Research on Electrode Kinetics in mRNA Delivery Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Delivery Systems Background and Objectives
Messenger RNA (mRNA) delivery systems represent a revolutionary approach in modern therapeutics, gaining significant attention following the successful development of mRNA-based COVID-19 vaccines. The technology's history dates back to the 1990s when researchers first demonstrated the potential of mRNA for protein expression in vivo. Since then, the field has witnessed remarkable advancements in understanding mRNA structure, stability, and delivery mechanisms.
The evolution of mRNA delivery technology has been characterized by progressive improvements in addressing key challenges, particularly concerning stability and efficient cellular uptake. Early research focused on naked mRNA delivery, which proved inefficient due to rapid degradation by nucleases. This led to the development of various protective delivery vehicles, with lipid nanoparticles (LNPs) emerging as the most successful approach to date.
Current technological trends indicate a shift toward more sophisticated delivery systems that incorporate electrokinetic principles to enhance cellular uptake and targeting specificity. Electrode kinetics in this context refers to the study of charge transfer processes at electrode-electrolyte interfaces, which can be leveraged to improve the efficiency of mRNA delivery through techniques such as electroporation, iontophoresis, and electrochemical cell-targeting approaches.
The primary objective of research in electrode kinetics for mRNA delivery systems is to develop more efficient, targeted, and controllable delivery mechanisms that overcome existing limitations. Specifically, this research aims to enhance transfection efficiency while minimizing cytotoxicity, improve cell-specific targeting to reduce off-target effects, and develop systems capable of controlled release profiles for optimized therapeutic outcomes.
Additionally, research objectives include understanding the fundamental electrochemical interactions between delivery vehicles and biological membranes, developing novel electrode materials and configurations for improved performance, and establishing standardized protocols for evaluating electrokinetic delivery systems. These advancements would address current challenges in mRNA therapeutics, including limited delivery efficiency, poor tissue penetration, and immune system activation.
The long-term technological goal is to create a versatile platform that enables precise control over mRNA delivery parameters, allowing for personalized therapeutic approaches. This would potentially expand the application of mRNA therapeutics beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments, representing a paradigm shift in how we approach disease treatment and prevention.
As the field continues to mature, integration of electrode kinetics with other emerging technologies, such as microfluidics and advanced biomaterials, is expected to further enhance the capabilities and applications of mRNA delivery systems, potentially revolutionizing the landscape of modern medicine.
The evolution of mRNA delivery technology has been characterized by progressive improvements in addressing key challenges, particularly concerning stability and efficient cellular uptake. Early research focused on naked mRNA delivery, which proved inefficient due to rapid degradation by nucleases. This led to the development of various protective delivery vehicles, with lipid nanoparticles (LNPs) emerging as the most successful approach to date.
Current technological trends indicate a shift toward more sophisticated delivery systems that incorporate electrokinetic principles to enhance cellular uptake and targeting specificity. Electrode kinetics in this context refers to the study of charge transfer processes at electrode-electrolyte interfaces, which can be leveraged to improve the efficiency of mRNA delivery through techniques such as electroporation, iontophoresis, and electrochemical cell-targeting approaches.
The primary objective of research in electrode kinetics for mRNA delivery systems is to develop more efficient, targeted, and controllable delivery mechanisms that overcome existing limitations. Specifically, this research aims to enhance transfection efficiency while minimizing cytotoxicity, improve cell-specific targeting to reduce off-target effects, and develop systems capable of controlled release profiles for optimized therapeutic outcomes.
Additionally, research objectives include understanding the fundamental electrochemical interactions between delivery vehicles and biological membranes, developing novel electrode materials and configurations for improved performance, and establishing standardized protocols for evaluating electrokinetic delivery systems. These advancements would address current challenges in mRNA therapeutics, including limited delivery efficiency, poor tissue penetration, and immune system activation.
The long-term technological goal is to create a versatile platform that enables precise control over mRNA delivery parameters, allowing for personalized therapeutic approaches. This would potentially expand the application of mRNA therapeutics beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments, representing a paradigm shift in how we approach disease treatment and prevention.
As the field continues to mature, integration of electrode kinetics with other emerging technologies, such as microfluidics and advanced biomaterials, is expected to further enhance the capabilities and applications of mRNA delivery systems, potentially revolutionizing the landscape of modern medicine.
Market Analysis for mRNA-Based Therapeutics
The mRNA therapeutics market has experienced unprecedented growth since the successful deployment of COVID-19 vaccines, catalyzing broader interest in this revolutionary technology. Current market valuations place the global mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 13.2% through 2030. This remarkable expansion is driven by several converging factors, including increased R&D investments, favorable regulatory pathways, and growing clinical validation across multiple therapeutic areas.
Oncology represents the largest application segment, accounting for roughly 38% of the current market share, followed by infectious diseases at 32%. Emerging applications in genetic disorders, cardiovascular diseases, and autoimmune conditions collectively constitute the remaining market distribution, with particularly strong growth trajectories observed in rare genetic disorder treatments.
Geographically, North America dominates the market landscape with 45% share, benefiting from robust research infrastructure and significant public-private funding initiatives. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with 18% annual growth, primarily driven by expanding healthcare infrastructure and increasing biopharmaceutical capabilities in China, Japan, and South Korea.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms. Key market players include Moderna, BioNTech, CureVac, Arcturus Therapeutics, and Translate Bio, alongside traditional pharmaceutical companies like Pfizer, Merck, and Sanofi that have established strategic partnerships or direct investments in mRNA technology platforms.
Investor confidence remains strong, with venture capital funding for mRNA-focused startups exceeding $12 billion since 2020. This investment surge has accelerated technological innovations in delivery systems, including advances in electrode kinetics for enhanced cellular uptake and targeted delivery mechanisms.
Market challenges persist, primarily centered around manufacturing scalability, cold chain logistics, and cost considerations. The average cost-per-dose for mRNA therapeutics remains significantly higher than conventional treatment modalities, potentially limiting market penetration in emerging economies without substantial price adjustments or innovative access programs.
Consumer awareness and acceptance of mRNA technology have dramatically improved following COVID-19 vaccine campaigns, with recent surveys indicating 76% of healthcare consumers now express familiarity with mRNA technology compared to just 12% pre-pandemic. This heightened awareness creates favorable conditions for broader therapeutic applications beyond vaccines.
Oncology represents the largest application segment, accounting for roughly 38% of the current market share, followed by infectious diseases at 32%. Emerging applications in genetic disorders, cardiovascular diseases, and autoimmune conditions collectively constitute the remaining market distribution, with particularly strong growth trajectories observed in rare genetic disorder treatments.
Geographically, North America dominates the market landscape with 45% share, benefiting from robust research infrastructure and significant public-private funding initiatives. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with 18% annual growth, primarily driven by expanding healthcare infrastructure and increasing biopharmaceutical capabilities in China, Japan, and South Korea.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms. Key market players include Moderna, BioNTech, CureVac, Arcturus Therapeutics, and Translate Bio, alongside traditional pharmaceutical companies like Pfizer, Merck, and Sanofi that have established strategic partnerships or direct investments in mRNA technology platforms.
Investor confidence remains strong, with venture capital funding for mRNA-focused startups exceeding $12 billion since 2020. This investment surge has accelerated technological innovations in delivery systems, including advances in electrode kinetics for enhanced cellular uptake and targeted delivery mechanisms.
Market challenges persist, primarily centered around manufacturing scalability, cold chain logistics, and cost considerations. The average cost-per-dose for mRNA therapeutics remains significantly higher than conventional treatment modalities, potentially limiting market penetration in emerging economies without substantial price adjustments or innovative access programs.
Consumer awareness and acceptance of mRNA technology have dramatically improved following COVID-19 vaccine campaigns, with recent surveys indicating 76% of healthcare consumers now express familiarity with mRNA technology compared to just 12% pre-pandemic. This heightened awareness creates favorable conditions for broader therapeutic applications beyond vaccines.
Current Electrode Kinetics Challenges in mRNA Delivery
The current landscape of electrode kinetics in mRNA delivery systems presents several significant challenges that impede optimal therapeutic outcomes. Traditional electroporation methods, while effective for cellular transfection, often result in substantial cell damage due to the high voltage requirements and uncontrolled pore formation. This cytotoxicity significantly limits clinical applications, particularly for sensitive cell types or in vivo delivery scenarios.
Electrode material biocompatibility remains a critical concern, as many highly conductive materials exhibit either poor biocompatibility or inadequate electrochemical stability in physiological environments. The trade-off between conductivity and biocompatibility continues to challenge researchers developing next-generation delivery systems, with noble metals offering excellent conductivity but limited functionality for biological interactions.
The spatial precision of current electrode-based delivery systems falls short of requirements for targeted tissue delivery. Most systems operate on a macro or bulk tissue level, lacking the resolution necessary for cell-specific or subcellular targeting. This limitation becomes particularly problematic when delivering mRNA to heterogeneous tissues where only specific cell populations are therapeutic targets.
Electrode fouling and degradation represent persistent technical hurdles, as protein adsorption and electrochemical reactions at the electrode surface progressively reduce performance during operation. This degradation not only affects delivery efficiency but also introduces potential contaminants into the biological system, raising safety concerns for clinical applications.
The kinetics of mRNA release from electrode-based systems remains poorly controlled, with most current technologies offering limited temporal control over the delivery process. The inability to precisely modulate release profiles restricts the potential for sequential or sustained delivery strategies that could enhance therapeutic outcomes through coordinated expression patterns.
Scaling challenges present significant barriers to clinical translation, as technologies demonstrating efficacy in laboratory settings often encounter substantial engineering hurdles during scale-up. The maintenance of uniform electric fields across larger electrode surfaces and ensuring consistent delivery to millions of cells simultaneously remain unresolved challenges.
Integration with real-time monitoring capabilities represents another frontier challenge, as current systems typically operate "blindly" without feedback mechanisms to adjust parameters based on delivery progress or cellular responses. This lack of adaptive capability limits optimization and introduces variability in treatment outcomes.
The energy efficiency of electrode-based delivery systems requires substantial improvement, particularly for implantable or portable applications where power constraints are significant. Current systems often demand high power inputs that are incompatible with miniaturized or long-term delivery platforms.
Electrode material biocompatibility remains a critical concern, as many highly conductive materials exhibit either poor biocompatibility or inadequate electrochemical stability in physiological environments. The trade-off between conductivity and biocompatibility continues to challenge researchers developing next-generation delivery systems, with noble metals offering excellent conductivity but limited functionality for biological interactions.
The spatial precision of current electrode-based delivery systems falls short of requirements for targeted tissue delivery. Most systems operate on a macro or bulk tissue level, lacking the resolution necessary for cell-specific or subcellular targeting. This limitation becomes particularly problematic when delivering mRNA to heterogeneous tissues where only specific cell populations are therapeutic targets.
Electrode fouling and degradation represent persistent technical hurdles, as protein adsorption and electrochemical reactions at the electrode surface progressively reduce performance during operation. This degradation not only affects delivery efficiency but also introduces potential contaminants into the biological system, raising safety concerns for clinical applications.
The kinetics of mRNA release from electrode-based systems remains poorly controlled, with most current technologies offering limited temporal control over the delivery process. The inability to precisely modulate release profiles restricts the potential for sequential or sustained delivery strategies that could enhance therapeutic outcomes through coordinated expression patterns.
Scaling challenges present significant barriers to clinical translation, as technologies demonstrating efficacy in laboratory settings often encounter substantial engineering hurdles during scale-up. The maintenance of uniform electric fields across larger electrode surfaces and ensuring consistent delivery to millions of cells simultaneously remain unresolved challenges.
Integration with real-time monitoring capabilities represents another frontier challenge, as current systems typically operate "blindly" without feedback mechanisms to adjust parameters based on delivery progress or cellular responses. This lack of adaptive capability limits optimization and introduces variability in treatment outcomes.
The energy efficiency of electrode-based delivery systems requires substantial improvement, particularly for implantable or portable applications where power constraints are significant. Current systems often demand high power inputs that are incompatible with miniaturized or long-term delivery platforms.
Current Electrode-Based mRNA Delivery Approaches
01 Electrode kinetics in electrochemical systems
Electrode kinetics plays a crucial role in electrochemical systems, focusing on the rate at which electron transfer occurs at electrode surfaces. These studies examine factors affecting reaction rates such as electrode material, surface conditions, electrolyte composition, and applied potential. Understanding these kinetics is essential for optimizing electrochemical processes, improving efficiency, and developing more effective electrode materials for applications in energy storage, sensors, and catalysis.- Electrochemical reaction kinetics measurement and analysis: Methods and systems for measuring and analyzing electrode kinetics in electrochemical reactions. These technologies involve monitoring reaction rates, electron transfer processes, and kinetic parameters at electrode surfaces. Advanced analytical techniques are employed to understand the fundamental mechanisms governing electrode reactions, which is crucial for optimizing electrochemical systems and improving their efficiency.
- Electrode materials and surface modifications for enhanced kinetics: Development of specialized electrode materials and surface modifications designed to improve reaction kinetics. This includes novel catalyst compositions, nanostructured surfaces, and functionalized electrodes that facilitate faster electron transfer and reduce activation energy barriers. These innovations lead to more efficient electrochemical processes by optimizing the interface between electrodes and electrolytes.
- Thermal management systems for electrode kinetics control: Systems and methods for controlling temperature in electrochemical cells to optimize electrode kinetics. These technologies include heat exchange mechanisms, thermal monitoring devices, and temperature regulation strategies that maintain optimal conditions for electrochemical reactions. Proper thermal management is essential for consistent reaction rates and preventing degradation of electrode materials during operation.
- Flow systems and microfluidic devices for electrode kinetics studies: Specialized flow systems and microfluidic platforms designed for studying electrode kinetics under controlled conditions. These devices enable precise manipulation of reactant transport, concentration gradients, and interfacial phenomena. By controlling mass transport and reaction environments at microscale, researchers can isolate and analyze specific aspects of electrode kinetics for fundamental research and application development.
- Computational methods and modeling for electrode kinetics prediction: Advanced computational approaches for modeling and predicting electrode kinetics in various electrochemical systems. These methods include simulation algorithms, machine learning techniques, and theoretical frameworks that can forecast reaction behavior under different conditions. Computational tools help researchers understand complex kinetic mechanisms, optimize electrode designs, and accelerate the development of new electrochemical technologies.
02 Measurement techniques for electrode kinetics
Various measurement techniques are employed to study electrode kinetics, including cyclic voltammetry, impedance spectroscopy, and chronoamperometry. These methods allow researchers to determine kinetic parameters such as exchange current density, transfer coefficients, and reaction rate constants. Advanced instrumentation and methodologies enable precise measurements of electron transfer processes, helping to characterize electrode behavior under different conditions and optimize electrochemical system performance.Expand Specific Solutions03 Electrode materials and surface modifications for enhanced kinetics
The development of specialized electrode materials and surface modifications significantly impacts electrode kinetics. Researchers focus on creating materials with optimized surface area, conductivity, and catalytic properties to enhance reaction rates. Surface treatments, nanostructuring, and composite formulations can improve electron transfer efficiency, reduce overpotential, and increase electrochemical performance. These advancements are critical for applications in fuel cells, batteries, and electrochemical sensors.Expand Specific Solutions04 Thermal management and temperature effects on electrode kinetics
Temperature significantly influences electrode kinetics, affecting reaction rates according to Arrhenius behavior. Thermal management systems are designed to control and optimize operating temperatures in electrochemical devices. These systems may include heat exchangers, cooling mechanisms, or thermal insulators to maintain ideal temperature conditions. Understanding and controlling temperature effects is crucial for maintaining consistent performance, extending device lifespan, and ensuring safety in electrochemical applications.Expand Specific Solutions05 Modeling and simulation of electrode kinetics
Computational modeling and simulation techniques are increasingly important for understanding electrode kinetics. These approaches include finite element analysis, molecular dynamics simulations, and quantum mechanical calculations to predict electron transfer behavior. Advanced models incorporate multiple variables such as mass transport, charge transfer, and surface interactions to provide comprehensive insights into electrochemical processes. These computational tools accelerate research and development by reducing experimental iterations and providing deeper mechanistic understanding.Expand Specific Solutions
Key Industry Players in mRNA Delivery Technologies
The electrode kinetics in mRNA delivery systems market is currently in a growth phase, with increasing research focus due to its critical role in enhancing therapeutic efficacy. The global market is expanding rapidly, driven by the success of mRNA vaccines and growing applications in gene therapy. Technologically, the field shows varying maturity levels across companies. Leading players like NeoCura Bio-Medical and Regis Biotechnology have established advanced mRNA delivery platforms, while academic institutions including MIT, Harvard, and Tsinghua University contribute significant research innovations. Pharmaceutical companies such as Translate Bio and Alnylam Pharmaceuticals bring commercial expertise, creating a competitive landscape where collaboration between academia and industry is accelerating technological advancement and clinical translation.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have made significant contributions to understanding electrode kinetics in mRNA delivery systems through innovative biomaterial design approaches. Their work has focused on developing lipid and polymer-based nanocarriers with precisely engineered surface properties to optimize electrochemical interactions with cellular membranes. Harvard's research has revealed that the molecular architecture of delivery materials, particularly the head group structure and charge density, critically influences cellular uptake mechanisms and endosomal escape efficiency. Their teams have developed ionizable lipid nanoparticles with optimized pKa values that undergo charge transitions in response to environmental pH changes, enhancing membrane fusion and cargo release. A notable innovation from Harvard involves the development of lipid-polymer hybrid nanoparticles that combine the advantages of both material classes, offering tunable surface charge characteristics and improved stability. Their electrode kinetics research has employed advanced biophysical techniques including surface plasmon resonance and electrochemical impedance spectroscopy to characterize the nano-bio interface in unprecedented detail. Harvard's work has also explored the impact of protein corona formation on the electrochemical properties of nanocarriers in biological environments.
Strengths: Multidisciplinary research approach combining expertise in materials science, biophysics, and pharmaceutical sciences; access to state-of-the-art characterization facilities; strong collaborative networks with clinical partners. Weaknesses: Some advanced formulations may present regulatory challenges due to novel components; potential gap between academic research focus and commercial manufacturing considerations.
Massachusetts Institute of Technology
Technical Solution: MIT has developed pioneering approaches to mRNA delivery focusing on electrode kinetics optimization. Their research teams have engineered lipid-like materials called lipidoids through combinatorial synthesis approaches, creating libraries of structurally diverse compounds with varied electrochemical properties. MIT's work has revealed that the molecular architecture of delivery materials significantly impacts electrode kinetics at the nano-bio interface. Their studies demonstrate that lipidoid nanoparticles with optimized head group pKa values (typically 6.2-6.8) exhibit superior endosomal escape capabilities through pH-dependent charge transitions. MIT researchers have also explored poly(β-amino ester) (PBAE) polymers with precisely tuned charge densities that enhance cellular uptake through electrostatic interactions with cell membranes. A significant innovation from MIT involves the development of ionizable lipid nanoparticles containing biodegradable linkages that improve safety profiles while maintaining efficient transfection. Their electrode kinetics research has led to the identification of structure-function relationships that guide rational design of next-generation delivery systems with enhanced performance characteristics.
Strengths: World-class research capabilities in materials science and nanotechnology; innovative approaches to rational design of delivery systems; strong track record of translating academic discoveries to commercial applications. Weaknesses: Some advanced formulations may face scalability challenges in manufacturing; academic focus may sometimes prioritize novelty over practical implementation considerations.
Critical Patents and Research in Electrode Kinetics
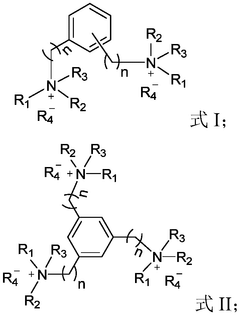
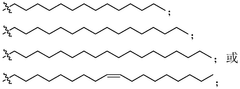
mRNA delivery system and preparation method therefor and application thereof
PatentWO2023045036A1
Innovation
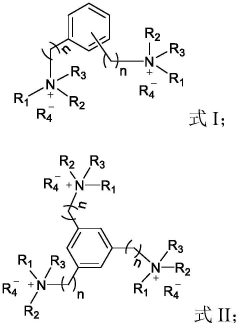
- Using lipid-like nanoparticles containing quaternary ammonium salt lipid-like molecules as carriers, a simple-component mRNA delivery system was developed by adjusting the nitrogen-phosphorus ratio of quaternary ammonium salt lipid-like molecules to mRNA and the molar ratio of lipid molecules. Achieve efficient and highly specific lung-targeted delivery.
MRNA delivery system as well as preparation method and application thereof
PatentActiveCN113786391A
Innovation
- Using lipid-like nanoparticles containing quaternary ammonium salt lipid-like molecules as carriers, the lung-targeting mRNA with high efficiency and high specificity is prepared by mixing the quaternary ammonium salt lipid-like molecules with organic solvents and then with water and mRNA solutions. delivery system.
Regulatory Framework for mRNA Delivery Technologies
The regulatory landscape for mRNA delivery technologies is complex and evolving rapidly as these innovative therapeutic approaches gain prominence in clinical applications. Currently, the FDA and EMA have established specific guidelines for lipid nanoparticle (LNP) delivery systems, which represent the primary vehicle for mRNA therapeutics. These guidelines address critical aspects such as characterization requirements, stability testing, and safety evaluations specific to electrokinetic properties that influence cellular uptake and distribution.
Regulatory bodies increasingly focus on the electrode kinetics of delivery systems, recognizing their crucial role in determining transfection efficiency and safety profiles. The FDA's guidance on liposomal drug products (2018) has been expanded to include considerations for surface charge characteristics and zeta potential measurements of mRNA-LNP complexes, acknowledging the impact of these parameters on biodistribution and cellular internalization.
International harmonization efforts through the International Council for Harmonisation (ICH) have resulted in the development of Q3D guidelines that specifically address elemental impurities in delivery systems, including those that might affect electrode kinetics. These guidelines establish permissible limits for metallic contaminants that could potentially interfere with the electrokinetic properties of mRNA delivery vehicles.
Regulatory requirements for manufacturing processes have become increasingly stringent, with particular emphasis on process analytical technology (PAT) for real-time monitoring of critical electrokinetic parameters during production. Companies must demonstrate robust control strategies for maintaining consistent surface charge characteristics across manufacturing batches, as variations can significantly impact in vivo performance and safety profiles.
The emergence of novel electrode-based delivery methods, such as electroporation and iontophoresis for mRNA delivery, has prompted regulatory agencies to develop new frameworks specifically addressing these technologies. The FDA's Center for Biologics Evaluation and Research (CBER) has established a specialized working group focused on developing guidance for electrode-assisted delivery technologies, with draft guidance expected by late 2023.
Post-market surveillance requirements have also been enhanced to monitor long-term safety implications related to the electrokinetic properties of approved mRNA therapeutics. Manufacturers must implement risk management plans that include specific monitoring for adverse events potentially linked to charge-related interactions between delivery vehicles and biological systems, particularly concerning inflammatory responses and tissue accumulation patterns.
Regulatory bodies increasingly focus on the electrode kinetics of delivery systems, recognizing their crucial role in determining transfection efficiency and safety profiles. The FDA's guidance on liposomal drug products (2018) has been expanded to include considerations for surface charge characteristics and zeta potential measurements of mRNA-LNP complexes, acknowledging the impact of these parameters on biodistribution and cellular internalization.
International harmonization efforts through the International Council for Harmonisation (ICH) have resulted in the development of Q3D guidelines that specifically address elemental impurities in delivery systems, including those that might affect electrode kinetics. These guidelines establish permissible limits for metallic contaminants that could potentially interfere with the electrokinetic properties of mRNA delivery vehicles.
Regulatory requirements for manufacturing processes have become increasingly stringent, with particular emphasis on process analytical technology (PAT) for real-time monitoring of critical electrokinetic parameters during production. Companies must demonstrate robust control strategies for maintaining consistent surface charge characteristics across manufacturing batches, as variations can significantly impact in vivo performance and safety profiles.
The emergence of novel electrode-based delivery methods, such as electroporation and iontophoresis for mRNA delivery, has prompted regulatory agencies to develop new frameworks specifically addressing these technologies. The FDA's Center for Biologics Evaluation and Research (CBER) has established a specialized working group focused on developing guidance for electrode-assisted delivery technologies, with draft guidance expected by late 2023.
Post-market surveillance requirements have also been enhanced to monitor long-term safety implications related to the electrokinetic properties of approved mRNA therapeutics. Manufacturers must implement risk management plans that include specific monitoring for adverse events potentially linked to charge-related interactions between delivery vehicles and biological systems, particularly concerning inflammatory responses and tissue accumulation patterns.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of electrode-based mRNA delivery systems represent critical considerations for clinical translation. These systems, which utilize electrical fields to enhance cellular uptake of mRNA therapeutics, must demonstrate minimal cytotoxicity and immunogenicity while maintaining therapeutic efficacy. Current research indicates that electrode material composition significantly impacts tissue compatibility, with gold and platinum electrodes showing superior biocompatibility compared to stainless steel or aluminum alternatives.
Electrode surface modifications have emerged as a promising approach to mitigate adverse biological responses. Hydrogel coatings and biomimetic membranes can effectively reduce electrode-tissue interface reactions while maintaining efficient charge transfer kinetics. These modifications create a buffer zone that minimizes direct contact between metallic surfaces and biological tissues, thereby reducing inflammatory responses and fibrotic encapsulation that could otherwise compromise long-term functionality.
The electrical parameters employed in mRNA delivery systems present another critical safety consideration. Pulse duration, amplitude, and frequency must be carefully optimized to prevent thermal damage and excessive reactive oxygen species (ROS) generation. Recent studies have demonstrated that low-voltage, multiple-pulse protocols achieve superior transfection efficiency with minimal cellular stress compared to high-voltage single pulses traditionally used in electroporation techniques.
Systemic toxicity assessments have revealed potential concerns regarding metal ion leaching from electrodes during repeated application. Chronic exposure to certain metal ions can lead to accumulation in vital organs and subsequent toxicity. This necessitates comprehensive biocompatibility testing that extends beyond acute cellular responses to include long-term biodistribution and clearance studies of electrode materials and their degradation products.
Immunological considerations present another dimension of safety evaluation. The interaction between electrode surfaces and immune cells can trigger inflammatory cascades that not only compromise patient safety but also potentially reduce therapeutic efficacy through accelerated clearance of mRNA therapeutics. Recent innovations in immunomodulatory coatings have shown promise in mitigating these responses while preserving electrode functionality.
Regulatory frameworks for electrode-based delivery systems continue to evolve, with the FDA and EMA developing specialized guidance for combination products that incorporate both device and biological components. Manufacturers must navigate complex regulatory pathways that evaluate both the electrode device safety and the mRNA therapeutic efficacy as an integrated system rather than as separate entities.
Electrode surface modifications have emerged as a promising approach to mitigate adverse biological responses. Hydrogel coatings and biomimetic membranes can effectively reduce electrode-tissue interface reactions while maintaining efficient charge transfer kinetics. These modifications create a buffer zone that minimizes direct contact between metallic surfaces and biological tissues, thereby reducing inflammatory responses and fibrotic encapsulation that could otherwise compromise long-term functionality.
The electrical parameters employed in mRNA delivery systems present another critical safety consideration. Pulse duration, amplitude, and frequency must be carefully optimized to prevent thermal damage and excessive reactive oxygen species (ROS) generation. Recent studies have demonstrated that low-voltage, multiple-pulse protocols achieve superior transfection efficiency with minimal cellular stress compared to high-voltage single pulses traditionally used in electroporation techniques.
Systemic toxicity assessments have revealed potential concerns regarding metal ion leaching from electrodes during repeated application. Chronic exposure to certain metal ions can lead to accumulation in vital organs and subsequent toxicity. This necessitates comprehensive biocompatibility testing that extends beyond acute cellular responses to include long-term biodistribution and clearance studies of electrode materials and their degradation products.
Immunological considerations present another dimension of safety evaluation. The interaction between electrode surfaces and immune cells can trigger inflammatory cascades that not only compromise patient safety but also potentially reduce therapeutic efficacy through accelerated clearance of mRNA therapeutics. Recent innovations in immunomodulatory coatings have shown promise in mitigating these responses while preserving electrode functionality.
Regulatory frameworks for electrode-based delivery systems continue to evolve, with the FDA and EMA developing specialized guidance for combination products that incorporate both device and biological components. Manufacturers must navigate complex regulatory pathways that evaluate both the electrode device safety and the mRNA therapeutic efficacy as an integrated system rather than as separate entities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!