance and product performance.
Cold chain management during manufacturing represents another significant challenge. Temperature excursions during production, filling, and transfer operations can trigger mRNA degradation through hydrolysis or enzymatic pathways. Implementing controlled environmental conditions throughout the manufacturing process requires substantial infrastructure investment but remains essential for maintaining product stability.
Automation technologies offer promising solutions for enhancing manufacturing consistency. Robotic systems for precise reagent handling, continuous processing equipment, and automated quality testing can minimize human error while increasing throughput. These technologies can help maintain the narrow processing parameters required for optimal mRNA nanoparticle stability, though their implementation requires significant capital investment and specialized expertise.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!