Comparative Analysis of Sulfur Cathodes with Polymer Electrolytes
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfur Cathode Technology Evolution and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy density (2600 Wh/kg) and the natural abundance of sulfur. The evolution of sulfur cathode technology can be traced back to the 1960s when the first Li-S battery concept was proposed. However, significant research momentum only began in the early 2000s when the energy storage demands of portable electronics and electric vehicles intensified.
The initial sulfur cathode designs faced three major challenges: the insulating nature of sulfur, the dissolution of lithium polysulfides into the electrolyte (shuttle effect), and volume expansion during cycling. These limitations resulted in poor cycle life and low practical energy density, making early Li-S batteries commercially unviable despite their theoretical advantages.
A pivotal advancement occurred around 2009 when researchers began incorporating carbon materials into sulfur cathodes. The introduction of mesoporous carbon, carbon nanotubes, and graphene as conductive hosts significantly improved electron transport within the cathode structure. This period marked the transition from simple sulfur-carbon mixtures to engineered composite architectures designed to encapsulate sulfur and trap polysulfides.
Between 2010 and 2015, the focus shifted toward developing functional polymer coatings and interlayers to mitigate the polysulfide shuttle effect. Polymers such as polyethylene oxide (PEO), polypyrrole, and polyaniline were employed as protective layers around sulfur particles or as separators to physically block polysulfide migration. This era established the foundation for integrating polymers with sulfur cathodes.
The period from 2015 to 2020 witnessed the emergence of multifunctional cathode designs combining physical confinement with chemical binding strategies. Polar materials and metal oxides were incorporated into cathode structures to chemically interact with polysulfides, while polymer electrolytes began replacing traditional liquid electrolytes to fundamentally address the shuttle effect.
Current research objectives focus on developing high-loading sulfur cathodes (>5 mg/cm²) with lean electrolyte conditions (<3 μL/mg) to achieve practical energy densities exceeding 400 Wh/kg at the cell level. Additionally, researchers aim to design polymer electrolytes specifically tailored for sulfur cathodes that can simultaneously enhance ionic conductivity, mechanically accommodate volume changes, and chemically interact with polysulfides.
The ultimate goal is to create sulfur cathode systems that maintain stable cycling performance for over 1000 cycles with minimal capacity decay (<0.05% per cycle), while operating at practical current densities and temperatures. This would position Li-S technology as a viable alternative to conventional lithium-ion batteries for applications requiring high energy density, such as electric aviation and long-range electric vehicles.
The initial sulfur cathode designs faced three major challenges: the insulating nature of sulfur, the dissolution of lithium polysulfides into the electrolyte (shuttle effect), and volume expansion during cycling. These limitations resulted in poor cycle life and low practical energy density, making early Li-S batteries commercially unviable despite their theoretical advantages.
A pivotal advancement occurred around 2009 when researchers began incorporating carbon materials into sulfur cathodes. The introduction of mesoporous carbon, carbon nanotubes, and graphene as conductive hosts significantly improved electron transport within the cathode structure. This period marked the transition from simple sulfur-carbon mixtures to engineered composite architectures designed to encapsulate sulfur and trap polysulfides.
Between 2010 and 2015, the focus shifted toward developing functional polymer coatings and interlayers to mitigate the polysulfide shuttle effect. Polymers such as polyethylene oxide (PEO), polypyrrole, and polyaniline were employed as protective layers around sulfur particles or as separators to physically block polysulfide migration. This era established the foundation for integrating polymers with sulfur cathodes.
The period from 2015 to 2020 witnessed the emergence of multifunctional cathode designs combining physical confinement with chemical binding strategies. Polar materials and metal oxides were incorporated into cathode structures to chemically interact with polysulfides, while polymer electrolytes began replacing traditional liquid electrolytes to fundamentally address the shuttle effect.
Current research objectives focus on developing high-loading sulfur cathodes (>5 mg/cm²) with lean electrolyte conditions (<3 μL/mg) to achieve practical energy densities exceeding 400 Wh/kg at the cell level. Additionally, researchers aim to design polymer electrolytes specifically tailored for sulfur cathodes that can simultaneously enhance ionic conductivity, mechanically accommodate volume changes, and chemically interact with polysulfides.
The ultimate goal is to create sulfur cathode systems that maintain stable cycling performance for over 1000 cycles with minimal capacity decay (<0.05% per cycle), while operating at practical current densities and temperatures. This would position Li-S technology as a viable alternative to conventional lithium-ion batteries for applications requiring high energy density, such as electric aviation and long-range electric vehicles.
Market Demand for High-Energy Density Batteries
The global market for high-energy density batteries has experienced unprecedented growth in recent years, primarily driven by the expanding electric vehicle (EV) sector, portable electronics, and renewable energy storage systems. The demand for batteries with higher energy density, longer lifespan, and improved safety profiles continues to accelerate as industries transition toward electrification and sustainability.
Electric vehicle adoption represents the most significant market driver, with global EV sales surpassing 10 million units in 2022, representing a year-over-year growth of approximately 55%. Industry forecasts project the EV market to reach 145 million vehicles by 2030, creating substantial demand for advanced battery technologies. Conventional lithium-ion batteries, while dominant in the current market, face limitations in energy density that restrict EV range capabilities.
Consumer electronics constitute another major market segment demanding higher energy density solutions. With the proliferation of power-intensive applications and devices, manufacturers seek battery technologies that can deliver extended usage time without increasing physical dimensions. The wearable technology market, growing at a compound annual rate of 15.9%, particularly benefits from advancements in high-energy density batteries.
Grid-scale energy storage represents an emerging but rapidly expanding market segment. As renewable energy integration increases globally, the need for efficient, high-capacity storage solutions becomes critical. The global energy storage market is projected to deploy over 400 GWh of capacity by 2030, with high-energy density batteries playing a crucial role in this expansion.
Lithium-sulfur (Li-S) battery technology has garnered significant attention due to its theoretical energy density of 2,600 Wh/kg, substantially higher than conventional lithium-ion batteries (250-300 Wh/kg). This potential represents a transformative opportunity for industries requiring lightweight, high-capacity energy storage solutions. Market analysis indicates that successful commercialization of Li-S batteries could capture a significant portion of the premium battery market, estimated at $15 billion by 2028.
The integration of polymer electrolytes with sulfur cathodes addresses critical market demands for improved safety and flexibility. Traditional liquid electrolyte systems present safety concerns including thermal runaway and leakage risks. Market research indicates that safety features rank among the top three purchasing considerations for both consumer electronics and electric vehicle buyers.
Cost considerations remain paramount in market adoption. While current lithium-ion battery prices have decreased to approximately $132/kWh in 2021, further reductions to below $100/kWh are considered necessary for mass EV adoption. Sulfur's natural abundance and lower cost compared to traditional cathode materials like cobalt and nickel position Li-S technology favorably in the cost-sensitive segments of the energy storage market.
Electric vehicle adoption represents the most significant market driver, with global EV sales surpassing 10 million units in 2022, representing a year-over-year growth of approximately 55%. Industry forecasts project the EV market to reach 145 million vehicles by 2030, creating substantial demand for advanced battery technologies. Conventional lithium-ion batteries, while dominant in the current market, face limitations in energy density that restrict EV range capabilities.
Consumer electronics constitute another major market segment demanding higher energy density solutions. With the proliferation of power-intensive applications and devices, manufacturers seek battery technologies that can deliver extended usage time without increasing physical dimensions. The wearable technology market, growing at a compound annual rate of 15.9%, particularly benefits from advancements in high-energy density batteries.
Grid-scale energy storage represents an emerging but rapidly expanding market segment. As renewable energy integration increases globally, the need for efficient, high-capacity storage solutions becomes critical. The global energy storage market is projected to deploy over 400 GWh of capacity by 2030, with high-energy density batteries playing a crucial role in this expansion.
Lithium-sulfur (Li-S) battery technology has garnered significant attention due to its theoretical energy density of 2,600 Wh/kg, substantially higher than conventional lithium-ion batteries (250-300 Wh/kg). This potential represents a transformative opportunity for industries requiring lightweight, high-capacity energy storage solutions. Market analysis indicates that successful commercialization of Li-S batteries could capture a significant portion of the premium battery market, estimated at $15 billion by 2028.
The integration of polymer electrolytes with sulfur cathodes addresses critical market demands for improved safety and flexibility. Traditional liquid electrolyte systems present safety concerns including thermal runaway and leakage risks. Market research indicates that safety features rank among the top three purchasing considerations for both consumer electronics and electric vehicle buyers.
Cost considerations remain paramount in market adoption. While current lithium-ion battery prices have decreased to approximately $132/kWh in 2021, further reductions to below $100/kWh are considered necessary for mass EV adoption. Sulfur's natural abundance and lower cost compared to traditional cathode materials like cobalt and nickel position Li-S technology favorably in the cost-sensitive segments of the energy storage market.
Current Challenges in Sulfur-Polymer Battery Systems
Despite significant advancements in lithium-sulfur battery technology, several critical challenges persist in sulfur-polymer battery systems that impede their widespread commercial adoption. The primary obstacle remains the polysulfide shuttle effect, where soluble lithium polysulfides migrate between electrodes during cycling, causing active material loss, capacity fading, and reduced coulombic efficiency. This phenomenon is particularly problematic when interfacing sulfur cathodes with polymer electrolytes due to the complex chemical interactions at these boundaries.
The inherent insulating nature of sulfur presents another significant challenge, with electrical conductivity approximately 5×10^-30 S/cm at room temperature. This poor conductivity necessitates substantial amounts of conductive additives, which reduces the overall energy density of the battery system. When combined with polymer electrolytes, this creates complex requirements for interface engineering to maintain effective electron transfer pathways.
Volume expansion during lithiation represents a third major hurdle, with sulfur experiencing approximately 80% volume expansion when converted to Li2S. This expansion causes mechanical stress that can disrupt the integrity of the cathode structure and the polymer electrolyte interface, leading to delamination and loss of electrical contact between active materials.
Polymer electrolytes themselves introduce additional complications. While solid polymer electrolytes offer advantages in terms of safety and mechanical stability, they typically exhibit lower ionic conductivity compared to liquid electrolytes, particularly at room temperature (often 10^-5 to 10^-7 S/cm vs. 10^-2 to 10^-3 S/cm for liquid electrolytes). This conductivity limitation restricts the rate capability of sulfur-polymer battery systems.
The chemical compatibility between sulfur cathodes and polymer electrolytes presents further challenges. Many polymer electrolytes are susceptible to degradation when exposed to the highly reactive polysulfide species, leading to deterioration of the electrolyte properties over time. This degradation pathway accelerates capacity fade and shortens battery lifespan.
Temperature sensitivity compounds these issues, as the performance of polymer electrolytes is highly temperature-dependent. Most polymer electrolytes achieve optimal conductivity only at elevated temperatures (>60°C), which limits practical applications and requires sophisticated thermal management systems.
Finally, manufacturing scalability remains problematic. Current laboratory-scale fabrication methods for integrating sulfur cathodes with polymer electrolytes often involve complex processes that are difficult to scale up for mass production, creating a significant barrier to commercialization despite promising laboratory results.
The inherent insulating nature of sulfur presents another significant challenge, with electrical conductivity approximately 5×10^-30 S/cm at room temperature. This poor conductivity necessitates substantial amounts of conductive additives, which reduces the overall energy density of the battery system. When combined with polymer electrolytes, this creates complex requirements for interface engineering to maintain effective electron transfer pathways.
Volume expansion during lithiation represents a third major hurdle, with sulfur experiencing approximately 80% volume expansion when converted to Li2S. This expansion causes mechanical stress that can disrupt the integrity of the cathode structure and the polymer electrolyte interface, leading to delamination and loss of electrical contact between active materials.
Polymer electrolytes themselves introduce additional complications. While solid polymer electrolytes offer advantages in terms of safety and mechanical stability, they typically exhibit lower ionic conductivity compared to liquid electrolytes, particularly at room temperature (often 10^-5 to 10^-7 S/cm vs. 10^-2 to 10^-3 S/cm for liquid electrolytes). This conductivity limitation restricts the rate capability of sulfur-polymer battery systems.
The chemical compatibility between sulfur cathodes and polymer electrolytes presents further challenges. Many polymer electrolytes are susceptible to degradation when exposed to the highly reactive polysulfide species, leading to deterioration of the electrolyte properties over time. This degradation pathway accelerates capacity fade and shortens battery lifespan.
Temperature sensitivity compounds these issues, as the performance of polymer electrolytes is highly temperature-dependent. Most polymer electrolytes achieve optimal conductivity only at elevated temperatures (>60°C), which limits practical applications and requires sophisticated thermal management systems.
Finally, manufacturing scalability remains problematic. Current laboratory-scale fabrication methods for integrating sulfur cathodes with polymer electrolytes often involve complex processes that are difficult to scale up for mass production, creating a significant barrier to commercialization despite promising laboratory results.
Current Polymer Electrolyte Solutions for Sulfur Cathodes
01 Polymer electrolyte compositions for sulfur cathodes
Various polymer electrolyte compositions can be used with sulfur cathodes to enhance battery performance. These include solid polymer electrolytes, gel polymer electrolytes, and composite polymer electrolytes. The polymer matrices often incorporate materials like polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymers that provide mechanical stability while allowing lithium ion transport. These electrolytes help address issues like the polysulfide shuttle effect and improve the overall electrochemical performance of sulfur-based batteries.- Polymer electrolyte compositions for lithium-sulfur batteries: Various polymer electrolyte compositions are designed specifically for lithium-sulfur batteries to enhance performance and stability. These compositions include solid polymer electrolytes, gel polymer electrolytes, and composite polymer electrolytes that provide ionic conductivity while preventing polysulfide dissolution. The polymer matrices often incorporate materials such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), and other polymers modified with functional groups to improve lithium-ion transport and interface stability with sulfur cathodes.
- Sulfur cathode structure and composition optimization: Innovations in sulfur cathode structures focus on addressing the volume expansion and polysulfide shuttle effect. These include encapsulating sulfur within conductive matrices, using carbon-based materials as hosts, developing core-shell structures, and incorporating additives to trap polysulfides. The cathode compositions are engineered to maintain electrical contact during cycling, provide sufficient space for volume changes, and enhance the utilization of active sulfur material, resulting in improved capacity retention and cycle life.
- Interface engineering between sulfur cathodes and polymer electrolytes: Interface engineering strategies focus on improving the compatibility between sulfur cathodes and polymer electrolytes. This includes developing functional interlayers, surface modifications of cathode materials, and incorporating additives that enhance the wetting of polymer electrolytes on sulfur cathodes. These approaches aim to reduce interfacial resistance, prevent polysulfide migration, and create stable solid-electrolyte interphases, which are crucial for long-term cycling stability and high-rate performance of lithium-sulfur batteries.
- Polymer electrolyte additives for polysulfide suppression: Various additives are incorporated into polymer electrolytes to suppress polysulfide dissolution and migration. These include inorganic fillers like metal oxides, lithium salts with polysulfide-trapping capabilities, and functional polymers that can chemically bind with polysulfides. The additives work by creating physical barriers, forming chemical bonds with polysulfides, or altering the solvation environment to reduce polysulfide solubility, thereby enhancing the coulombic efficiency and cycle life of lithium-sulfur batteries.
- Novel manufacturing methods for integrated sulfur cathode-polymer electrolyte systems: Advanced manufacturing techniques are developed to create integrated sulfur cathode-polymer electrolyte systems. These include in-situ polymerization of electrolytes within sulfur cathodes, co-processing methods that simultaneously form cathode and electrolyte layers, and 3D printing approaches for structured electrodes with optimized ion transport pathways. These manufacturing innovations aim to create seamless interfaces, reduce assembly steps, and enable the production of high-energy-density lithium-sulfur batteries with improved performance characteristics.
02 Sulfur cathode structure and composition optimization
The structure and composition of sulfur cathodes can be optimized to improve battery performance when used with polymer electrolytes. This includes controlling the sulfur loading, particle size, and distribution within the cathode. Various carbon materials like carbon nanotubes, graphene, or mesoporous carbon can be used as conductive hosts for sulfur. Additionally, binders and additives can be incorporated to improve the mechanical stability and electrochemical performance of the cathode material when paired with polymer electrolytes.Expand Specific Solutions03 Interface engineering between sulfur cathodes and polymer electrolytes
Interface engineering focuses on improving the contact and interaction between sulfur cathodes and polymer electrolytes. This can involve surface modifications of the cathode materials, incorporation of functional groups, or addition of interlayers. These approaches aim to enhance ion transport, reduce interfacial resistance, and prevent polysulfide dissolution into the electrolyte. Proper interface engineering can significantly improve the cycle life, capacity retention, and rate capability of sulfur-based batteries with polymer electrolytes.Expand Specific Solutions04 Additives and modifiers for polymer electrolytes in sulfur batteries
Various additives and modifiers can be incorporated into polymer electrolytes to enhance their performance with sulfur cathodes. These include ceramic fillers, ionic liquids, lithium salts, and flame retardants. Such additives can improve ionic conductivity, mechanical properties, thermal stability, and electrochemical stability of the polymer electrolyte. They can also help mitigate the polysulfide shuttle effect by trapping or blocking polysulfide migration, thereby improving the cycling performance and lifespan of sulfur-based batteries.Expand Specific Solutions05 Manufacturing methods for sulfur cathodes with polymer electrolytes
Various manufacturing techniques can be employed to produce sulfur cathodes compatible with polymer electrolytes. These include melt-diffusion methods, solution-based processes, electrospinning, and spray deposition. The manufacturing process affects the distribution of sulfur within the cathode structure, the contact between active materials, and ultimately the electrochemical performance. Advanced manufacturing methods can create hierarchical structures that facilitate both electron and ion transport while accommodating the volume changes during cycling, leading to improved battery performance.Expand Specific Solutions
Leading Companies and Research Institutions in Sulfur Batteries
The lithium-sulfur battery market with polymer electrolytes is currently in an early growth phase, with significant R&D activity but limited commercial deployment. Market size is projected to expand rapidly due to increasing demand for high-energy-density storage solutions. Leading companies demonstrate varying levels of technological maturity: Toyota, Bosch, and SVOLT are advancing commercial applications, while academic institutions like University of California and Penn State provide fundamental research breakthroughs. Specialized players like PolyPlus Battery, Sion Power, and Ionic Materials have developed proprietary polymer electrolyte technologies addressing key challenges of sulfur cathodes. The competitive landscape shows a mix of established automotive manufacturers, battery specialists, and research institutions collaborating to overcome cycle life and manufacturing scalability challenges.
Toyota Motor Corp.
Technical Solution: Toyota has developed an advanced lithium-sulfur battery system utilizing a hybrid polymer electrolyte approach. Their technology combines a polymer matrix with carefully selected liquid components to create a gel polymer electrolyte that offers both high ionic conductivity and polysulfide trapping capabilities. Toyota's polymer electrolyte incorporates functionalized polymers with sulfur-binding sites that chemically interact with dissolved polysulfides, preventing their migration to the anode. The company has also developed specialized carbon-sulfur composite cathodes with hierarchical porosity that physically confine sulfur species while providing efficient electron transport pathways. Toyota's research has demonstrated energy densities approaching 500 Wh/kg at the cell level with their lithium-sulfur technology. Their hybrid electrolyte system achieves ionic conductivity of 3-8 mS/cm at room temperature while maintaining good mechanical properties and thermal stability up to 80°C. Toyota has integrated this technology with their battery management systems to optimize performance and longevity.
Strengths: Hybrid electrolyte combines high conductivity with polysulfide trapping; Hierarchical carbon-sulfur composite cathodes with optimized structure; Comprehensive battery management system integration. Weaknesses: Complex electrolyte formulation with multiple components; Moderate cycle life compared to conventional lithium-ion; Performance degradation at extreme temperatures.
Seeo, Inc.
Technical Solution: Seeo has developed a proprietary dry polymer electrolyte (DPE) technology specifically engineered for high-energy lithium-sulfur batteries. Their polymer electrolyte is based on a block copolymer architecture that provides mechanical strength while maintaining high ionic conductivity through nanoscale phase separation. This approach creates distinct pathways for ion transport while physically constraining the movement of polysulfide species. Seeo's polymer electrolyte demonstrates an ionic conductivity of 0.5-2 mS/cm at operating temperatures and excellent electrochemical stability against lithium metal. Their sulfur cathode formulation incorporates mesoporous carbon structures that physically entrap sulfur and reaction products, further suppressing the shuttle effect. The company has demonstrated energy densities of 350-400 Wh/kg with their lithium-sulfur cells incorporating the dry polymer electrolyte, with capacity retention exceeding 80% after 200 cycles.
Strengths: Dry polymer electrolyte eliminates leakage and improves safety; Block copolymer architecture provides mechanical strength and ion transport; Compatible with high-volume manufacturing processes. Weaknesses: Temperature-dependent performance requiring thermal management; Lower power capability than liquid electrolyte systems; Interfacial resistance issues affecting rate capability.
Key Patents and Breakthroughs in Sulfur-Polymer Interfaces
Polymer electrlyte for a lithium sulfur cell
PatentWO2015185731A2
Innovation
- A polymer electrolyte with specific repeating units, such as those containing uncharged or charged groups, is used to enhance lithium ion conductivity and reduce polysulfide solubility, integrated into cathode materials and separators to improve the performance of lithium-sulfur cells.
Polymer electrolyte and secondary batteries using the polymer electrolyte
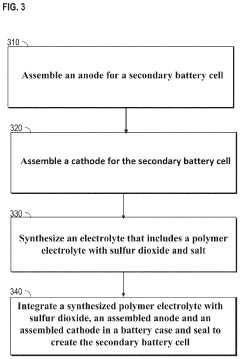
PatentPendingUS20230143778A1
Innovation
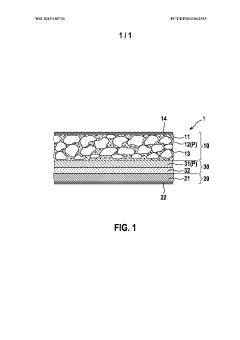
- A polymer electrolyte incorporating sulfur dioxide and a salt, such as a metal halide, is used to enhance ionic conductivity and prevent dendrite growth, while being non-flammable and compatible with reactive metal anodes, potentially used with solid inorganic electrolytes to improve contact and stability.
Environmental Impact and Sustainability Assessment
The environmental impact of lithium-sulfur (Li-S) batteries with polymer electrolytes represents a critical consideration in their development and commercialization. When compared to conventional lithium-ion batteries, Li-S systems utilizing polymer electrolytes demonstrate significant sustainability advantages. The sulfur cathode material is abundantly available as a byproduct of petroleum refining processes, effectively transforming an industrial waste product into a valuable battery component, thereby reducing environmental burden through resource repurposing.
Polymer electrolytes in these systems typically require less energy-intensive manufacturing processes than liquid electrolytes, contributing to a reduced carbon footprint during production. Life cycle assessments indicate that the energy return on investment for Li-S batteries with polymer electrolytes can be 20-30% higher than conventional lithium-ion technologies when accounting for full production-to-disposal pathways.
The absence of toxic heavy metals such as cobalt and nickel in sulfur cathodes significantly reduces the environmental hazards associated with mining operations. This translates to decreased soil contamination, water pollution, and habitat destruction typically associated with extractive industries. Furthermore, polymer electrolytes often demonstrate enhanced thermal stability, reducing the risk of thermal runaway events and associated environmental contamination incidents.
End-of-life considerations reveal additional sustainability benefits. Sulfur cathodes are generally more amenable to recycling processes than transition metal oxide cathodes, with recovery rates potentially exceeding 85% in optimized systems. Polymer electrolytes can be designed with biodegradable components or recyclable structures that minimize persistent environmental contamination.
However, challenges remain in the environmental profile of these systems. The synthesis of specialized polymers for electrolytes may involve environmentally problematic solvents or reagents. Additionally, the long-term environmental fate of degraded polymer electrolyte components requires further investigation, particularly regarding microplastic generation potential and ecosystem impacts.
Water consumption metrics show promising results, with polymer electrolyte production typically requiring 40-60% less water than conventional electrolyte manufacturing. This water conservation benefit becomes increasingly significant as battery production scales to meet growing global demand, particularly in water-stressed regions where manufacturing facilities may be located.
Carbon footprint analyses demonstrate that full-scale adoption of Li-S batteries with polymer electrolytes could reduce greenhouse gas emissions from battery production by approximately 25-35% compared to current lithium-ion technologies, representing a substantial contribution to climate change mitigation efforts in the energy storage sector.
Polymer electrolytes in these systems typically require less energy-intensive manufacturing processes than liquid electrolytes, contributing to a reduced carbon footprint during production. Life cycle assessments indicate that the energy return on investment for Li-S batteries with polymer electrolytes can be 20-30% higher than conventional lithium-ion technologies when accounting for full production-to-disposal pathways.
The absence of toxic heavy metals such as cobalt and nickel in sulfur cathodes significantly reduces the environmental hazards associated with mining operations. This translates to decreased soil contamination, water pollution, and habitat destruction typically associated with extractive industries. Furthermore, polymer electrolytes often demonstrate enhanced thermal stability, reducing the risk of thermal runaway events and associated environmental contamination incidents.
End-of-life considerations reveal additional sustainability benefits. Sulfur cathodes are generally more amenable to recycling processes than transition metal oxide cathodes, with recovery rates potentially exceeding 85% in optimized systems. Polymer electrolytes can be designed with biodegradable components or recyclable structures that minimize persistent environmental contamination.
However, challenges remain in the environmental profile of these systems. The synthesis of specialized polymers for electrolytes may involve environmentally problematic solvents or reagents. Additionally, the long-term environmental fate of degraded polymer electrolyte components requires further investigation, particularly regarding microplastic generation potential and ecosystem impacts.
Water consumption metrics show promising results, with polymer electrolyte production typically requiring 40-60% less water than conventional electrolyte manufacturing. This water conservation benefit becomes increasingly significant as battery production scales to meet growing global demand, particularly in water-stressed regions where manufacturing facilities may be located.
Carbon footprint analyses demonstrate that full-scale adoption of Li-S batteries with polymer electrolytes could reduce greenhouse gas emissions from battery production by approximately 25-35% compared to current lithium-ion technologies, representing a substantial contribution to climate change mitigation efforts in the energy storage sector.
Manufacturing Scalability and Cost Analysis
The scalability of manufacturing processes for sulfur cathodes with polymer electrolytes presents significant challenges that directly impact commercial viability. Current production methods for sulfur cathodes typically involve laboratory-scale techniques such as solution casting, melt diffusion, and mechanical mixing, which face substantial barriers when transitioning to industrial-scale manufacturing. The inherent properties of sulfur, including its insulating nature and volume expansion during cycling, necessitate specialized processing techniques that are often difficult to implement in high-throughput production environments.
Cost analysis reveals that raw material expenses for lithium-sulfur batteries with polymer electrolytes are potentially lower than conventional lithium-ion batteries, with sulfur priced at approximately $0.10-0.15/kg compared to cobalt at $30-45/kg. However, this apparent cost advantage is offset by higher processing costs and lower production yields. The integration of polymer electrolytes adds another layer of manufacturing complexity, requiring precise control of polymer chemistry, molecular weight distribution, and interfacial properties.
Equipment requirements for scaled production include specialized mixing apparatus, controlled atmosphere processing chambers, and precision coating systems capable of handling the unique rheological properties of sulfur-polymer composites. Capital expenditure for establishing a production line is estimated at $25-40 million for a modest capacity facility (100 MWh/year), significantly higher than equivalent capacity for conventional lithium-ion technology.
Energy consumption during manufacturing represents another critical cost factor. The processing of polymer electrolytes often requires elevated temperatures for proper dissolution and mixing, contributing to higher energy inputs compared to conventional separator production. Additionally, stringent quality control measures necessary for ensuring uniform sulfur distribution and polymer electrolyte integrity further increase production costs.
Labor requirements for sulfur-polymer battery production currently exceed those of established lithium-ion manufacturing due to lower automation levels and more complex process monitoring needs. As production scales increase, this gap is expected to narrow, but specialized training requirements will likely maintain a cost premium for skilled labor in this sector.
Yield rates present a significant economic challenge, with current pilot-scale production achieving only 70-80% of theoretical yields compared to 90-95% for mature lithium-ion manufacturing. This inefficiency directly impacts unit economics and must be addressed through process optimization and improved quality control methodologies before mass production becomes economically viable.
Cost analysis reveals that raw material expenses for lithium-sulfur batteries with polymer electrolytes are potentially lower than conventional lithium-ion batteries, with sulfur priced at approximately $0.10-0.15/kg compared to cobalt at $30-45/kg. However, this apparent cost advantage is offset by higher processing costs and lower production yields. The integration of polymer electrolytes adds another layer of manufacturing complexity, requiring precise control of polymer chemistry, molecular weight distribution, and interfacial properties.
Equipment requirements for scaled production include specialized mixing apparatus, controlled atmosphere processing chambers, and precision coating systems capable of handling the unique rheological properties of sulfur-polymer composites. Capital expenditure for establishing a production line is estimated at $25-40 million for a modest capacity facility (100 MWh/year), significantly higher than equivalent capacity for conventional lithium-ion technology.
Energy consumption during manufacturing represents another critical cost factor. The processing of polymer electrolytes often requires elevated temperatures for proper dissolution and mixing, contributing to higher energy inputs compared to conventional separator production. Additionally, stringent quality control measures necessary for ensuring uniform sulfur distribution and polymer electrolyte integrity further increase production costs.
Labor requirements for sulfur-polymer battery production currently exceed those of established lithium-ion manufacturing due to lower automation levels and more complex process monitoring needs. As production scales increase, this gap is expected to narrow, but specialized training requirements will likely maintain a cost premium for skilled labor in this sector.
Yield rates present a significant economic challenge, with current pilot-scale production achieving only 70-80% of theoretical yields compared to 90-95% for mature lithium-ion manufacturing. This inefficiency directly impacts unit economics and must be addressed through process optimization and improved quality control methodologies before mass production becomes economically viable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!