How mRNA Nanoparticle Delivery Modifies Pharmaceutical Landscapes
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Technology Evolution and Objectives
The evolution of mRNA nanoparticle technology represents one of the most significant breakthroughs in modern pharmaceutical science. Initially conceptualized in the 1990s, mRNA therapeutics remained largely theoretical until the development of effective delivery systems, particularly lipid nanoparticles (LNPs), overcame the inherent instability and immunogenicity challenges of naked mRNA. The watershed moment came with the COVID-19 pandemic, which catalyzed unprecedented acceleration in mRNA vaccine development and deployment, demonstrating the platform's remarkable adaptability and speed.
The technological trajectory has evolved from rudimentary lipid formulations to sophisticated nanoparticle designs incorporating ionizable lipids, PEGylated lipids, and structural lipids that enhance stability, cellular uptake, and endosomal escape. This evolution has been marked by progressive improvements in encapsulation efficiency, targeted delivery capabilities, and reduced immunogenicity profiles, enabling broader therapeutic applications beyond vaccines.
Current research objectives focus on several critical fronts: enhancing tissue-specific targeting to minimize off-target effects; improving mRNA stability and translation efficiency to reduce dosage requirements; developing alternative non-lipid nanocarriers such as polymer-based systems and hybrid nanoparticles; and creating formulations suitable for oral and other non-injectable administration routes to expand accessibility and patient compliance.
The pharmaceutical landscape modification stems from mRNA nanoparticle technology's unique advantages: unprecedented manufacturing flexibility allowing rapid adaptation to emerging pathogens; the capacity for multiplexed delivery of multiple mRNA constructs simultaneously; and the transient nature of mRNA expression, which offers improved safety profiles compared to DNA-based gene therapies. These attributes position mRNA therapeutics to potentially address previously untreatable conditions.
Looking forward, the field aims to achieve ambient temperature stability to overcome cold-chain logistics challenges, develop self-amplifying mRNA constructs to enhance potency at lower doses, and create programmable delivery systems responsive to specific physiological triggers. The ultimate objective is to establish mRNA therapeutics as a versatile platform technology capable of addressing diverse medical needs from infectious diseases to cancer immunotherapy, protein replacement therapies, and regenerative medicine.
The convergence of advances in computational design, high-throughput screening methodologies, and mechanistic understanding of nanoparticle-cellular interactions is expected to accelerate innovation in this space, potentially revolutionizing pharmaceutical development paradigms by enabling personalized, on-demand medicine production with unprecedented speed and precision.
The technological trajectory has evolved from rudimentary lipid formulations to sophisticated nanoparticle designs incorporating ionizable lipids, PEGylated lipids, and structural lipids that enhance stability, cellular uptake, and endosomal escape. This evolution has been marked by progressive improvements in encapsulation efficiency, targeted delivery capabilities, and reduced immunogenicity profiles, enabling broader therapeutic applications beyond vaccines.
Current research objectives focus on several critical fronts: enhancing tissue-specific targeting to minimize off-target effects; improving mRNA stability and translation efficiency to reduce dosage requirements; developing alternative non-lipid nanocarriers such as polymer-based systems and hybrid nanoparticles; and creating formulations suitable for oral and other non-injectable administration routes to expand accessibility and patient compliance.
The pharmaceutical landscape modification stems from mRNA nanoparticle technology's unique advantages: unprecedented manufacturing flexibility allowing rapid adaptation to emerging pathogens; the capacity for multiplexed delivery of multiple mRNA constructs simultaneously; and the transient nature of mRNA expression, which offers improved safety profiles compared to DNA-based gene therapies. These attributes position mRNA therapeutics to potentially address previously untreatable conditions.
Looking forward, the field aims to achieve ambient temperature stability to overcome cold-chain logistics challenges, develop self-amplifying mRNA constructs to enhance potency at lower doses, and create programmable delivery systems responsive to specific physiological triggers. The ultimate objective is to establish mRNA therapeutics as a versatile platform technology capable of addressing diverse medical needs from infectious diseases to cancer immunotherapy, protein replacement therapies, and regenerative medicine.
The convergence of advances in computational design, high-throughput screening methodologies, and mechanistic understanding of nanoparticle-cellular interactions is expected to accelerate innovation in this space, potentially revolutionizing pharmaceutical development paradigms by enabling personalized, on-demand medicine production with unprecedented speed and precision.
Market Demand Analysis for mRNA Therapeutics
The mRNA therapeutics market has experienced unprecedented growth following the successful deployment of COVID-19 vaccines, establishing a new paradigm in pharmaceutical development. Current market valuations place the global mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 13.2% through 2030, potentially reaching $109.8 billion by decade's end. This remarkable trajectory reflects both immediate pandemic-driven demand and long-term therapeutic potential across multiple disease categories.
Primary market drivers include the demonstrated efficacy of mRNA technology in vaccine development, significantly shorter production timelines compared to traditional approaches, and the versatility of the platform for addressing various diseases. The COVID-19 pandemic served as a catalyst, accelerating both regulatory pathways and public acceptance of mRNA-based interventions, while simultaneously attracting substantial investment capital to the sector.
Beyond vaccines, oncology represents the fastest-growing application segment for mRNA therapeutics, with personalized cancer vaccines and immunotherapies showing promising clinical results. Market research indicates that approximately 35% of the mRNA therapeutics pipeline is now focused on oncology applications, with particular emphasis on difficult-to-treat solid tumors and hematological malignancies.
Rare genetic disorders constitute another significant market opportunity, with several mRNA-based treatments for conditions like cystic fibrosis, sickle cell disease, and various metabolic disorders advancing through clinical trials. The addressable patient population for these conditions, while individually small, collectively represents a substantial market estimated at $15.3 billion annually.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the highest growth rates are projected in emerging markets, particularly China and India, where domestic mRNA development programs are receiving significant government support and investment.
Consumer and healthcare provider sentiment surveys indicate growing acceptance of mRNA therapeutics, with 78% of physicians expressing willingness to prescribe mRNA-based treatments beyond vaccines. Patient awareness has increased dramatically, with 67% of surveyed individuals now familiar with mRNA technology compared to just 12% pre-pandemic.
Key challenges affecting market demand include cold chain logistics requirements, production scaling limitations, and pricing concerns. The temperature-sensitive nature of mRNA formulations necessitates sophisticated distribution infrastructure, potentially limiting access in resource-constrained regions. Additionally, manufacturing capacity constraints and high development costs may impact broader market penetration in the near term.
Primary market drivers include the demonstrated efficacy of mRNA technology in vaccine development, significantly shorter production timelines compared to traditional approaches, and the versatility of the platform for addressing various diseases. The COVID-19 pandemic served as a catalyst, accelerating both regulatory pathways and public acceptance of mRNA-based interventions, while simultaneously attracting substantial investment capital to the sector.
Beyond vaccines, oncology represents the fastest-growing application segment for mRNA therapeutics, with personalized cancer vaccines and immunotherapies showing promising clinical results. Market research indicates that approximately 35% of the mRNA therapeutics pipeline is now focused on oncology applications, with particular emphasis on difficult-to-treat solid tumors and hematological malignancies.
Rare genetic disorders constitute another significant market opportunity, with several mRNA-based treatments for conditions like cystic fibrosis, sickle cell disease, and various metabolic disorders advancing through clinical trials. The addressable patient population for these conditions, while individually small, collectively represents a substantial market estimated at $15.3 billion annually.
Regional analysis reveals North America currently dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the highest growth rates are projected in emerging markets, particularly China and India, where domestic mRNA development programs are receiving significant government support and investment.
Consumer and healthcare provider sentiment surveys indicate growing acceptance of mRNA therapeutics, with 78% of physicians expressing willingness to prescribe mRNA-based treatments beyond vaccines. Patient awareness has increased dramatically, with 67% of surveyed individuals now familiar with mRNA technology compared to just 12% pre-pandemic.
Key challenges affecting market demand include cold chain logistics requirements, production scaling limitations, and pricing concerns. The temperature-sensitive nature of mRNA formulations necessitates sophisticated distribution infrastructure, potentially limiting access in resource-constrained regions. Additionally, manufacturing capacity constraints and high development costs may impact broader market penetration in the near term.
Current Challenges in mRNA Delivery Systems
Despite the revolutionary potential of mRNA therapeutics, several significant challenges persist in current delivery systems that hinder their widespread clinical application. The primary obstacle remains the inherent instability of mRNA molecules, which are highly susceptible to enzymatic degradation by ubiquitous ribonucleases in biological environments. This instability necessitates sophisticated delivery vehicles to protect the cargo during transport to target cells.
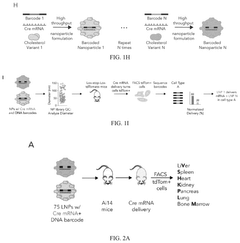
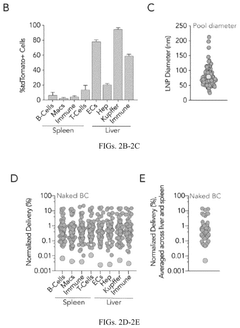
Lipid nanoparticles (LNPs), while currently the most advanced delivery platform, face challenges related to their biodistribution profiles. Most LNPs accumulate predominantly in the liver, limiting their utility for targeting other tissues and organs. This hepatic tropism restricts the application of mRNA therapeutics to liver-specific diseases, leaving many potential therapeutic areas unexplored.
Immunogenicity presents another significant hurdle, as both the mRNA molecule itself and components of delivery systems can trigger innate immune responses. These responses not only reduce therapeutic efficacy but may also lead to adverse effects ranging from mild inflammation to potentially severe systemic reactions, complicating the safety profile of mRNA-based pharmaceuticals.
Manufacturing scalability and reproducibility remain problematic for complex nanoparticle formulations. Current production processes often yield heterogeneous populations of nanoparticles with variable size distributions, surface properties, and encapsulation efficiencies, leading to batch-to-batch inconsistencies that complicate regulatory approval pathways.
The cold chain requirements for mRNA formulations represent a logistical and economic challenge, particularly for global distribution. Most mRNA-LNP formulations require ultra-cold storage conditions (-70°C to -20°C), creating barriers to access in resource-limited settings and increasing overall healthcare costs.
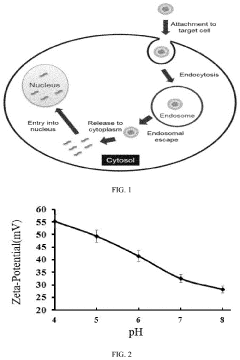
Cellular uptake and endosomal escape efficiency continue to limit the therapeutic index of mRNA therapeutics. Once internalized by target cells, a significant portion of nanoparticles become trapped in endosomes and are ultimately degraded in lysosomes, dramatically reducing the amount of mRNA available for translation.
Targeted delivery remains an elusive goal, with current systems lacking the specificity needed for precise tissue targeting beyond liver accumulation. The development of delivery vehicles capable of crossing biological barriers such as the blood-brain barrier or placental barrier would significantly expand the therapeutic potential of mRNA technologies.
Cost considerations also present substantial challenges, as the complex manufacturing processes, specialized delivery materials, and cold chain requirements contribute to high production costs that may limit accessibility and commercial viability in competitive pharmaceutical markets.
Lipid nanoparticles (LNPs), while currently the most advanced delivery platform, face challenges related to their biodistribution profiles. Most LNPs accumulate predominantly in the liver, limiting their utility for targeting other tissues and organs. This hepatic tropism restricts the application of mRNA therapeutics to liver-specific diseases, leaving many potential therapeutic areas unexplored.
Immunogenicity presents another significant hurdle, as both the mRNA molecule itself and components of delivery systems can trigger innate immune responses. These responses not only reduce therapeutic efficacy but may also lead to adverse effects ranging from mild inflammation to potentially severe systemic reactions, complicating the safety profile of mRNA-based pharmaceuticals.
Manufacturing scalability and reproducibility remain problematic for complex nanoparticle formulations. Current production processes often yield heterogeneous populations of nanoparticles with variable size distributions, surface properties, and encapsulation efficiencies, leading to batch-to-batch inconsistencies that complicate regulatory approval pathways.
The cold chain requirements for mRNA formulations represent a logistical and economic challenge, particularly for global distribution. Most mRNA-LNP formulations require ultra-cold storage conditions (-70°C to -20°C), creating barriers to access in resource-limited settings and increasing overall healthcare costs.
Cellular uptake and endosomal escape efficiency continue to limit the therapeutic index of mRNA therapeutics. Once internalized by target cells, a significant portion of nanoparticles become trapped in endosomes and are ultimately degraded in lysosomes, dramatically reducing the amount of mRNA available for translation.
Targeted delivery remains an elusive goal, with current systems lacking the specificity needed for precise tissue targeting beyond liver accumulation. The development of delivery vehicles capable of crossing biological barriers such as the blood-brain barrier or placental barrier would significantly expand the therapeutic potential of mRNA technologies.
Cost considerations also present substantial challenges, as the complex manufacturing processes, specialized delivery materials, and cold chain requirements contribute to high production costs that may limit accessibility and commercial viability in competitive pharmaceutical markets.
Current Delivery Platforms and Formulation Strategies
01 Lipid nanoparticle formulations for mRNA delivery
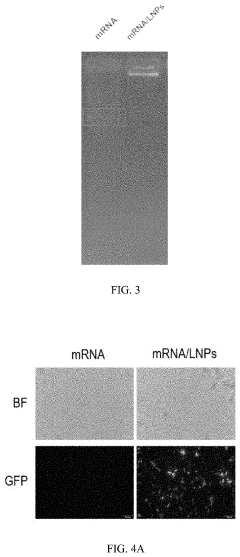
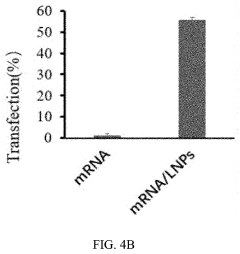
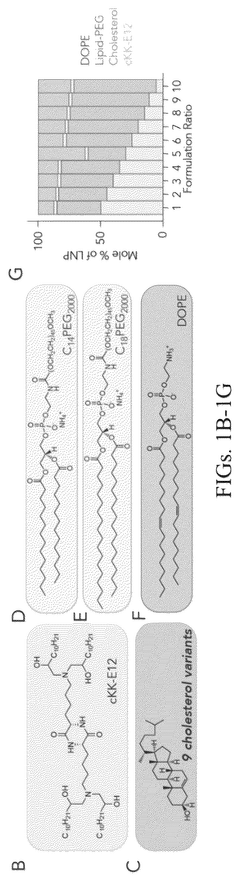
Lipid nanoparticles (LNPs) represent a leading delivery system for mRNA therapeutics, offering protection from degradation and facilitating cellular uptake. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize transfection efficiency. The composition can be tailored to target specific tissues, enhance stability, and improve the pharmacokinetic profile of the encapsulated mRNA, making them particularly valuable for vaccine and gene therapy applications.- Lipid nanoparticle formulations for mRNA delivery: Lipid nanoparticles (LNPs) have emerged as a leading delivery system for mRNA therapeutics. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate and protect mRNA molecules. The composition and structure of these lipids can be optimized to enhance cellular uptake, endosomal escape, and targeted delivery to specific tissues. Advanced LNP formulations have demonstrated improved stability, reduced toxicity, and increased transfection efficiency for mRNA delivery in various therapeutic applications.
- Polymer-based nanoparticles for mRNA delivery: Polymer-based nanoparticles represent an alternative approach to lipid-based systems for mRNA delivery. These formulations utilize biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), and chitosan derivatives to form complexes with mRNA. The polymeric structure can be engineered to control release kinetics, improve stability, and enhance cellular uptake. Recent innovations include stimuli-responsive polymers that can trigger mRNA release under specific physiological conditions, as well as hybrid polymer-lipid systems that combine the advantages of both delivery platforms.
- Targeted delivery strategies for mRNA nanoparticles: Targeted delivery approaches aim to enhance the specificity of mRNA nanoparticles for particular tissues or cell types while minimizing off-target effects. These strategies include surface modification of nanoparticles with ligands such as antibodies, peptides, aptamers, or small molecules that recognize specific cellular receptors. Additionally, physical targeting methods using external stimuli like magnetic fields, ultrasound, or light can direct nanoparticles to desired locations. Recent advances have focused on developing multi-functional targeting systems that respond to the tumor microenvironment or other disease-specific conditions.
- Manufacturing and scale-up technologies for mRNA nanoparticles: The commercial viability of mRNA therapeutics depends on efficient manufacturing processes that can produce consistent, high-quality nanoparticle formulations at scale. Recent innovations include microfluidic mixing devices, continuous flow systems, and automated production platforms that enable precise control over particle size, polydispersity, and encapsulation efficiency. Quality control methods have also advanced, with new analytical techniques for characterizing nanoparticle properties and mRNA integrity. Regulatory frameworks are evolving to address the unique challenges of manufacturing complex mRNA-nanoparticle formulations for clinical applications.
- Novel applications and therapeutic areas for mRNA nanoparticle delivery: The pharmaceutical landscape for mRNA nanoparticle delivery has expanded beyond vaccines to encompass diverse therapeutic applications. These include cancer immunotherapy, protein replacement therapies for rare genetic disorders, regenerative medicine, and treatment of infectious diseases. Emerging applications involve in vivo gene editing using CRISPR-Cas9 delivered via mRNA nanoparticles, as well as mRNA-based treatments for autoimmune disorders and inflammatory conditions. The versatility of mRNA as a therapeutic modality, combined with advances in nanoparticle delivery systems, continues to open new possibilities for addressing previously untreatable medical conditions.
02 Polymer-based nanoparticle systems for mRNA delivery
Polymer-based nanoparticles offer an alternative approach to mRNA delivery with advantages including biodegradability, biocompatibility, and versatility in design. These systems utilize cationic polymers such as polyethylenimine (PEI), poly(lactic-co-glycolic acid) (PLGA), or chitosan derivatives that can complex with negatively charged mRNA. The polymer composition can be modified to control release kinetics, improve stability, and reduce toxicity, making them suitable for various therapeutic applications including cancer treatment and genetic disorders.Expand Specific Solutions03 Targeted delivery strategies for mRNA nanoparticles
Targeted delivery approaches enhance the specificity of mRNA nanoparticles for particular tissues or cell types, improving therapeutic efficacy while reducing off-target effects. These strategies include surface modification with ligands such as antibodies, peptides, or aptamers that recognize specific cellular receptors. Additionally, stimuli-responsive nanoparticles that release their cargo in response to environmental triggers like pH, temperature, or enzymatic activity can further improve targeted delivery to disease sites such as tumors or inflamed tissues.Expand Specific Solutions04 Manufacturing and scale-up technologies for mRNA nanoparticles
The commercial viability of mRNA therapeutics depends on efficient manufacturing processes that can produce consistent, high-quality nanoparticles at scale. Advanced technologies include microfluidic mixing devices, continuous flow systems, and automated production lines that enable precise control over particle size, polydispersity, and encapsulation efficiency. These manufacturing innovations address critical challenges in transitioning from laboratory-scale production to commercial manufacturing, ensuring batch-to-batch reproducibility and compliance with regulatory standards for pharmaceutical products.Expand Specific Solutions05 Stability enhancement and storage solutions for mRNA nanoparticles
Maintaining the stability of mRNA nanoparticles during storage and distribution remains a significant challenge in pharmaceutical development. Innovative approaches include lyophilization (freeze-drying) with appropriate cryoprotectants, specialized buffer systems that prevent degradation, and novel formulation additives that enhance thermostability. These technologies aim to extend shelf-life, reduce cold chain requirements, and maintain the integrity of the mRNA cargo until administration, which is particularly important for global distribution of mRNA vaccines and therapeutics to regions with limited refrigeration infrastructure.Expand Specific Solutions
Key Industry Players in mRNA Nanoparticle Development
The mRNA nanoparticle delivery landscape is evolving rapidly, currently in a growth phase characterized by expanding applications beyond vaccines into therapeutics. The global market is projected to reach $15-20 billion by 2030, driven by post-COVID momentum. Technical maturity varies across applications, with vaccine delivery more advanced than therapeutic applications. Key players include ModernaTX leading commercial implementation, while academic-industry partnerships flourish through institutions like UNC Chapel Hill, Zhejiang University, and Cornell. Emerging companies like Orna Therapeutics and Quantoom Biosciences are advancing circular RNA and manufacturing technologies. Chinese entities including Shenzhen Houcun Nano Pharmaceutical and Nanjing Luye Pharmaceutical are rapidly expanding capabilities, suggesting a globally competitive landscape with significant innovation potential across multiple delivery platforms.
ModernaTX, Inc.
Technical Solution: Moderna has pioneered lipid nanoparticle (LNP) delivery systems for mRNA therapeutics, with their platform featuring ionizable lipids that protect mRNA from degradation while facilitating cellular uptake. Their proprietary LNP formulation includes SM-102 ionizable lipid, cholesterol, DSPC, and PEG2000-DMG, creating nanoparticles with optimal size (80-100nm) for efficient tissue distribution[1]. Moderna's technology enables precise control over mRNA encapsulation efficiency (>90%) and has demonstrated remarkable stability profiles allowing for standard refrigeration storage. Their delivery system incorporates modifications to reduce immunogenicity while enhancing translation efficiency, including 5' cap modifications and engineered untranslated regions (UTRs)[2]. The company has developed tissue-specific targeting capabilities by modifying the lipid composition and surface properties of their nanoparticles, enabling preferential delivery to liver, lung, or muscle tissues depending on therapeutic application requirements.
Strengths: Industry-leading encapsulation efficiency and stability profiles; extensive clinical validation through COVID-19 vaccine success; proprietary ionizable lipid formulations optimized for specific tissue targeting. Weaknesses: Higher manufacturing costs compared to traditional pharmaceuticals; potential cold-chain requirements for some formulations; limited long-term human safety data for repeated administration of LNP-based therapeutics.
The University of North Carolina at Chapel Hill
Technical Solution: The University of North Carolina at Chapel Hill has developed innovative mRNA nanoparticle delivery systems focusing on polymeric and lipid-polymer hybrid nanoparticles. Their technology platform features biodegradable poly(beta-amino ester) (PBAE) and poly(lactic-co-glycolic acid) (PLGA) based nanoparticles that offer tunable degradation profiles and reduced cytotoxicity compared to conventional lipid systems[9]. UNC researchers have engineered these nanoparticles with precise control over surface charge characteristics, achieving zeta potentials optimized for both colloidal stability and cellular interaction. Their delivery system incorporates pH-responsive elements that facilitate endosomal escape through the proton sponge effect, with buffering capacity carefully calibrated to maximize cytosolic mRNA delivery while minimizing membrane disruption[10]. The university has pioneered approaches for tissue-specific targeting through both passive targeting (leveraging the enhanced permeability and retention effect) and active targeting (incorporating ligands such as folate, transferrin, and cell-penetrating peptides). Their platform includes innovations in lyophilization techniques that enhance storage stability without cryopreservation, potentially addressing cold-chain challenges in mRNA therapeutics.
Strengths: Reduced cytotoxicity compared to some lipid-based systems; enhanced stability profiles enabling less stringent storage conditions; versatile platform adaptable to multiple RNA cargo types. Weaknesses: Generally lower transfection efficiency compared to optimized LNP systems; more complex manufacturing processes for polymer-based nanoparticles; less extensive clinical validation compared to leading LNP technologies.
Breakthrough Patents in Lipid Nanoparticle Technology
Intracellular Delivery System for mRNA Nucleic Acid Drugs, Preparation Method and Application Thereof
PatentInactiveUS20210299058A1
Innovation
- A delivery system comprising lipid nanoparticles made from ionizable cationic lipids, phospholipid auxiliary lipids, cholesterol, and polyethylene glycol-derivatized phospholipids, which enables efficient loading and targeted intracellular delivery of mRNA by electrostatic interactions, pH sensitivity, and reduced immunogenicity.
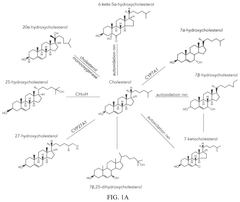
Drug delivery systems containing oxidized cholesterols
PatentInactiveUS20240315983A1
Innovation
- Development of lipid nanoparticles containing oxidized cholesterol, which modifies the tropism of nanoparticles to target specific cell types by incorporating ionizable lipids, phospholipids, and PEG lipids, allowing for preferential delivery of therapeutic agents to non-hepatocytes in the liver and other cell types.
Regulatory Framework for mRNA-Based Pharmaceuticals
The regulatory landscape for mRNA-based pharmaceuticals has evolved rapidly following the unprecedented success of mRNA vaccines during the COVID-19 pandemic. Regulatory agencies worldwide have been compelled to develop new frameworks that address the unique characteristics and challenges of this novel therapeutic modality.
The FDA has established specific guidance documents for mRNA product development, focusing on chemistry, manufacturing, and controls (CMC) considerations that differ significantly from traditional pharmaceuticals. These guidelines emphasize the importance of lipid nanoparticle characterization, mRNA sequence integrity, and process validation methods specific to nucleic acid-based therapeutics.
In Europe, the EMA has implemented an adaptive licensing pathway for mRNA therapeutics, recognizing the need for flexibility in evaluating these rapidly evolving technologies. This approach allows for conditional approval based on preliminary data, with requirements for continued post-market surveillance and additional clinical evidence generation.
Regulatory considerations for mRNA nanoparticle delivery systems present unique challenges due to their complex composition. Authorities now require comprehensive characterization of lipid components, particle size distribution, encapsulation efficiency, and stability profiles. The potential for immunogenicity and inflammatory responses associated with lipid nanoparticles has prompted specific safety assessment requirements beyond those typical for conventional pharmaceuticals.
Global harmonization efforts are underway through the International Council for Harmonisation (ICH), which is developing specialized guidelines for nucleic acid-based therapeutics including mRNA products. These initiatives aim to standardize regulatory expectations across major markets, facilitating more efficient global development programs.
Accelerated approval pathways have been established in several jurisdictions specifically for mRNA therapeutics addressing unmet medical needs. These pathways incorporate novel endpoints and surrogate markers relevant to mRNA mechanism of action, acknowledging that traditional clinical endpoints may not fully capture the therapeutic benefit of these innovative treatments.
Regulatory requirements for manufacturing facilities have also been adapted, with increased emphasis on containment strategies for nucleic acid handling, specialized equipment validation, and environmental monitoring protocols designed to detect potential nucleic acid contamination. These specialized GMP considerations reflect the unique production processes involved in mRNA pharmaceutical manufacturing.
Looking forward, regulatory frameworks continue to evolve as scientific understanding of mRNA therapeutics advances. Agencies are increasingly incorporating real-world evidence and patient-reported outcomes into their assessment processes, recognizing the need for innovative approaches to evaluate these transformative medicines.
The FDA has established specific guidance documents for mRNA product development, focusing on chemistry, manufacturing, and controls (CMC) considerations that differ significantly from traditional pharmaceuticals. These guidelines emphasize the importance of lipid nanoparticle characterization, mRNA sequence integrity, and process validation methods specific to nucleic acid-based therapeutics.
In Europe, the EMA has implemented an adaptive licensing pathway for mRNA therapeutics, recognizing the need for flexibility in evaluating these rapidly evolving technologies. This approach allows for conditional approval based on preliminary data, with requirements for continued post-market surveillance and additional clinical evidence generation.
Regulatory considerations for mRNA nanoparticle delivery systems present unique challenges due to their complex composition. Authorities now require comprehensive characterization of lipid components, particle size distribution, encapsulation efficiency, and stability profiles. The potential for immunogenicity and inflammatory responses associated with lipid nanoparticles has prompted specific safety assessment requirements beyond those typical for conventional pharmaceuticals.
Global harmonization efforts are underway through the International Council for Harmonisation (ICH), which is developing specialized guidelines for nucleic acid-based therapeutics including mRNA products. These initiatives aim to standardize regulatory expectations across major markets, facilitating more efficient global development programs.
Accelerated approval pathways have been established in several jurisdictions specifically for mRNA therapeutics addressing unmet medical needs. These pathways incorporate novel endpoints and surrogate markers relevant to mRNA mechanism of action, acknowledging that traditional clinical endpoints may not fully capture the therapeutic benefit of these innovative treatments.
Regulatory requirements for manufacturing facilities have also been adapted, with increased emphasis on containment strategies for nucleic acid handling, specialized equipment validation, and environmental monitoring protocols designed to detect potential nucleic acid contamination. These specialized GMP considerations reflect the unique production processes involved in mRNA pharmaceutical manufacturing.
Looking forward, regulatory frameworks continue to evolve as scientific understanding of mRNA therapeutics advances. Agencies are increasingly incorporating real-world evidence and patient-reported outcomes into their assessment processes, recognizing the need for innovative approaches to evaluate these transformative medicines.
Manufacturing Scalability and Cost Considerations
The scalability of mRNA nanoparticle manufacturing represents a critical challenge in the pharmaceutical industry's transition toward mRNA-based therapeutics. Current production processes involve complex multi-step procedures including in vitro transcription (IVT), purification, and lipid nanoparticle formulation, each requiring specialized equipment and expertise. These processes, while effective at laboratory and clinical trial scales, face significant hurdles when scaled to commercial production volumes necessary for global distribution.
Manufacturing costs remain prohibitively high, with estimates suggesting that producing mRNA vaccines costs 5-10 times more per dose than traditional protein-based vaccines. Key cost drivers include specialized raw materials such as modified nucleotides, enzymes, and lipid components, which often have limited suppliers and premium pricing. The cold chain requirements for mRNA products (-70°C for some formulations) further add to the overall cost structure through specialized storage and transportation needs.
Quality control presents another dimension of manufacturing complexity, as mRNA products require sophisticated analytical methods to ensure integrity, purity, and potency. These testing procedures add significant time and cost to production cycles, impacting overall manufacturing efficiency. Batch-to-batch consistency remains challenging at larger scales, requiring substantial investment in process development and validation.
Regulatory frameworks for large-scale mRNA manufacturing are still evolving, creating uncertainty for pharmaceutical companies investing in production capacity. The novelty of the technology means that regulatory agencies and manufacturers are simultaneously developing standards and best practices, potentially leading to costly redesigns of manufacturing processes as requirements solidify.
Recent innovations show promise for addressing these challenges, including continuous manufacturing approaches that could replace traditional batch processing, potentially reducing costs by 30-50%. Advances in enzymatic synthesis of mRNA and improved lipid formulation technologies are gradually reducing raw material costs and improving production yields. Several major pharmaceutical companies have announced significant investments in dedicated mRNA manufacturing facilities, indicating confidence in eventual resolution of scalability issues.
The economic viability of mRNA therapeutics will ultimately depend on achieving manufacturing economies of scale similar to those realized in other biopharmaceutical sectors. Industry analysts project that manufacturing costs could decrease by 40-60% over the next five years as production volumes increase and technologies mature, potentially expanding mRNA applications beyond high-value vaccines into broader therapeutic areas.
Manufacturing costs remain prohibitively high, with estimates suggesting that producing mRNA vaccines costs 5-10 times more per dose than traditional protein-based vaccines. Key cost drivers include specialized raw materials such as modified nucleotides, enzymes, and lipid components, which often have limited suppliers and premium pricing. The cold chain requirements for mRNA products (-70°C for some formulations) further add to the overall cost structure through specialized storage and transportation needs.
Quality control presents another dimension of manufacturing complexity, as mRNA products require sophisticated analytical methods to ensure integrity, purity, and potency. These testing procedures add significant time and cost to production cycles, impacting overall manufacturing efficiency. Batch-to-batch consistency remains challenging at larger scales, requiring substantial investment in process development and validation.
Regulatory frameworks for large-scale mRNA manufacturing are still evolving, creating uncertainty for pharmaceutical companies investing in production capacity. The novelty of the technology means that regulatory agencies and manufacturers are simultaneously developing standards and best practices, potentially leading to costly redesigns of manufacturing processes as requirements solidify.
Recent innovations show promise for addressing these challenges, including continuous manufacturing approaches that could replace traditional batch processing, potentially reducing costs by 30-50%. Advances in enzymatic synthesis of mRNA and improved lipid formulation technologies are gradually reducing raw material costs and improving production yields. Several major pharmaceutical companies have announced significant investments in dedicated mRNA manufacturing facilities, indicating confidence in eventual resolution of scalability issues.
The economic viability of mRNA therapeutics will ultimately depend on achieving manufacturing economies of scale similar to those realized in other biopharmaceutical sectors. Industry analysts project that manufacturing costs could decrease by 40-60% over the next five years as production volumes increase and technologies mature, potentially expanding mRNA applications beyond high-value vaccines into broader therapeutic areas.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!