What Defines Efficiency in mRNA Lipid Nanoparticle Delivery
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Delivery Evolution and Objectives
The evolution of mRNA delivery systems represents one of the most significant advancements in modern biotechnology, culminating in the breakthrough application of lipid nanoparticles (LNPs) for COVID-19 vaccines. This technology's journey began in the 1990s with rudimentary liposomal delivery systems that faced substantial challenges in stability and cellular uptake. The field progressed through several critical phases, from basic lipid formulations to sophisticated engineered nanoparticles capable of targeted delivery with reduced immunogenicity.
The fundamental objective of mRNA LNP technology centers on achieving efficient cellular delivery while maintaining RNA integrity. This involves overcoming multiple biological barriers: protection from nuclease degradation, efficient endosomal escape, and precise targeting to specific cell types. The technology aims to optimize the delicate balance between transfection efficiency and cytotoxicity, a persistent challenge in the field.
Recent technological evolution has focused on developing ionizable lipids with improved pKa profiles, enabling pH-dependent changes that facilitate both endosomal escape and reduced toxicity. Parallel advancements in mRNA modification technologies have complemented these delivery systems, with innovations in 5' capping, poly(A) tail optimization, and nucleoside modifications significantly enhancing translation efficiency and reducing immunogenicity.
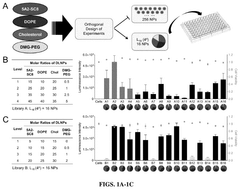
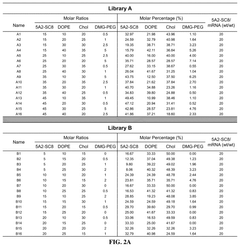
The field is currently trending toward precision engineering of LNP components, with particular emphasis on lipid chemistry optimization. Research indicates that minor structural modifications in the lipid components can dramatically alter biodistribution profiles and cellular uptake mechanisms. This has led to the development of libraries of structurally diverse lipids, systematically evaluated for their delivery properties across different tissue types.
Another significant trend involves the integration of computational approaches with high-throughput screening methodologies. Machine learning algorithms are increasingly being employed to predict structure-function relationships in LNP formulations, accelerating the discovery of optimal delivery systems for specific applications.
The ultimate technological objective extends beyond vaccine applications to realize the full therapeutic potential of mRNA. This includes developing delivery systems capable of targeting previously inaccessible tissues such as the central nervous system, enabling in vivo gene editing applications, and creating platforms for protein replacement therapies for genetic disorders. The field is progressively moving toward personalized medicine applications, where mRNA therapeutics can be rapidly designed and deployed for individual patient needs.
The fundamental objective of mRNA LNP technology centers on achieving efficient cellular delivery while maintaining RNA integrity. This involves overcoming multiple biological barriers: protection from nuclease degradation, efficient endosomal escape, and precise targeting to specific cell types. The technology aims to optimize the delicate balance between transfection efficiency and cytotoxicity, a persistent challenge in the field.
Recent technological evolution has focused on developing ionizable lipids with improved pKa profiles, enabling pH-dependent changes that facilitate both endosomal escape and reduced toxicity. Parallel advancements in mRNA modification technologies have complemented these delivery systems, with innovations in 5' capping, poly(A) tail optimization, and nucleoside modifications significantly enhancing translation efficiency and reducing immunogenicity.
The field is currently trending toward precision engineering of LNP components, with particular emphasis on lipid chemistry optimization. Research indicates that minor structural modifications in the lipid components can dramatically alter biodistribution profiles and cellular uptake mechanisms. This has led to the development of libraries of structurally diverse lipids, systematically evaluated for their delivery properties across different tissue types.
Another significant trend involves the integration of computational approaches with high-throughput screening methodologies. Machine learning algorithms are increasingly being employed to predict structure-function relationships in LNP formulations, accelerating the discovery of optimal delivery systems for specific applications.
The ultimate technological objective extends beyond vaccine applications to realize the full therapeutic potential of mRNA. This includes developing delivery systems capable of targeting previously inaccessible tissues such as the central nervous system, enabling in vivo gene editing applications, and creating platforms for protein replacement therapies for genetic disorders. The field is progressively moving toward personalized medicine applications, where mRNA therapeutics can be rapidly designed and deployed for individual patient needs.
Market Analysis for mRNA LNP Therapeutics
The mRNA therapeutics market is experiencing unprecedented growth, propelled by the success of COVID-19 vaccines. The global market value reached $46.7 billion in 2022 and is projected to grow at a CAGR of 12.8% through 2030, potentially reaching $137.4 billion. This remarkable expansion represents one of the fastest-growing segments within the broader biopharmaceutical industry.
Lipid nanoparticle (LNP) delivery systems have emerged as the dominant vehicle for mRNA therapeutics, accounting for approximately 85% of all mRNA delivery methods in clinical development. The LNP delivery market itself was valued at $3.2 billion in 2022 and is expected to grow at a CAGR of 15.6% through 2028, outpacing the overall mRNA therapeutics market.
Oncology represents the largest application segment for mRNA LNP therapeutics, comprising 42% of the current pipeline. Infectious diseases follow at 31%, with rare genetic disorders at 18%, and other applications including cardiovascular and metabolic diseases making up the remainder. The oncology segment is expected to maintain dominance due to the pressing need for personalized cancer vaccines and immunotherapies.
Geographically, North America leads the market with 58% share, followed by Europe at 27% and Asia-Pacific at 12%. The United States hosts the majority of companies developing mRNA LNP technologies, though European players like BioNTech and CureVac have established significant market positions. China is rapidly expanding its presence with substantial government investment in mRNA technology development.
Key market drivers include the proven efficacy of mRNA vaccines during the COVID-19 pandemic, increasing investment in RNA therapeutics, advances in LNP formulation technology, and expanding applications beyond vaccines into protein replacement therapies and gene editing. The versatility of mRNA as a therapeutic platform continues to attract significant venture capital and pharmaceutical industry interest.
Market challenges include cold chain requirements for mRNA stability, manufacturing scalability issues, high production costs, and regulatory uncertainties for novel delivery systems. The average cost of goods for mRNA LNP therapeutics remains 3-5 times higher than traditional biologics, presenting a significant barrier to market expansion in cost-sensitive regions.
Customer segments include large pharmaceutical companies seeking to build mRNA capabilities through partnerships or acquisitions, biotech companies focused on specific therapeutic applications, academic research institutions, and government agencies interested in pandemic preparedness and biodefense applications.
Lipid nanoparticle (LNP) delivery systems have emerged as the dominant vehicle for mRNA therapeutics, accounting for approximately 85% of all mRNA delivery methods in clinical development. The LNP delivery market itself was valued at $3.2 billion in 2022 and is expected to grow at a CAGR of 15.6% through 2028, outpacing the overall mRNA therapeutics market.
Oncology represents the largest application segment for mRNA LNP therapeutics, comprising 42% of the current pipeline. Infectious diseases follow at 31%, with rare genetic disorders at 18%, and other applications including cardiovascular and metabolic diseases making up the remainder. The oncology segment is expected to maintain dominance due to the pressing need for personalized cancer vaccines and immunotherapies.
Geographically, North America leads the market with 58% share, followed by Europe at 27% and Asia-Pacific at 12%. The United States hosts the majority of companies developing mRNA LNP technologies, though European players like BioNTech and CureVac have established significant market positions. China is rapidly expanding its presence with substantial government investment in mRNA technology development.
Key market drivers include the proven efficacy of mRNA vaccines during the COVID-19 pandemic, increasing investment in RNA therapeutics, advances in LNP formulation technology, and expanding applications beyond vaccines into protein replacement therapies and gene editing. The versatility of mRNA as a therapeutic platform continues to attract significant venture capital and pharmaceutical industry interest.
Market challenges include cold chain requirements for mRNA stability, manufacturing scalability issues, high production costs, and regulatory uncertainties for novel delivery systems. The average cost of goods for mRNA LNP therapeutics remains 3-5 times higher than traditional biologics, presenting a significant barrier to market expansion in cost-sensitive regions.
Customer segments include large pharmaceutical companies seeking to build mRNA capabilities through partnerships or acquisitions, biotech companies focused on specific therapeutic applications, academic research institutions, and government agencies interested in pandemic preparedness and biodefense applications.
Current Challenges in mRNA LNP Delivery Efficiency
Despite significant advancements in mRNA lipid nanoparticle (LNP) delivery systems, several critical challenges continue to impede optimal efficiency. The foremost challenge remains the targeted delivery of mRNA to specific tissues and cell types. Current LNP formulations exhibit preferential accumulation in the liver, limiting their therapeutic potential for non-hepatic diseases. This tissue tropism presents a substantial hurdle for treating conditions affecting other organs such as the lungs, heart, or central nervous system.
Immunogenicity represents another significant barrier to efficient mRNA delivery. LNPs can trigger innate immune responses through pattern recognition receptors, leading to cytokine production and inflammatory reactions. These immune responses not only reduce therapeutic efficacy but may also cause adverse effects ranging from mild fever to potentially severe cytokine storms, particularly with repeated administrations.
Endosomal escape efficiency remains suboptimal in current LNP systems. While LNPs successfully enter cells through endocytosis, a significant portion of the payload becomes trapped in endosomes and ultimately degraded in lysosomes. This bottleneck substantially reduces the amount of mRNA reaching the cytoplasm for translation, diminishing overall therapeutic effect despite successful cellular uptake.
Stability issues present multifaceted challenges for mRNA-LNP formulations. The inherent instability of mRNA molecules necessitates careful formulation and storage conditions. Current LNP systems require ultra-cold chain storage (-70°C for some COVID-19 vaccines), creating logistical complications for global distribution, particularly in resource-limited settings.
Manufacturing scalability and reproducibility pose significant technical hurdles. The production of consistent LNP formulations with precise size distributions, uniform mRNA encapsulation efficiency, and batch-to-batch reproducibility remains challenging at commercial scale. Minor variations in manufacturing parameters can significantly impact in vivo performance, necessitating stringent quality control measures.
Pharmacokinetic and biodistribution optimization represents an ongoing challenge. The rapid clearance of LNPs from circulation, primarily through interactions with serum proteins and uptake by the mononuclear phagocyte system, limits their therapeutic window. Additionally, the potential for off-target effects due to non-specific distribution requires careful consideration in therapeutic development.
Regulatory and safety concerns further complicate the advancement of mRNA-LNP technologies. Long-term safety data remains limited, particularly regarding repeated administrations and potential cumulative effects of lipid components. Regulatory frameworks continue to evolve as this relatively new therapeutic modality advances toward broader clinical applications.
Immunogenicity represents another significant barrier to efficient mRNA delivery. LNPs can trigger innate immune responses through pattern recognition receptors, leading to cytokine production and inflammatory reactions. These immune responses not only reduce therapeutic efficacy but may also cause adverse effects ranging from mild fever to potentially severe cytokine storms, particularly with repeated administrations.
Endosomal escape efficiency remains suboptimal in current LNP systems. While LNPs successfully enter cells through endocytosis, a significant portion of the payload becomes trapped in endosomes and ultimately degraded in lysosomes. This bottleneck substantially reduces the amount of mRNA reaching the cytoplasm for translation, diminishing overall therapeutic effect despite successful cellular uptake.
Stability issues present multifaceted challenges for mRNA-LNP formulations. The inherent instability of mRNA molecules necessitates careful formulation and storage conditions. Current LNP systems require ultra-cold chain storage (-70°C for some COVID-19 vaccines), creating logistical complications for global distribution, particularly in resource-limited settings.
Manufacturing scalability and reproducibility pose significant technical hurdles. The production of consistent LNP formulations with precise size distributions, uniform mRNA encapsulation efficiency, and batch-to-batch reproducibility remains challenging at commercial scale. Minor variations in manufacturing parameters can significantly impact in vivo performance, necessitating stringent quality control measures.
Pharmacokinetic and biodistribution optimization represents an ongoing challenge. The rapid clearance of LNPs from circulation, primarily through interactions with serum proteins and uptake by the mononuclear phagocyte system, limits their therapeutic window. Additionally, the potential for off-target effects due to non-specific distribution requires careful consideration in therapeutic development.
Regulatory and safety concerns further complicate the advancement of mRNA-LNP technologies. Long-term safety data remains limited, particularly regarding repeated administrations and potential cumulative effects of lipid components. Regulatory frameworks continue to evolve as this relatively new therapeutic modality advances toward broader clinical applications.
Established Approaches to Enhance LNP Delivery Efficiency
01 Lipid composition optimization for mRNA delivery
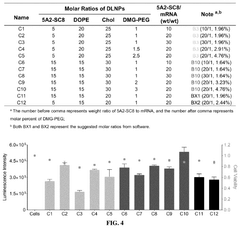
The efficiency of mRNA lipid nanoparticles can be significantly improved by optimizing the lipid composition. This includes adjusting the ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids. Specific lipid structures and combinations can enhance cellular uptake, endosomal escape, and overall transfection efficiency. These optimizations can lead to more effective mRNA delivery systems with improved therapeutic outcomes.- Lipid composition optimization for mRNA delivery: The efficiency of mRNA lipid nanoparticles can be significantly improved by optimizing the lipid composition. This includes adjusting the ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids to enhance stability, cellular uptake, and endosomal escape. Specific lipid structures and their molecular arrangements within the nanoparticle can dramatically affect transfection efficiency and protein expression levels.
- mRNA structural modifications for enhanced stability: Structural modifications to the mRNA molecule itself can enhance the efficiency of lipid nanoparticle delivery systems. These modifications include optimized 5' and 3' untranslated regions, modified nucleosides (such as pseudouridine and 5-methylcytidine), codon optimization, and poly(A) tail engineering. Such modifications can improve mRNA stability, reduce immunogenicity, and increase protein expression levels after delivery.
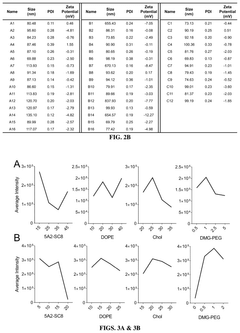
- Formulation techniques for improved nanoparticle homogeneity: Advanced formulation techniques can enhance the efficiency of mRNA lipid nanoparticles by improving size distribution, morphology, and encapsulation efficiency. Methods such as microfluidic mixing, ethanol injection, and controlled precipitation can produce more homogeneous nanoparticle populations with optimal size ranges (typically 70-100 nm). These techniques result in better biodistribution profiles and more consistent transfection efficiency across target tissues.
- Surface modifications for targeted delivery: Surface modifications of lipid nanoparticles can significantly improve the efficiency of mRNA delivery to specific cell types or tissues. These modifications include the attachment of targeting ligands, antibodies, peptides, or aptamers that recognize receptors on target cells. Additionally, surface charge optimization and stealth coatings can enhance circulation time and reduce non-specific uptake, resulting in more efficient delivery to the intended site of action.
- Endosomal escape enhancement strategies: Improving endosomal escape is crucial for enhancing the efficiency of mRNA lipid nanoparticles, as it prevents mRNA degradation in lysosomes. Strategies include incorporating pH-responsive lipids that become fusogenic in the acidic endosomal environment, using endosomolytic peptides, or adding compounds that induce the proton sponge effect. These approaches facilitate the release of mRNA into the cytoplasm where translation can occur, significantly increasing transfection efficiency and protein expression levels.
02 Surface modification techniques for targeted delivery
Surface modifications of lipid nanoparticles can enhance the targeting efficiency of mRNA delivery. By incorporating targeting ligands, antibodies, or peptides on the nanoparticle surface, the specificity for certain tissues or cell types can be improved. These modifications can reduce off-target effects and increase the therapeutic index of mRNA-based treatments, allowing for more precise delivery to intended sites of action.Expand Specific Solutions03 Manufacturing process improvements for enhanced stability
Advancements in manufacturing processes can significantly impact the stability and efficiency of mRNA lipid nanoparticles. Techniques such as microfluidic mixing, controlled temperature formulation, and lyophilization methods can produce more uniform particles with better size distribution and encapsulation efficiency. These improved manufacturing approaches result in more stable formulations with extended shelf-life and preserved therapeutic activity.Expand Specific Solutions04 Novel ionizable lipids for improved transfection
Development of novel ionizable lipids has led to significant improvements in mRNA transfection efficiency. These specially designed lipids can better facilitate endosomal escape, allowing more mRNA to reach the cytoplasm where translation occurs. The molecular structure of these lipids, including head group composition, linker chemistry, and hydrophobic tail configuration, plays a crucial role in determining transfection efficiency and can be tailored for specific applications.Expand Specific Solutions05 Formulation additives for enhanced biodistribution
Various additives incorporated into mRNA lipid nanoparticle formulations can enhance biodistribution profiles and cellular uptake. These include antioxidants, pH buffers, cryoprotectants, and specific helper lipids that can improve stability in circulation and facilitate interaction with target cell membranes. The careful selection of these additives can significantly impact the pharmacokinetics and ultimately the therapeutic efficacy of mRNA-based treatments.Expand Specific Solutions
Leading Companies in mRNA LNP Development
The mRNA lipid nanoparticle (LNP) delivery field is currently in a rapid growth phase, with the market expanding significantly following COVID-19 vaccine successes. Efficiency in mRNA LNP delivery is defined by multiple factors including nanoparticle composition, size uniformity, stability, and targeted tissue distribution. The competitive landscape features established pharmaceutical companies like Sanofi alongside specialized biotechnology firms such as Arbutus Biopharma, GreenLight Biosciences, and Translate Bio. Academic institutions including Tsinghua University, University of Copenhagen, and University of Pennsylvania are driving fundamental research advances. The technology is approaching commercial maturity for certain applications while still evolving for targeted delivery to tissues beyond the liver, with companies like Genevant Sciences and Orna Therapeutics developing proprietary LNP formulations to overcome current delivery limitations.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences has developed a sophisticated lipid nanoparticle (LNP) platform specifically engineered for efficient mRNA delivery. Their technology centers on proprietary ionizable lipids with optimized pKa values (typically 6.0-6.5) that facilitate efficient endosomal escape—a critical efficiency factor in mRNA delivery. Genevant's LNP formulations incorporate precisely controlled ratios of four key components: ionizable amino lipids, helper phospholipids, cholesterol, and PEG-lipids, with compositions tailored for specific tissue targets. Their manufacturing process employs advanced microfluidic mixing technology that ensures narrow particle size distributions (typically 60-90 nm) and high encapsulation efficiency (>95%), both critical parameters for reproducible in vivo performance. Genevant has developed specialized LNP formulations with enhanced stability profiles that reduce cold chain requirements while maintaining potency. Their technology includes surface modifications that improve biodistribution beyond traditional liver targeting, with demonstrated success in delivering mRNA to lung, spleen, and tumor tissues. The company has established structure-activity relationships for their ionizable lipids, optimizing factors such as lipid tail length, saturation, linker chemistry, and headgroup structure to maximize transfection efficiency while minimizing toxicity.
Strengths: Extensive intellectual property portfolio covering fundamental LNP technology; demonstrated expertise in scalable manufacturing processes; advanced understanding of structure-function relationships in LNP components. Weaknesses: Potential challenges in achieving efficient delivery to tissues beyond traditional LNP targets; complex formulations may present manufacturing challenges at commercial scale; possible immunogenicity concerns with repeated administration of certain formulations.
Arbutus Biopharma Corp.
Technical Solution: Arbutus Biopharma has developed proprietary lipid nanoparticle (LNP) delivery systems specifically optimized for mRNA therapeutics. Their technology focuses on ionizable lipids with unique structural properties that enhance endosomal escape, a critical efficiency factor in mRNA delivery. Their LNP formulations typically contain four components: ionizable amino lipids, helper phospholipids, cholesterol, and PEG-lipids, with precise ratios calibrated for different tissue targets. Arbutus has pioneered the development of LNP systems with improved biodistribution profiles, particularly for liver-directed therapies. Their third-generation LNP technology demonstrates enhanced potency with reduced lipid doses, addressing toxicity concerns associated with earlier formulations. The company has established manufacturing processes that ensure consistent particle size distribution (typically 60-100 nm) and high encapsulation efficiency (>90%), both critical parameters for therapeutic efficacy.
Strengths: Industry-leading expertise in ionizable lipid chemistry with proven clinical applications; established manufacturing processes ensuring consistent quality; extensive intellectual property portfolio covering LNP composition and manufacturing. Weaknesses: Primary focus on liver-targeted delivery limits applications in other tissues; potential immunogenicity concerns with repeated administration; higher production costs compared to some competing delivery technologies.
Critical Patents and Innovations in LNP Formulation
Lipid compositions and methods for nucleic acid delivery
PatentPendingUS20250275918A1
Innovation
- Lipid nanoparticle compositions comprising ionizable lipids with nitrogen atoms in the main chain and lipophilic substituents, allowing for enhanced interaction with encapsulated nucleic acids, particularly RNA, to improve delivery efficiency and endosomal escape.
Lipid nanoparticle compositions for delivery of mRNA and long nucleic acids
PatentActiveUS20240325315A1
Innovation
- The development of lipid nanoparticle compositions comprising specific molar ratios of cationic ionizable lipids, phospholipids, steroids, and PEGylated lipids, optimized for the delivery of nucleic acids greater than 80 nucleotides, including mRNAs and sgRNAs, to enhance stability and efficacy.
Regulatory Framework for mRNA LNP Therapeutics
The regulatory landscape for mRNA lipid nanoparticle (LNP) therapeutics has evolved rapidly, particularly following the accelerated approval of COVID-19 vaccines. Regulatory agencies worldwide have established frameworks that address the unique characteristics of these advanced therapeutic modalities, balancing the need for innovation with patient safety considerations.
The U.S. Food and Drug Administration (FDA) has developed specific guidance documents for mRNA LNP products, categorizing them under both biological products and nanomedicine frameworks. These guidelines emphasize characterization requirements for lipid components, encapsulation efficiency, and in vivo biodistribution profiles. The European Medicines Agency (EMA) has similarly established the Advanced Therapy Medicinal Products (ATMP) classification, which includes provisions specifically addressing mRNA delivery technologies.
Regulatory considerations for mRNA LNP therapeutics extend beyond traditional pharmaceutical requirements, incorporating specialized assessments of lipid excipient safety, immunogenicity potential, and manufacturing consistency. Authorities require comprehensive characterization of particle size distribution, polydispersity, zeta potential, and encapsulation efficiency as critical quality attributes that directly impact delivery efficiency.
Manufacturing process validation represents a significant regulatory hurdle, with agencies demanding robust control strategies for critical process parameters that affect LNP formation and mRNA integrity. The scalability of manufacturing processes must be demonstrated without compromising product quality, particularly regarding lipid-to-RNA ratios and particle homogeneity.
Toxicological assessment frameworks have been adapted to address the unique considerations of LNP systems, including potential accumulation in tissues, inflammatory responses, and the biodegradability of novel lipid components. Regulatory bodies increasingly require comparative biodistribution studies to establish safety margins and appropriate dosing regimens.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH), which is developing specific guidelines for nucleic acid-based therapeutics. However, significant regional differences persist in regulatory approaches, creating challenges for companies pursuing multinational development programs.
Accelerated approval pathways established during the pandemic have created precedents for expedited review of mRNA therapeutics addressing unmet medical needs. These pathways typically require robust post-marketing surveillance commitments to gather long-term safety data, particularly regarding repeated administration scenarios not extensively studied in pre-approval trials.
As the field advances, regulatory frameworks continue to evolve, with increasing focus on establishing standardized analytical methods for LNP characterization and developing reference standards to facilitate comparability assessments across different mRNA LNP platforms and manufacturing processes.
The U.S. Food and Drug Administration (FDA) has developed specific guidance documents for mRNA LNP products, categorizing them under both biological products and nanomedicine frameworks. These guidelines emphasize characterization requirements for lipid components, encapsulation efficiency, and in vivo biodistribution profiles. The European Medicines Agency (EMA) has similarly established the Advanced Therapy Medicinal Products (ATMP) classification, which includes provisions specifically addressing mRNA delivery technologies.
Regulatory considerations for mRNA LNP therapeutics extend beyond traditional pharmaceutical requirements, incorporating specialized assessments of lipid excipient safety, immunogenicity potential, and manufacturing consistency. Authorities require comprehensive characterization of particle size distribution, polydispersity, zeta potential, and encapsulation efficiency as critical quality attributes that directly impact delivery efficiency.
Manufacturing process validation represents a significant regulatory hurdle, with agencies demanding robust control strategies for critical process parameters that affect LNP formation and mRNA integrity. The scalability of manufacturing processes must be demonstrated without compromising product quality, particularly regarding lipid-to-RNA ratios and particle homogeneity.
Toxicological assessment frameworks have been adapted to address the unique considerations of LNP systems, including potential accumulation in tissues, inflammatory responses, and the biodegradability of novel lipid components. Regulatory bodies increasingly require comparative biodistribution studies to establish safety margins and appropriate dosing regimens.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH), which is developing specific guidelines for nucleic acid-based therapeutics. However, significant regional differences persist in regulatory approaches, creating challenges for companies pursuing multinational development programs.
Accelerated approval pathways established during the pandemic have created precedents for expedited review of mRNA therapeutics addressing unmet medical needs. These pathways typically require robust post-marketing surveillance commitments to gather long-term safety data, particularly regarding repeated administration scenarios not extensively studied in pre-approval trials.
As the field advances, regulatory frameworks continue to evolve, with increasing focus on establishing standardized analytical methods for LNP characterization and developing reference standards to facilitate comparability assessments across different mRNA LNP platforms and manufacturing processes.
Manufacturing Scalability and Quality Control Considerations
The scalability of mRNA lipid nanoparticle (LNP) manufacturing represents a critical determinant of efficiency in mRNA delivery systems. Current manufacturing processes face significant challenges when transitioning from laboratory-scale production to industrial-scale manufacturing. The microfluidic mixing techniques widely employed for LNP formulation require specialized equipment and precise control parameters that become increasingly complex at larger scales. This scaling difficulty directly impacts batch-to-batch consistency, which is paramount for therapeutic applications.
Quality control considerations in mRNA LNP production encompass multiple dimensions that collectively define delivery efficiency. Particle size distribution and polydispersity index (PDI) serve as primary quality indicators, with optimal therapeutic efficacy typically achieved with LNPs ranging from 70-100 nm and PDI values below 0.2. Deviations from these specifications can significantly alter biodistribution profiles and cellular uptake efficiency, ultimately compromising therapeutic outcomes.
Encapsulation efficiency represents another critical quality parameter, with current technologies achieving approximately 80-95% mRNA encapsulation within LNPs. Manufacturing processes must maintain this high efficiency while ensuring minimal exposure of mRNA to degradative conditions. The development of in-line monitoring technologies capable of real-time assessment of encapsulation efficiency would substantially enhance manufacturing control capabilities.
Lipid composition consistency presents unique challenges in scaled production. The precise ratios of structural lipids, ionizable lipids, PEG-lipids, and cholesterol must be maintained across batches to ensure consistent performance. Minor variations in these components can dramatically alter the LNP's physicochemical properties and biological performance. Advanced analytical methods including HPLC-MS and NMR spectroscopy are increasingly employed to verify compositional consistency.
Sterility and endotoxin control represent non-negotiable quality requirements that become more challenging at industrial scales. Aseptic processing environments and validated sterilization methods compatible with thermolabile mRNA cargo are essential. The industry is increasingly adopting closed-system manufacturing approaches to minimize contamination risks while enhancing scalability.
Regulatory frameworks for mRNA LNP manufacturing continue to evolve, with agencies including FDA and EMA developing specialized guidance. These frameworks emphasize the need for robust process analytical technology (PAT) implementation to ensure consistent quality. The integration of artificial intelligence and machine learning approaches for manufacturing optimization represents an emerging trend that promises to enhance both scalability and quality control in next-generation mRNA LNP production systems.
Quality control considerations in mRNA LNP production encompass multiple dimensions that collectively define delivery efficiency. Particle size distribution and polydispersity index (PDI) serve as primary quality indicators, with optimal therapeutic efficacy typically achieved with LNPs ranging from 70-100 nm and PDI values below 0.2. Deviations from these specifications can significantly alter biodistribution profiles and cellular uptake efficiency, ultimately compromising therapeutic outcomes.
Encapsulation efficiency represents another critical quality parameter, with current technologies achieving approximately 80-95% mRNA encapsulation within LNPs. Manufacturing processes must maintain this high efficiency while ensuring minimal exposure of mRNA to degradative conditions. The development of in-line monitoring technologies capable of real-time assessment of encapsulation efficiency would substantially enhance manufacturing control capabilities.
Lipid composition consistency presents unique challenges in scaled production. The precise ratios of structural lipids, ionizable lipids, PEG-lipids, and cholesterol must be maintained across batches to ensure consistent performance. Minor variations in these components can dramatically alter the LNP's physicochemical properties and biological performance. Advanced analytical methods including HPLC-MS and NMR spectroscopy are increasingly employed to verify compositional consistency.
Sterility and endotoxin control represent non-negotiable quality requirements that become more challenging at industrial scales. Aseptic processing environments and validated sterilization methods compatible with thermolabile mRNA cargo are essential. The industry is increasingly adopting closed-system manufacturing approaches to minimize contamination risks while enhancing scalability.
Regulatory frameworks for mRNA LNP manufacturing continue to evolve, with agencies including FDA and EMA developing specialized guidance. These frameworks emphasize the need for robust process analytical technology (PAT) implementation to ensure consistent quality. The integration of artificial intelligence and machine learning approaches for manufacturing optimization represents an emerging trend that promises to enhance both scalability and quality control in next-generation mRNA LNP production systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!