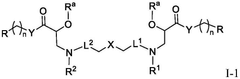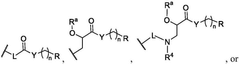How mRNA Lipid Nanoparticles Revolutionize Vaccine Development
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Background and Objectives
Messenger RNA (mRNA) technology has evolved significantly since its discovery in the early 1960s. Initially considered merely an intermediary between DNA and proteins, mRNA has transformed into a revolutionary platform for vaccine development. The trajectory of mRNA research accelerated in the 1990s when researchers overcame key stability challenges, but it wasn't until the 2010s that lipid nanoparticle (LNP) delivery systems emerged as the critical enabler for therapeutic applications.
The convergence of mRNA and LNP technologies represents a paradigm shift in vaccinology. Traditional vaccines typically require months or years of development, utilizing attenuated pathogens or protein subunits. In contrast, mRNA vaccines leverage the body's cellular machinery to produce antigenic proteins, dramatically reducing development timelines while maintaining high efficacy profiles.
LNPs serve as sophisticated delivery vehicles that protect fragile mRNA molecules from degradation and facilitate their cellular uptake. These nanoparticles typically comprise four components: ionizable lipids that complex with mRNA, helper phospholipids that stabilize the bilayer structure, cholesterol that provides structural integrity, and PEG-lipids that extend circulation time and prevent aggregation.
The technical evolution of mRNA LNP platforms has been marked by progressive improvements in lipid chemistry, formulation processes, and manufacturing scalability. Early iterations faced challenges with inflammatory responses and delivery efficiency, while contemporary formulations demonstrate remarkable stability profiles and targeted delivery capabilities.
The COVID-19 pandemic catalyzed unprecedented acceleration in mRNA LNP technology development, compressing what might have been a decade of incremental progress into less than a year. This acceleration validated the platform's adaptability and responsiveness to emerging pathogens, establishing mRNA vaccines as a transformative approach to infectious disease prevention.
The primary technical objectives for advancing mRNA LNP technology include enhancing thermostability to reduce cold chain requirements, optimizing lipid compositions to minimize reactogenicity while maintaining potency, developing targeted delivery mechanisms for specific cell types, and establishing scalable manufacturing processes that can support global vaccine distribution.
Beyond infectious diseases, the mRNA LNP platform shows promising applications in cancer immunotherapy, protein replacement therapies, and gene editing. The versatility of this technology stems from its modular nature—the lipid delivery system remains largely consistent while the mRNA payload can be readily modified to address diverse therapeutic targets.
The convergence of mRNA and LNP technologies represents a paradigm shift in vaccinology. Traditional vaccines typically require months or years of development, utilizing attenuated pathogens or protein subunits. In contrast, mRNA vaccines leverage the body's cellular machinery to produce antigenic proteins, dramatically reducing development timelines while maintaining high efficacy profiles.
LNPs serve as sophisticated delivery vehicles that protect fragile mRNA molecules from degradation and facilitate their cellular uptake. These nanoparticles typically comprise four components: ionizable lipids that complex with mRNA, helper phospholipids that stabilize the bilayer structure, cholesterol that provides structural integrity, and PEG-lipids that extend circulation time and prevent aggregation.
The technical evolution of mRNA LNP platforms has been marked by progressive improvements in lipid chemistry, formulation processes, and manufacturing scalability. Early iterations faced challenges with inflammatory responses and delivery efficiency, while contemporary formulations demonstrate remarkable stability profiles and targeted delivery capabilities.
The COVID-19 pandemic catalyzed unprecedented acceleration in mRNA LNP technology development, compressing what might have been a decade of incremental progress into less than a year. This acceleration validated the platform's adaptability and responsiveness to emerging pathogens, establishing mRNA vaccines as a transformative approach to infectious disease prevention.
The primary technical objectives for advancing mRNA LNP technology include enhancing thermostability to reduce cold chain requirements, optimizing lipid compositions to minimize reactogenicity while maintaining potency, developing targeted delivery mechanisms for specific cell types, and establishing scalable manufacturing processes that can support global vaccine distribution.
Beyond infectious diseases, the mRNA LNP platform shows promising applications in cancer immunotherapy, protein replacement therapies, and gene editing. The versatility of this technology stems from its modular nature—the lipid delivery system remains largely consistent while the mRNA payload can be readily modified to address diverse therapeutic targets.
Market Demand Analysis for mRNA Vaccines
The global mRNA vaccine market has experienced unprecedented growth following the successful deployment of COVID-19 vaccines, with market valuations reaching approximately $36.9 billion in 2022. Projections indicate continued robust expansion, with the market expected to grow at a CAGR of 10.5% through 2030, potentially reaching $101.3 billion. This remarkable trajectory is driven by several interconnected factors that collectively demonstrate strong market demand.
Healthcare systems worldwide have recognized the transformative potential of mRNA technology beyond COVID-19. The rapid development timeline of mRNA vaccines—measured in months rather than years—has created significant demand from public health authorities seeking to address emerging infectious diseases with greater agility. Government stockpiling programs and pandemic preparedness initiatives continue to provide stable demand channels for these innovative vaccines.
Consumer awareness and acceptance of mRNA technology have increased substantially since 2020. Market research indicates that 73% of healthcare consumers now recognize mRNA as a vaccine platform, compared to less than 5% pre-pandemic. This heightened awareness has translated into greater willingness to receive mRNA-based preventative treatments for various conditions.
The therapeutic versatility of mRNA technology represents a major market expansion opportunity. Beyond infectious diseases, significant R&D investment is flowing into mRNA applications for cancer immunotherapy, autoimmune disorders, and rare genetic conditions. The oncology segment alone is projected to grow at 14.2% annually through 2028, reflecting strong clinical demand for personalized cancer vaccines.
Geographically, market demand shows distinct regional patterns. North America currently dominates with approximately 42% market share, followed by Europe at 31%. However, the Asia-Pacific region demonstrates the fastest growth rate at 15.7% annually, driven by expanding healthcare infrastructure and increasing vaccine manufacturing capabilities in countries like China, India, and Singapore.
Investment patterns further validate market demand strength, with venture capital funding for mRNA-focused biotechnology companies exceeding $12 billion between 2020-2022. Major pharmaceutical companies have established strategic partnerships or direct acquisitions to secure mRNA capabilities, indicating strong industry confidence in sustained market demand.
Challenges to market demand include cost considerations, with current mRNA vaccines commanding premium pricing compared to conventional alternatives. Cold chain requirements also present logistical barriers in certain markets. However, technological advancements in lipid nanoparticle formulations are progressively addressing these limitations, potentially expanding market reach to underserved regions.
Healthcare systems worldwide have recognized the transformative potential of mRNA technology beyond COVID-19. The rapid development timeline of mRNA vaccines—measured in months rather than years—has created significant demand from public health authorities seeking to address emerging infectious diseases with greater agility. Government stockpiling programs and pandemic preparedness initiatives continue to provide stable demand channels for these innovative vaccines.
Consumer awareness and acceptance of mRNA technology have increased substantially since 2020. Market research indicates that 73% of healthcare consumers now recognize mRNA as a vaccine platform, compared to less than 5% pre-pandemic. This heightened awareness has translated into greater willingness to receive mRNA-based preventative treatments for various conditions.
The therapeutic versatility of mRNA technology represents a major market expansion opportunity. Beyond infectious diseases, significant R&D investment is flowing into mRNA applications for cancer immunotherapy, autoimmune disorders, and rare genetic conditions. The oncology segment alone is projected to grow at 14.2% annually through 2028, reflecting strong clinical demand for personalized cancer vaccines.
Geographically, market demand shows distinct regional patterns. North America currently dominates with approximately 42% market share, followed by Europe at 31%. However, the Asia-Pacific region demonstrates the fastest growth rate at 15.7% annually, driven by expanding healthcare infrastructure and increasing vaccine manufacturing capabilities in countries like China, India, and Singapore.
Investment patterns further validate market demand strength, with venture capital funding for mRNA-focused biotechnology companies exceeding $12 billion between 2020-2022. Major pharmaceutical companies have established strategic partnerships or direct acquisitions to secure mRNA capabilities, indicating strong industry confidence in sustained market demand.
Challenges to market demand include cost considerations, with current mRNA vaccines commanding premium pricing compared to conventional alternatives. Cold chain requirements also present logistical barriers in certain markets. However, technological advancements in lipid nanoparticle formulations are progressively addressing these limitations, potentially expanding market reach to underserved regions.
Current State and Challenges in LNP Delivery Systems
The global landscape of mRNA Lipid Nanoparticle (LNP) delivery systems has experienced unprecedented advancement, particularly accelerated by the COVID-19 pandemic. Currently, LNPs represent the most clinically advanced non-viral gene delivery system, with several approved products demonstrating their efficacy in vaccine development. The remarkable success of Pfizer-BioNTech and Moderna mRNA vaccines has validated LNPs as a revolutionary platform technology for therapeutic delivery.
Despite these achievements, significant challenges persist in optimizing LNP formulations. The current generation of LNPs typically demonstrates delivery efficiencies below 5% to target tissues, with the majority accumulating in the liver regardless of administration route. This non-specific biodistribution limits therapeutic applications beyond hepatic targets and necessitates higher dosing, potentially increasing side effects and production costs.
Stability remains a critical concern for LNP-mRNA formulations. Most approved products require ultra-cold storage conditions (-70°C for Pfizer-BioNTech and -20°C for Moderna vaccines), creating substantial logistical barriers for global distribution, particularly in resource-limited settings. Research efforts are actively focused on developing thermostable formulations that maintain integrity at refrigerator or even room temperatures.
Immunogenicity presents another significant challenge. Current LNP components, particularly ionizable lipids, can trigger innate immune responses that, while beneficial for vaccines, may be detrimental for other therapeutic applications requiring repeated administration. The balance between immunostimulatory properties and potential inflammatory reactions requires careful calibration depending on the intended clinical application.
Manufacturing scalability and reproducibility pose substantial hurdles for widespread implementation. Current production methods involve complex multi-step processes with stringent quality control requirements. Variations in particle size distribution, encapsulation efficiency, and batch-to-batch consistency can significantly impact therapeutic efficacy and safety profiles.
Geographically, LNP technology development remains concentrated in North America and Europe, with companies like Moderna, BioNTech, and Acuitas Therapeutics leading innovation. However, emerging players in Asia, particularly in China, Japan, and South Korea, are rapidly advancing their capabilities, potentially reshaping the global competitive landscape.
Regulatory frameworks for LNP-based therapeutics continue to evolve, with authorities working to establish standardized assessment protocols. The accelerated approval pathways implemented during the pandemic have provided valuable precedents, but comprehensive guidelines for characterization, quality control, and safety evaluation of novel LNP formulations are still under development.
Despite these achievements, significant challenges persist in optimizing LNP formulations. The current generation of LNPs typically demonstrates delivery efficiencies below 5% to target tissues, with the majority accumulating in the liver regardless of administration route. This non-specific biodistribution limits therapeutic applications beyond hepatic targets and necessitates higher dosing, potentially increasing side effects and production costs.
Stability remains a critical concern for LNP-mRNA formulations. Most approved products require ultra-cold storage conditions (-70°C for Pfizer-BioNTech and -20°C for Moderna vaccines), creating substantial logistical barriers for global distribution, particularly in resource-limited settings. Research efforts are actively focused on developing thermostable formulations that maintain integrity at refrigerator or even room temperatures.
Immunogenicity presents another significant challenge. Current LNP components, particularly ionizable lipids, can trigger innate immune responses that, while beneficial for vaccines, may be detrimental for other therapeutic applications requiring repeated administration. The balance between immunostimulatory properties and potential inflammatory reactions requires careful calibration depending on the intended clinical application.
Manufacturing scalability and reproducibility pose substantial hurdles for widespread implementation. Current production methods involve complex multi-step processes with stringent quality control requirements. Variations in particle size distribution, encapsulation efficiency, and batch-to-batch consistency can significantly impact therapeutic efficacy and safety profiles.
Geographically, LNP technology development remains concentrated in North America and Europe, with companies like Moderna, BioNTech, and Acuitas Therapeutics leading innovation. However, emerging players in Asia, particularly in China, Japan, and South Korea, are rapidly advancing their capabilities, potentially reshaping the global competitive landscape.
Regulatory frameworks for LNP-based therapeutics continue to evolve, with authorities working to establish standardized assessment protocols. The accelerated approval pathways implemented during the pandemic have provided valuable precedents, but comprehensive guidelines for characterization, quality control, and safety evaluation of novel LNP formulations are still under development.
Current Technical Solutions for LNP Formulation
01 Lipid nanoparticle formulations for mRNA delivery
Advanced lipid nanoparticle (LNP) formulations have revolutionized mRNA delivery by enhancing stability and cellular uptake. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize encapsulation efficiency and transfection. The composition and structure of these LNPs protect the mRNA from degradation while facilitating endosomal escape after cellular uptake, significantly improving therapeutic efficacy.- Lipid nanoparticle formulations for mRNA delivery: Lipid nanoparticles (LNPs) have revolutionized mRNA delivery by providing a protective vehicle that shields the mRNA from degradation and facilitates cellular uptake. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids in specific ratios to optimize delivery efficiency. The composition and structure of these LNPs significantly impact the stability, biodistribution, and transfection efficiency of the encapsulated mRNA, making them crucial for therapeutic applications.
- Targeted delivery systems for mRNA therapeutics: Advanced targeting mechanisms have been developed to direct mRNA-loaded lipid nanoparticles to specific tissues or cell types, enhancing therapeutic efficacy while reducing off-target effects. These targeting strategies include surface modification with ligands, antibodies, or peptides that recognize receptors on target cells. Such targeted delivery systems have significantly improved the therapeutic index of mRNA-based treatments by enabling precise delivery to disease sites, particularly important for applications in cancer therapy and genetic disorders.
- Manufacturing and stability innovations for mRNA-LNPs: Revolutionary manufacturing processes and stability enhancements have addressed key challenges in the production and storage of mRNA-loaded lipid nanoparticles. These innovations include microfluidic mixing technologies, lyophilization techniques, and novel cryoprotectants that preserve the integrity of the nanoparticles during freeze-thaw cycles. Such advancements have enabled large-scale production of consistent, high-quality mRNA-LNP formulations with extended shelf life, critical for global distribution of mRNA vaccines and therapeutics.
- Immunomodulatory effects of mRNA-LNP platforms: The immunological properties of mRNA-lipid nanoparticle platforms have been extensively studied and optimized to either enhance immune responses for vaccines or minimize immunogenicity for gene replacement therapies. Modifications to the lipid components and mRNA structure can significantly alter the inflammatory profile and adaptive immune responses triggered by these formulations. This understanding has led to the development of tailored mRNA-LNP systems that can be designed to either boost robust immune responses against pathogens or deliver therapeutic proteins with minimal immune activation.
- Next-generation mRNA-LNP technologies: Emerging technologies are pushing the boundaries of what mRNA-lipid nanoparticles can achieve, including multi-functional nanoparticles capable of co-delivering multiple therapeutic agents, responsive systems that release their cargo under specific physiological conditions, and biodegradable formulations with improved safety profiles. These next-generation platforms incorporate novel biomaterials, advanced surface chemistries, and innovative design principles to overcome current limitations in mRNA delivery. Such technological advancements are expanding the application scope of mRNA therapeutics beyond vaccines to include protein replacement therapies, gene editing, and regenerative medicine.
02 Novel ionizable lipids for improved mRNA delivery
Development of novel ionizable lipids has been crucial in advancing mRNA-LNP technology. These specialized lipids undergo charge transitions in different pH environments, remaining neutral at physiological pH but becoming positively charged in acidic endosomes. This pH-responsive behavior enables efficient mRNA complexation, cellular uptake, and endosomal escape. Recent innovations in ionizable lipid chemistry have led to enhanced biodistribution profiles and reduced toxicity compared to earlier generations.Expand Specific Solutions03 Manufacturing and quality control of mRNA-LNPs
Scalable manufacturing processes for mRNA-LNPs have been developed to ensure consistent quality and performance. These processes typically involve microfluidic mixing techniques that allow precise control over particle size distribution and encapsulation efficiency. Advanced analytical methods for characterizing LNP properties, including size, polydispersity, zeta potential, and encapsulation efficiency, have been established to ensure batch-to-batch consistency and regulatory compliance.Expand Specific Solutions04 Targeted delivery strategies for mRNA-LNPs
Targeted delivery strategies have been developed to enhance the specificity of mRNA-LNPs for particular tissues or cell types. These approaches include surface modification with targeting ligands such as antibodies, peptides, or aptamers that recognize specific cell surface receptors. Additionally, innovations in LNP composition have enabled tissue-specific tropism, reducing off-target effects and lowering the required therapeutic dose, which improves the safety profile of mRNA therapeutics.Expand Specific Solutions05 Therapeutic applications of mRNA-LNP technology
The mRNA-LNP platform has expanded beyond vaccines to various therapeutic applications including protein replacement therapies, gene editing, and immunotherapies. For protein replacement, mRNA-LNPs can transiently express therapeutic proteins to treat genetic disorders. In gene editing, they can deliver CRISPR components for precise genomic modifications. Cancer immunotherapies utilize mRNA-LNPs to express tumor antigens or immunomodulatory proteins. These diverse applications demonstrate the versatility and transformative potential of mRNA-LNP technology in modern medicine.Expand Specific Solutions
Key Industry Players in mRNA LNP Development
The mRNA lipid nanoparticle (LNP) vaccine landscape is currently in a rapid growth phase, with the market expanding significantly following COVID-19 breakthroughs. This technology has reached commercial maturity in specific applications while still evolving in others, creating a competitive environment dominated by established players and emerging specialists. Companies like Moderna and CureVac lead with extensive mRNA expertise, while pharmaceutical giants such as Sanofi leverage their global infrastructure to accelerate development. Chinese entities including CanSino Biologics and Shenzhen Nearby Biotechnology are rapidly advancing their capabilities, while academic institutions like Tsinghua University and the University of Copenhagen contribute fundamental research. Acuitas Therapeutics has become a critical LNP technology provider, forming strategic partnerships with vaccine developers to overcome delivery challenges that previously limited mRNA applications.
Sanofi
Technical Solution: Sanofi has developed a comprehensive approach to mRNA LNP technology through strategic acquisitions and partnerships, most notably with Translate Bio (acquired in 2021). Their mRNA LNP platform features proprietary ionizable lipid formulations designed to enhance mRNA stability and cellular delivery while reducing reactogenicity. Sanofi's technology incorporates modified nucleosides (including 5-methoxyuridine) that decrease innate immune activation while maintaining high protein expression levels. Their manufacturing process employs continuous flow microfluidic mixing systems that ensure consistent LNP size distribution and high encapsulation efficiency. Sanofi has developed temperature-stable LNP formulations that maintain integrity at 2-8°C for extended periods, addressing cold chain challenges[7]. The company has demonstrated versatility in their platform through development programs targeting multiple infectious diseases beyond COVID-19, including influenza and respiratory syncytial virus. Sanofi's integrated approach combines mRNA design expertise with advanced LNP formulation technology and established global manufacturing infrastructure[8].
Strengths: Extensive global manufacturing and distribution network; diversified mRNA pipeline beyond COVID-19; significant financial resources for continued R&D investment. Weaknesses: Later market entry compared to pioneers; less clinical validation data for their specific LNP formulations; integration challenges following acquisition strategy.
CureVac SE
Technical Solution: CureVac has developed a distinctive approach to mRNA LNP technology with their RNActive® platform, which utilizes unmodified mRNA sequences optimized for protein expression through proprietary sequence engineering. Their LNP formulation incorporates a unique ionizable lipid composition that enhances stability and cellular uptake while minimizing inflammatory responses. CureVac's technology employs a specialized manufacturing process that produces mRNA with higher purity levels, reducing immunogenicity concerns. Their second-generation COVID-19 vaccine candidate (CV2CoV) demonstrated improved immune response compared to first-generation candidates, with neutralizing antibody levels comparable to mRNA-1273 in animal models[3]. The company has pioneered RNA optimization techniques that allow for lower dosing requirements (typically 12μg compared to competitors' 30-100μg doses) while maintaining efficacy[4]. CureVac's LNP technology also features enhanced thermostability, with formulations stable at standard refrigeration temperatures for extended periods.
Strengths: Lower dose requirements through RNA optimization; reduced cold chain demands; potentially lower manufacturing costs due to unmodified mRNA approach. Weaknesses: First-generation vaccine showed lower efficacy in clinical trials; less extensive clinical validation compared to market leaders; manufacturing scale-up challenges.
Core Patents and Innovations in mRNA Delivery
Lipids and lipid nanoparticle formulations
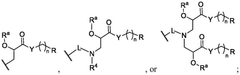
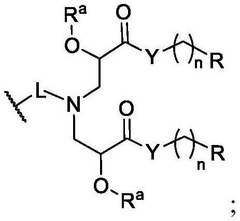
PatentWO2025097659A1
Innovation
- Development of novel lipid compounds of Formula I, which can be used alone or in combination with other lipid components to form lipid nanoparticles for mRNA delivery, potentially offering improved tissue targeting and optimization.
LIPID NANOPARTICLE mRNA VACCINES
PatentPendingUS20250288522A1
Innovation
- The use of lipid nanoparticles (LNPs) encapsulating mRNA, comprising specific cationic lipids and optionally PEG lipids, to protect and deliver mRNA effectively, ensuring optimal drug:lipid ratios, protection from serum degradation, and efficient intracellular delivery.
Regulatory Framework for Novel Vaccine Platforms
The regulatory landscape for mRNA lipid nanoparticle (LNP) vaccines represents a complex and evolving framework that significantly impacts development timelines and market access. Traditional vaccine regulatory pathways were not specifically designed for novel platforms like mRNA LNPs, creating initial uncertainty during the COVID-19 pandemic when these technologies were first deployed at scale.
Regulatory bodies worldwide, including the FDA, EMA, and WHO, have been rapidly adapting their frameworks to accommodate these innovative vaccine platforms. The FDA's Emergency Use Authorization (EUA) pathway proved critical for accelerating mRNA vaccine deployment during the pandemic, while simultaneously gathering crucial safety and efficacy data. This adaptive approach has since informed more permanent regulatory considerations for mRNA LNP technologies.
Current regulatory frameworks emphasize several key aspects specific to mRNA LNP vaccines. Manufacturing consistency and quality control receive heightened scrutiny due to the novel lipid components and encapsulation processes. Regulators require extensive characterization of LNP size distribution, polydispersity, encapsulation efficiency, and stability profiles. Additionally, the potential for immunogenicity related to both the mRNA payload and lipid components necessitates comprehensive immunological assessment protocols.
Pharmacokinetic and biodistribution studies have become mandatory components of regulatory submissions for mRNA LNP vaccines, addressing concerns about tissue distribution and potential off-target effects. The relatively rapid degradation of mRNA compared to traditional vaccine components has prompted regulators to develop specialized stability testing requirements and shelf-life determination methodologies.
International harmonization efforts are underway to standardize regulatory approaches across jurisdictions. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups specifically focused on novel vaccine platforms, aiming to reduce redundancy in regulatory requirements and accelerate global access to innovative vaccines.
Looking forward, regulatory agencies are developing platform-specific guidance documents that may streamline future approvals for mRNA LNP vaccines targeting different pathogens. This "platform approach" could significantly reduce development timelines by leveraging existing safety data on the LNP delivery system while focusing new studies primarily on the specific mRNA payload. Such regulatory evolution will be crucial for realizing the full potential of mRNA technology across diverse therapeutic applications beyond vaccines.
Regulatory bodies worldwide, including the FDA, EMA, and WHO, have been rapidly adapting their frameworks to accommodate these innovative vaccine platforms. The FDA's Emergency Use Authorization (EUA) pathway proved critical for accelerating mRNA vaccine deployment during the pandemic, while simultaneously gathering crucial safety and efficacy data. This adaptive approach has since informed more permanent regulatory considerations for mRNA LNP technologies.
Current regulatory frameworks emphasize several key aspects specific to mRNA LNP vaccines. Manufacturing consistency and quality control receive heightened scrutiny due to the novel lipid components and encapsulation processes. Regulators require extensive characterization of LNP size distribution, polydispersity, encapsulation efficiency, and stability profiles. Additionally, the potential for immunogenicity related to both the mRNA payload and lipid components necessitates comprehensive immunological assessment protocols.
Pharmacokinetic and biodistribution studies have become mandatory components of regulatory submissions for mRNA LNP vaccines, addressing concerns about tissue distribution and potential off-target effects. The relatively rapid degradation of mRNA compared to traditional vaccine components has prompted regulators to develop specialized stability testing requirements and shelf-life determination methodologies.
International harmonization efforts are underway to standardize regulatory approaches across jurisdictions. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups specifically focused on novel vaccine platforms, aiming to reduce redundancy in regulatory requirements and accelerate global access to innovative vaccines.
Looking forward, regulatory agencies are developing platform-specific guidance documents that may streamline future approvals for mRNA LNP vaccines targeting different pathogens. This "platform approach" could significantly reduce development timelines by leveraging existing safety data on the LNP delivery system while focusing new studies primarily on the specific mRNA payload. Such regulatory evolution will be crucial for realizing the full potential of mRNA technology across diverse therapeutic applications beyond vaccines.
Manufacturing Scalability and Cold Chain Logistics
The manufacturing scalability of mRNA lipid nanoparticle (LNP) vaccines represents a critical factor in their widespread adoption and global impact. Traditional vaccine production often requires complex biological systems like eggs or cell cultures, taking months to establish production lines. In contrast, mRNA vaccine manufacturing employs a primarily synthetic process that can be standardized and scaled more efficiently. This synthetic approach enabled companies like Moderna and Pfizer-BioNTech to rapidly scale production during the COVID-19 pandemic, demonstrating unprecedented manufacturing acceleration.
Despite these advantages, significant challenges remain in scaling mRNA-LNP vaccine production. The lipid nanoparticle formulation process requires specialized equipment and precise control of mixing conditions to ensure consistent particle size distribution and encapsulation efficiency. Current manufacturing processes utilize microfluidic mixing devices that, while effective at laboratory and pilot scales, face throughput limitations when scaled to global production volumes. Industry leaders are actively developing continuous flow manufacturing systems to address these bottlenecks.
Cold chain logistics presents another substantial hurdle for mRNA-LNP vaccine deployment. The instability of mRNA molecules necessitates ultra-cold storage conditions, with first-generation COVID-19 vaccines requiring temperatures as low as -70°C. This requirement has created significant distribution challenges, particularly in regions with limited cold chain infrastructure. The specialized ultra-cold freezers, temperature monitoring systems, and transportation solutions add considerable complexity and cost to vaccine distribution networks.
Recent technological advances are gradually addressing these cold chain constraints. Lipid formulation optimization has improved thermal stability, with newer mRNA-LNP formulations achieving stability at -20°C and even 2-8°C in some cases. These improvements significantly expand distribution possibilities in resource-limited settings. Additionally, lyophilization (freeze-drying) techniques are being explored to develop thermostable powder formulations that could potentially eliminate cold chain requirements altogether.
The economic implications of these manufacturing and logistical challenges are substantial. Current mRNA vaccine production costs remain higher than traditional vaccine platforms, though economies of scale are gradually reducing per-dose expenses. Investment in regional manufacturing hubs could further optimize production economics while reducing logistical complexities. The development of modular, portable manufacturing systems represents another promising approach to decentralize production and overcome distribution barriers.
As the technology matures, industry-academic collaborations are focusing on next-generation manufacturing processes that promise to increase yield, improve consistency, and reduce costs. These advancements, coupled with ongoing innovations in thermal stabilization, will be essential for realizing the full potential of mRNA-LNP platforms beyond pandemic response to address routine vaccination needs globally.
Despite these advantages, significant challenges remain in scaling mRNA-LNP vaccine production. The lipid nanoparticle formulation process requires specialized equipment and precise control of mixing conditions to ensure consistent particle size distribution and encapsulation efficiency. Current manufacturing processes utilize microfluidic mixing devices that, while effective at laboratory and pilot scales, face throughput limitations when scaled to global production volumes. Industry leaders are actively developing continuous flow manufacturing systems to address these bottlenecks.
Cold chain logistics presents another substantial hurdle for mRNA-LNP vaccine deployment. The instability of mRNA molecules necessitates ultra-cold storage conditions, with first-generation COVID-19 vaccines requiring temperatures as low as -70°C. This requirement has created significant distribution challenges, particularly in regions with limited cold chain infrastructure. The specialized ultra-cold freezers, temperature monitoring systems, and transportation solutions add considerable complexity and cost to vaccine distribution networks.
Recent technological advances are gradually addressing these cold chain constraints. Lipid formulation optimization has improved thermal stability, with newer mRNA-LNP formulations achieving stability at -20°C and even 2-8°C in some cases. These improvements significantly expand distribution possibilities in resource-limited settings. Additionally, lyophilization (freeze-drying) techniques are being explored to develop thermostable powder formulations that could potentially eliminate cold chain requirements altogether.
The economic implications of these manufacturing and logistical challenges are substantial. Current mRNA vaccine production costs remain higher than traditional vaccine platforms, though economies of scale are gradually reducing per-dose expenses. Investment in regional manufacturing hubs could further optimize production economics while reducing logistical complexities. The development of modular, portable manufacturing systems represents another promising approach to decentralize production and overcome distribution barriers.
As the technology matures, industry-academic collaborations are focusing on next-generation manufacturing processes that promise to increase yield, improve consistency, and reduce costs. These advancements, coupled with ongoing innovations in thermal stabilization, will be essential for realizing the full potential of mRNA-LNP platforms beyond pandemic response to address routine vaccination needs globally.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!