Exploring Thermal Stability in Lipid Nanoparticle Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lipid Nanoparticle Thermal Stability Background and Objectives
Lipid nanoparticles (LNPs) have emerged as revolutionary delivery systems for nucleic acid therapeutics, most notably demonstrated in their pivotal role in mRNA COVID-19 vaccines. The development of LNPs dates back to the 1960s with liposomal research, but significant advancements in the past decade have transformed these systems into sophisticated delivery vehicles capable of protecting sensitive cargo like mRNA and siRNA from degradation while facilitating cellular uptake.
Thermal stability represents a critical challenge in LNP development and application. The complex multicomponent structure of LNPs—typically comprising ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids—creates inherent vulnerabilities to temperature fluctuations. Historical approaches to lipid-based delivery systems have consistently encountered stability limitations, with temperature excursions causing phase transitions, component segregation, and cargo degradation.
The evolution of LNP technology has been marked by progressive improvements in formulation techniques, from thin-film hydration methods to more sophisticated microfluidic approaches. Each advancement has contributed incrementally to thermal resilience, yet temperature sensitivity remains a significant barrier to widespread implementation across diverse therapeutic applications and global distribution networks.
Current thermal stability challenges manifest in several critical ways: lipid bilayer phase transitions at specific temperatures that compromise structural integrity; oxidative degradation of unsaturated lipid components accelerated by elevated temperatures; payload degradation particularly with nucleic acid cargoes; and particle aggregation or fusion during temperature fluctuations. These phenomena collectively impact critical quality attributes including particle size distribution, encapsulation efficiency, and ultimately therapeutic efficacy.
The objective of this technical exploration is multifaceted: to comprehensively map the thermal behavior of various LNP compositions across temperature ranges relevant to manufacturing, storage, and administration; to identify molecular and structural determinants of thermal stability; to develop predictive models correlating formulation parameters with stability profiles; and to establish innovative approaches for enhancing thermal resilience without compromising functional performance.
This investigation aims to bridge fundamental physicochemical understanding with practical formulation strategies, ultimately enabling the development of thermally robust LNP systems capable of withstanding real-world temperature challenges. Success in this domain would significantly expand the therapeutic potential of LNP-delivered modalities by reducing cold chain dependencies, extending shelf-life, and enabling access in regions with limited refrigeration infrastructure.
The technological trajectory suggests promising avenues for innovation, including rational lipid design, novel stabilizing excipients, and advanced manufacturing processes specifically engineered to enhance thermal stability while maintaining the critical functional attributes that have made LNPs transformative delivery vehicles.
Thermal stability represents a critical challenge in LNP development and application. The complex multicomponent structure of LNPs—typically comprising ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids—creates inherent vulnerabilities to temperature fluctuations. Historical approaches to lipid-based delivery systems have consistently encountered stability limitations, with temperature excursions causing phase transitions, component segregation, and cargo degradation.
The evolution of LNP technology has been marked by progressive improvements in formulation techniques, from thin-film hydration methods to more sophisticated microfluidic approaches. Each advancement has contributed incrementally to thermal resilience, yet temperature sensitivity remains a significant barrier to widespread implementation across diverse therapeutic applications and global distribution networks.
Current thermal stability challenges manifest in several critical ways: lipid bilayer phase transitions at specific temperatures that compromise structural integrity; oxidative degradation of unsaturated lipid components accelerated by elevated temperatures; payload degradation particularly with nucleic acid cargoes; and particle aggregation or fusion during temperature fluctuations. These phenomena collectively impact critical quality attributes including particle size distribution, encapsulation efficiency, and ultimately therapeutic efficacy.
The objective of this technical exploration is multifaceted: to comprehensively map the thermal behavior of various LNP compositions across temperature ranges relevant to manufacturing, storage, and administration; to identify molecular and structural determinants of thermal stability; to develop predictive models correlating formulation parameters with stability profiles; and to establish innovative approaches for enhancing thermal resilience without compromising functional performance.
This investigation aims to bridge fundamental physicochemical understanding with practical formulation strategies, ultimately enabling the development of thermally robust LNP systems capable of withstanding real-world temperature challenges. Success in this domain would significantly expand the therapeutic potential of LNP-delivered modalities by reducing cold chain dependencies, extending shelf-life, and enabling access in regions with limited refrigeration infrastructure.
The technological trajectory suggests promising avenues for innovation, including rational lipid design, novel stabilizing excipients, and advanced manufacturing processes specifically engineered to enhance thermal stability while maintaining the critical functional attributes that have made LNPs transformative delivery vehicles.
Market Analysis for Thermally Stable LNP Applications
The global market for thermally stable Lipid Nanoparticle (LNP) systems is experiencing significant growth, driven primarily by the expanding applications in pharmaceutical delivery systems, particularly for mRNA vaccines and therapeutics. The market size for LNP-based drug delivery systems reached approximately $5.1 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 13.2% through 2028, potentially reaching $10.7 billion by the end of the forecast period.
The COVID-19 pandemic served as a catalyst for LNP market expansion, with the successful deployment of mRNA vaccines highlighting the critical importance of thermal stability in these delivery systems. Current market demand is concentrated in three primary segments: vaccines (46% of market share), cancer therapeutics (28%), and rare disease treatments (17%), with the remaining 9% distributed across various other applications.
Geographically, North America dominates the market with 42% share, followed by Europe (31%), Asia-Pacific (18%), and rest of the world (9%). The Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, driven by increasing healthcare infrastructure investments and growing pharmaceutical manufacturing capabilities in countries like China, India, and South Korea.
Key market drivers include the expanding pipeline of RNA therapeutics, with over 180 candidates currently in clinical trials globally. Additionally, the shift toward personalized medicine and targeted drug delivery systems is creating substantial demand for thermally stable LNP formulations that can maintain integrity across diverse supply chain conditions.
Market challenges primarily revolve around cost factors, with current thermally stable LNP formulations adding 15-30% to production costs compared to standard formulations. Regulatory hurdles also present significant barriers to market entry, with approval processes for novel LNP formulations typically taking 12-18 months longer than conventional alternatives.
Consumer demand patterns indicate growing preference for pharmaceutical products with reduced cold chain dependencies, with 78% of healthcare providers citing storage requirements as a critical factor in vaccine and therapeutic selection. This trend is particularly pronounced in emerging markets where cold chain infrastructure remains limited.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms focusing on proprietary LNP formulation technologies. Market concentration remains moderate, with the top five players controlling approximately 63% of market share, though this is expected to decrease as new entrants with innovative thermal stability solutions enter the space.
The COVID-19 pandemic served as a catalyst for LNP market expansion, with the successful deployment of mRNA vaccines highlighting the critical importance of thermal stability in these delivery systems. Current market demand is concentrated in three primary segments: vaccines (46% of market share), cancer therapeutics (28%), and rare disease treatments (17%), with the remaining 9% distributed across various other applications.
Geographically, North America dominates the market with 42% share, followed by Europe (31%), Asia-Pacific (18%), and rest of the world (9%). The Asia-Pacific region is expected to witness the fastest growth rate of 15.7% annually, driven by increasing healthcare infrastructure investments and growing pharmaceutical manufacturing capabilities in countries like China, India, and South Korea.
Key market drivers include the expanding pipeline of RNA therapeutics, with over 180 candidates currently in clinical trials globally. Additionally, the shift toward personalized medicine and targeted drug delivery systems is creating substantial demand for thermally stable LNP formulations that can maintain integrity across diverse supply chain conditions.
Market challenges primarily revolve around cost factors, with current thermally stable LNP formulations adding 15-30% to production costs compared to standard formulations. Regulatory hurdles also present significant barriers to market entry, with approval processes for novel LNP formulations typically taking 12-18 months longer than conventional alternatives.
Consumer demand patterns indicate growing preference for pharmaceutical products with reduced cold chain dependencies, with 78% of healthcare providers citing storage requirements as a critical factor in vaccine and therapeutic selection. This trend is particularly pronounced in emerging markets where cold chain infrastructure remains limited.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms focusing on proprietary LNP formulation technologies. Market concentration remains moderate, with the top five players controlling approximately 63% of market share, though this is expected to decrease as new entrants with innovative thermal stability solutions enter the space.
Current Challenges in LNP Thermal Stability
Lipid Nanoparticle (LNP) systems have emerged as critical delivery vehicles for nucleic acid therapeutics, with their recent success in mRNA vaccine delivery highlighting their importance. However, thermal stability remains a significant challenge that limits their broader application and commercial viability. Current LNP formulations exhibit sensitivity to temperature fluctuations, leading to structural changes, payload degradation, and reduced efficacy.
One of the primary challenges is the inherent thermodynamic instability of lipid bilayers at elevated temperatures. When exposed to temperatures above their phase transition point, lipids undergo conformational changes that can disrupt the nanoparticle structure. This results in increased particle size, altered surface charge, and potential leakage of encapsulated materials. Studies have shown that even brief exposure to temperatures above 40°C can lead to irreversible changes in LNP morphology.
The heterogeneous composition of LNPs further complicates thermal stability issues. Modern LNP formulations typically contain four components: ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. Each component responds differently to temperature changes, creating complex phase behaviors that are difficult to predict and control. The ionizable lipids, which are crucial for endosomal escape, are particularly susceptible to oxidation at elevated temperatures, compromising their functionality.
Payload protection presents another significant challenge. Nucleic acids encapsulated within LNPs can degrade when exposed to thermal stress, with RNA being especially vulnerable due to its inherent instability. The degradation pathways are multifaceted, involving hydrolysis, oxidation, and enzymatic breakdown, all of which are accelerated at higher temperatures. Current stabilization strategies often fail to adequately protect the payload during temperature excursions.
Storage and transportation requirements pose practical limitations for LNP-based therapeutics. Many current formulations require ultra-cold chain storage (-70°C for some mRNA vaccines), creating logistical hurdles and increasing costs, particularly in regions with limited infrastructure. This cold chain dependency significantly restricts global access to these advanced therapeutics.
Manufacturing processes also contribute to thermal stability challenges. High-pressure homogenization and microfluidic mixing, commonly used for LNP production, can introduce thermal stress during formulation. These processes may create inconsistencies in particle size distribution and lipid organization that predispose the final product to thermal instability.
Analytical limitations further complicate efforts to address thermal stability. Current characterization techniques often provide incomplete information about structural changes occurring at the molecular level during thermal stress. This knowledge gap hinders the development of rational design approaches for thermally stable LNPs and makes quality control during manufacturing challenging.
One of the primary challenges is the inherent thermodynamic instability of lipid bilayers at elevated temperatures. When exposed to temperatures above their phase transition point, lipids undergo conformational changes that can disrupt the nanoparticle structure. This results in increased particle size, altered surface charge, and potential leakage of encapsulated materials. Studies have shown that even brief exposure to temperatures above 40°C can lead to irreversible changes in LNP morphology.
The heterogeneous composition of LNPs further complicates thermal stability issues. Modern LNP formulations typically contain four components: ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. Each component responds differently to temperature changes, creating complex phase behaviors that are difficult to predict and control. The ionizable lipids, which are crucial for endosomal escape, are particularly susceptible to oxidation at elevated temperatures, compromising their functionality.
Payload protection presents another significant challenge. Nucleic acids encapsulated within LNPs can degrade when exposed to thermal stress, with RNA being especially vulnerable due to its inherent instability. The degradation pathways are multifaceted, involving hydrolysis, oxidation, and enzymatic breakdown, all of which are accelerated at higher temperatures. Current stabilization strategies often fail to adequately protect the payload during temperature excursions.
Storage and transportation requirements pose practical limitations for LNP-based therapeutics. Many current formulations require ultra-cold chain storage (-70°C for some mRNA vaccines), creating logistical hurdles and increasing costs, particularly in regions with limited infrastructure. This cold chain dependency significantly restricts global access to these advanced therapeutics.
Manufacturing processes also contribute to thermal stability challenges. High-pressure homogenization and microfluidic mixing, commonly used for LNP production, can introduce thermal stress during formulation. These processes may create inconsistencies in particle size distribution and lipid organization that predispose the final product to thermal instability.
Analytical limitations further complicate efforts to address thermal stability. Current characterization techniques often provide incomplete information about structural changes occurring at the molecular level during thermal stress. This knowledge gap hinders the development of rational design approaches for thermally stable LNPs and makes quality control during manufacturing challenging.
Current Approaches to Enhance LNP Thermal Stability
01 Lipid composition for enhanced thermal stability
Specific lipid compositions can significantly enhance the thermal stability of lipid nanoparticle systems. These compositions often include combinations of ionizable lipids, helper lipids, and cholesterol in optimized ratios. The selection of lipids with higher melting points and the incorporation of saturated lipids can improve the structural integrity of nanoparticles when exposed to elevated temperatures, preventing premature degradation and maintaining encapsulation efficiency.- Lipid composition optimization for thermal stability: Optimizing the lipid composition in nanoparticle systems can significantly enhance thermal stability. This includes selecting specific lipid types, adjusting lipid ratios, and incorporating stabilizing lipids such as cholesterol or PEGylated lipids. The careful balance of saturated and unsaturated lipids can create formulations that maintain structural integrity across a wider temperature range, preventing aggregation and content leakage during storage and transportation.
- Cryoprotectants and lyophilization techniques: The use of cryoprotectants and lyophilization (freeze-drying) techniques can dramatically improve the thermal stability of lipid nanoparticle systems. Sugars like trehalose, sucrose, and mannitol protect the nanoparticle structure during freezing and drying processes. These approaches allow for the conversion of liquid formulations into solid, thermally stable powders that can be reconstituted before use, significantly extending shelf life and reducing cold chain requirements.
- Surface modification and stabilizing agents: Surface modification of lipid nanoparticles with polymers or stabilizing agents can enhance thermal stability. Techniques include PEGylation, coating with hydrophilic polymers, or incorporation of surfactants that create steric barriers preventing particle aggregation at elevated temperatures. These modifications can alter the surface charge and hydration layer of nanoparticles, providing protection against temperature-induced destabilization and maintaining colloidal stability across broader temperature ranges.
- pH and buffer system optimization: The optimization of pH and buffer systems plays a crucial role in maintaining lipid nanoparticle thermal stability. Carefully selected buffer components and pH ranges can minimize hydrolysis of lipid components and prevent degradation at elevated temperatures. Some formulations incorporate pH-responsive elements that adapt to environmental changes, providing additional protection against thermal stress and maintaining the integrity of encapsulated active ingredients.
- Manufacturing process parameters for enhanced stability: Manufacturing process parameters significantly impact the thermal stability of lipid nanoparticle systems. Controlled heating rates, precise mixing conditions, homogenization techniques, and post-formation treatments can create more thermally resilient structures. Advanced production methods like microfluidics allow for precise control over particle formation, resulting in more uniform and stable nanoparticles with improved resistance to thermal degradation and better long-term stability profiles.
02 Cryoprotectants and lyophilization techniques
The use of cryoprotectants and lyophilization (freeze-drying) techniques can significantly improve the thermal stability of lipid nanoparticle systems during storage and transportation. Cryoprotectants such as sugars (trehalose, sucrose) and polyols protect the nanoparticle structure during freezing and drying processes. Lyophilization removes water content, reducing hydrolysis reactions and extending shelf-life at various temperatures, while maintaining the integrity and functionality of the encapsulated active ingredients.Expand Specific Solutions03 Surface modification and stabilizing agents
Surface modification of lipid nanoparticles and the addition of stabilizing agents can enhance thermal stability. PEGylation (coating with polyethylene glycol) creates a hydrophilic shield that prevents aggregation at elevated temperatures. Other stabilizing agents such as poloxamers, polysorbates, and specific polymers can be incorporated to maintain particle size distribution and prevent phase separation when exposed to thermal stress. These modifications create steric barriers that maintain colloidal stability across a wider temperature range.Expand Specific Solutions04 Antioxidants and pH stabilization
Incorporating antioxidants and pH stabilizers into lipid nanoparticle formulations can significantly improve their thermal stability. Antioxidants such as tocopherols, ascorbic acid derivatives, and butylated hydroxytoluene (BHT) prevent lipid oxidation at elevated temperatures. Maintaining optimal pH through appropriate buffer systems helps prevent hydrolysis of lipid components and degradation of encapsulated compounds. These approaches minimize chemical degradation pathways that are accelerated by heat exposure.Expand Specific Solutions05 Processing methods for thermally stable formulations
Advanced processing methods can enhance the thermal stability of lipid nanoparticle systems. Techniques such as microfluidic mixing, high-pressure homogenization with controlled temperature parameters, and post-formation thermal cycling can create more ordered lipid structures with improved resistance to thermal stress. Controlled cooling rates during production and specialized heat treatment protocols can induce favorable crystalline arrangements within the lipid matrix, resulting in nanoparticles with superior stability at elevated temperatures.Expand Specific Solutions
Leading Organizations in LNP Thermal Research
The thermal stability of lipid nanoparticle (LNP) systems represents a critical challenge in the rapidly evolving field of mRNA therapeutics, currently in its growth phase with an expanding market exceeding $5 billion. The competitive landscape features established pharmaceutical leaders like BioNTech, Moderna, and Eli Lilly alongside specialized players such as Arcturus Therapeutics and ETHRIS GmbH. Technical maturity varies significantly across applications, with vaccine delivery systems (pioneered by BioNTech/Moderna) achieving commercial deployment while therapeutic applications remain predominantly in clinical development. Academic institutions including MIT, University of Copenhagen, and Heidelberg University contribute fundamental research, while industrial players focus on proprietary formulation technologies to overcome thermal stability challenges that currently necessitate cold-chain distribution, representing a key competitive differentiator in this emerging field.
BioNTech SE
Technical Solution: BioNTech has pioneered thermally-stabilized LNP systems through their proprietary "RiboCure" technology platform. Their approach focuses on modifying the lipid bilayer composition with specialized saturated phospholipids and cholesterol derivatives that maintain membrane fluidity within specific temperature ranges. BioNTech employs a unique pH-responsive ionizable lipid structure that forms more compact and thermally resistant particles. Their technology incorporates antioxidants and metal chelators to prevent lipid oxidation during thermal stress, while utilizing proprietary lyoprotectants that form a glassy matrix around LNPs during freeze-drying. This enables storage at refrigerated or even room temperatures for extended periods. BioNTech has also developed specialized manufacturing processes that produce LNPs with more homogeneous size distribution, contributing to enhanced thermal stability by reducing particle fusion during temperature fluctuations.
Strengths: Robust intellectual property portfolio covering stabilization techniques; proven clinical success with thermally-optimized formulations; advanced analytical capabilities for stability assessment. Weaknesses: Complex manufacturing processes may limit scalability; some components have limited sourcing options, potentially affecting supply chain resilience.
ModernaTX, Inc.
Technical Solution: ModernaTX has developed proprietary lipid nanoparticle (LNP) formulations with enhanced thermal stability through strategic lipid composition engineering. Their approach involves incorporating specialized ionizable lipids with optimized pKa values and tailored hydrocarbon chains that maintain structural integrity at elevated temperatures. Moderna's LNPs utilize a unique combination of helper phospholipids, cholesterol, and PEG-lipids in precise ratios that create a protective shell around mRNA cargo. Their technology employs specific buffer systems and cryoprotectants that prevent aggregation during temperature fluctuations. Additionally, Moderna has implemented lyophilization techniques that transform liquid LNP formulations into stable dry powders, significantly extending shelf-life at various temperatures without compromising transfection efficiency upon reconstitution.
Strengths: Advanced lipid chemistry expertise allowing for customized formulations; established manufacturing infrastructure; extensive clinical validation data. Weaknesses: Higher production costs compared to conventional delivery systems; some formulations still require cold chain logistics for long-term storage.
Key Patents and Innovations in LNP Thermal Protection
A nanoparticle composition and related methods thereof
PatentWO2024253582A1
Innovation
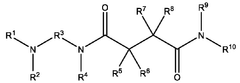
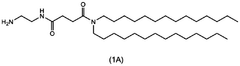
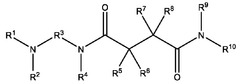
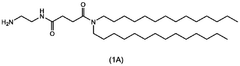
- A nanoparticle composition comprising a compound represented by general formula (1), an ionizable lipid, a neutral/helper lipid, a sterol, and a PEG-modified lipid, along with a cryoprotectant, which forms stable nanoparticles suitable for lyophilization and maintains encapsulation efficiency and immune response induction.
Lipid nanoparticle formulations and methods of synthesis thereof
PatentPendingUS20250281418A1
Innovation
- A method of producing lipid nanoparticles (LNPs) with improved biophysical properties and stability by incorporating specific concentrations of PEG-lipids during different stages of the manufacturing process, including the nanoprecipitation step and excipient addition, to enhance long-term stability and preserve mRNA functionality.
Regulatory Considerations for LNP-based Products
The regulatory landscape for Lipid Nanoparticle (LNP) systems presents a complex framework that developers must navigate carefully. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established specific guidelines for nanomedicine products, with particular attention to LNP-based formulations following their successful application in mRNA vaccines. These guidelines emphasize thermal stability as a critical quality attribute that must be thoroughly documented throughout the product lifecycle.
Regulatory submissions for LNP-based products require comprehensive stability data across various temperature conditions, including accelerated aging studies that demonstrate product integrity under thermal stress. Authorities typically require stability profiles at recommended storage temperatures, as well as excursion studies that simulate real-world distribution challenges. The ICH Q1A(R2) guidelines serve as the foundation for stability testing protocols, though regulatory bodies often request additional LNP-specific thermal stability assessments.
Manufacturing consistency represents another key regulatory consideration, with authorities requiring validation that thermal processes during production yield consistent LNP characteristics. This includes demonstration of batch-to-batch reproducibility in critical quality attributes such as particle size distribution, encapsulation efficiency, and lipid composition ratios—all of which can be affected by thermal variations during manufacturing.
Analytical method validation for thermal stability assessment presents unique regulatory challenges for LNP developers. Methods must be sufficiently sensitive to detect subtle changes in LNP structure and functionality resulting from thermal stress. Regulatory agencies increasingly expect orthogonal analytical approaches that can comprehensively characterize thermal degradation pathways specific to LNP systems.
Global regulatory harmonization efforts for LNP thermal stability requirements are progressing, though significant regional differences persist. The FDA's approach emphasizes risk-based assessment with particular focus on in-use stability after thawing, while the EMA has developed more detailed guidance on stability testing for nanomedicines. Japanese authorities have established specific guidelines for liposomal products that include thermal cycling studies to assess freeze-thaw stability.
Recent regulatory trends indicate increasing scrutiny of thermal stability during shipping and distribution, with authorities requesting real-time monitoring data from clinical and commercial supply chains. Developers are advised to engage in early regulatory consultation regarding their thermal stability testing strategy, as requirements continue to evolve with the rapid advancement of LNP technology and expanding application areas beyond vaccines.
Regulatory submissions for LNP-based products require comprehensive stability data across various temperature conditions, including accelerated aging studies that demonstrate product integrity under thermal stress. Authorities typically require stability profiles at recommended storage temperatures, as well as excursion studies that simulate real-world distribution challenges. The ICH Q1A(R2) guidelines serve as the foundation for stability testing protocols, though regulatory bodies often request additional LNP-specific thermal stability assessments.
Manufacturing consistency represents another key regulatory consideration, with authorities requiring validation that thermal processes during production yield consistent LNP characteristics. This includes demonstration of batch-to-batch reproducibility in critical quality attributes such as particle size distribution, encapsulation efficiency, and lipid composition ratios—all of which can be affected by thermal variations during manufacturing.
Analytical method validation for thermal stability assessment presents unique regulatory challenges for LNP developers. Methods must be sufficiently sensitive to detect subtle changes in LNP structure and functionality resulting from thermal stress. Regulatory agencies increasingly expect orthogonal analytical approaches that can comprehensively characterize thermal degradation pathways specific to LNP systems.
Global regulatory harmonization efforts for LNP thermal stability requirements are progressing, though significant regional differences persist. The FDA's approach emphasizes risk-based assessment with particular focus on in-use stability after thawing, while the EMA has developed more detailed guidance on stability testing for nanomedicines. Japanese authorities have established specific guidelines for liposomal products that include thermal cycling studies to assess freeze-thaw stability.
Recent regulatory trends indicate increasing scrutiny of thermal stability during shipping and distribution, with authorities requesting real-time monitoring data from clinical and commercial supply chains. Developers are advised to engage in early regulatory consultation regarding their thermal stability testing strategy, as requirements continue to evolve with the rapid advancement of LNP technology and expanding application areas beyond vaccines.
Scale-up Manufacturing Challenges for Stable LNPs
The transition from laboratory-scale production to industrial manufacturing of lipid nanoparticle (LNP) systems presents significant challenges in maintaining thermal stability. As production volumes increase, heat management becomes increasingly complex due to larger batch sizes and extended processing times. The thermal sensitivity of lipids, particularly those with lower melting points, creates a narrow operational window that must be precisely controlled throughout the manufacturing process.
Equipment design for large-scale production must incorporate sophisticated temperature control systems that can maintain consistent thermal conditions across larger volumes. Traditional jacketed vessels often struggle with uniform heat distribution in scaled-up operations, leading to temperature gradients that can compromise LNP integrity. Advanced heat exchange technologies, such as microfluidic-based continuous manufacturing platforms, offer promising alternatives but require substantial capital investment and specialized expertise.
Process parameters require significant recalibration during scale-up. Flow rates, mixing intensities, and residence times must be carefully optimized to prevent localized heating that could destabilize lipid components. The exothermic nature of certain formulation steps further complicates thermal management, necessitating precise cooling strategies that can respond rapidly to temperature fluctuations without disrupting the production flow.
Raw material consistency becomes increasingly critical at industrial scale. Batch-to-batch variations in lipid thermal properties, which might be manageable in laboratory settings, can lead to unpredictable stability profiles during large-scale manufacturing. Implementing robust supplier qualification programs and comprehensive incoming material characterization becomes essential to ensure consistent thermal behavior throughout the production process.
Quality control systems must evolve to accommodate the challenges of scale-up. Real-time monitoring of temperature across multiple points in the manufacturing line is necessary but technically demanding. The development of in-line analytical methods capable of detecting early signs of thermal degradation without disrupting production flow represents a significant technical hurdle that few manufacturers have successfully overcome.
Regulatory considerations add another layer of complexity to thermal stability challenges during scale-up. Demonstrating consistent thermal control across batches requires extensive validation studies and the establishment of meaningful acceptance criteria that correlate with product performance. The lack of harmonized standards specifically addressing thermal aspects of LNP manufacturing necessitates case-by-case approaches that can delay commercialization timelines and increase development costs.
Equipment design for large-scale production must incorporate sophisticated temperature control systems that can maintain consistent thermal conditions across larger volumes. Traditional jacketed vessels often struggle with uniform heat distribution in scaled-up operations, leading to temperature gradients that can compromise LNP integrity. Advanced heat exchange technologies, such as microfluidic-based continuous manufacturing platforms, offer promising alternatives but require substantial capital investment and specialized expertise.
Process parameters require significant recalibration during scale-up. Flow rates, mixing intensities, and residence times must be carefully optimized to prevent localized heating that could destabilize lipid components. The exothermic nature of certain formulation steps further complicates thermal management, necessitating precise cooling strategies that can respond rapidly to temperature fluctuations without disrupting the production flow.
Raw material consistency becomes increasingly critical at industrial scale. Batch-to-batch variations in lipid thermal properties, which might be manageable in laboratory settings, can lead to unpredictable stability profiles during large-scale manufacturing. Implementing robust supplier qualification programs and comprehensive incoming material characterization becomes essential to ensure consistent thermal behavior throughout the production process.
Quality control systems must evolve to accommodate the challenges of scale-up. Real-time monitoring of temperature across multiple points in the manufacturing line is necessary but technically demanding. The development of in-line analytical methods capable of detecting early signs of thermal degradation without disrupting production flow represents a significant technical hurdle that few manufacturers have successfully overcome.
Regulatory considerations add another layer of complexity to thermal stability challenges during scale-up. Demonstrating consistent thermal control across batches requires extensive validation studies and the establishment of meaningful acceptance criteria that correlate with product performance. The lack of harmonized standards specifically addressing thermal aspects of LNP manufacturing necessitates case-by-case approaches that can delay commercialization timelines and increase development costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!