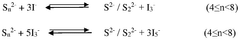Sulfur Cathodes Implementations in Advanced Energy Solutions
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfur Cathode Technology Evolution and Objectives
Sulfur cathode technology has undergone significant evolution since its initial conceptualization in the 1960s. The journey began with rudimentary lithium-sulfur battery designs that demonstrated promising theoretical energy density but suffered from severe practical limitations. Early research focused primarily on understanding the fundamental electrochemistry of sulfur reduction reactions, which laid the groundwork for subsequent technological advancements.
The 1980s and 1990s marked a period of incremental progress, with researchers addressing the polysulfide shuttle effect—a major challenge where soluble lithium polysulfides dissolve in the electrolyte, causing capacity fading and shortened battery life. This era saw the introduction of carbon-based materials as conductive hosts for sulfur, attempting to mitigate conductivity issues inherent to elemental sulfur.
A significant acceleration in sulfur cathode development occurred in the early 2000s, coinciding with the growing demand for high-energy storage solutions. This period witnessed the emergence of nanostructured carbon materials, including carbon nanotubes and graphene, as sophisticated sulfur hosts. These materials offered enhanced electrical conductivity and physical confinement of polysulfides, addressing two critical challenges simultaneously.
The 2010s represented a transformative decade for sulfur cathode technology, characterized by multidisciplinary approaches combining materials science, electrochemistry, and engineering. Researchers developed advanced composite cathodes incorporating functional polymers, metal oxides, and tailored porous structures. These innovations significantly improved cycle life and practical energy density, bringing lithium-sulfur batteries closer to commercial viability.
Current technological objectives for sulfur cathodes center on several key areas. First, increasing the practical energy density to approach the theoretical maximum of 2,600 Wh/kg, which far exceeds conventional lithium-ion batteries. Second, extending cycle life to thousands of cycles while maintaining high capacity retention, comparable to commercial lithium-ion systems. Third, improving rate capability to enable fast charging applications, addressing the inherently slow reaction kinetics of sulfur electrochemistry.
Additional objectives include enhancing operational safety through stable electrolyte systems and protective interfaces, reducing manufacturing costs to achieve price parity with conventional batteries, and developing scalable production methods suitable for mass manufacturing. The ultimate goal is to create sulfur cathode technologies that can revolutionize energy storage across multiple sectors, from portable electronics to electric vehicles and grid-scale applications, while offering environmental benefits through the utilization of abundant and low-cost sulfur resources.
The 1980s and 1990s marked a period of incremental progress, with researchers addressing the polysulfide shuttle effect—a major challenge where soluble lithium polysulfides dissolve in the electrolyte, causing capacity fading and shortened battery life. This era saw the introduction of carbon-based materials as conductive hosts for sulfur, attempting to mitigate conductivity issues inherent to elemental sulfur.
A significant acceleration in sulfur cathode development occurred in the early 2000s, coinciding with the growing demand for high-energy storage solutions. This period witnessed the emergence of nanostructured carbon materials, including carbon nanotubes and graphene, as sophisticated sulfur hosts. These materials offered enhanced electrical conductivity and physical confinement of polysulfides, addressing two critical challenges simultaneously.
The 2010s represented a transformative decade for sulfur cathode technology, characterized by multidisciplinary approaches combining materials science, electrochemistry, and engineering. Researchers developed advanced composite cathodes incorporating functional polymers, metal oxides, and tailored porous structures. These innovations significantly improved cycle life and practical energy density, bringing lithium-sulfur batteries closer to commercial viability.
Current technological objectives for sulfur cathodes center on several key areas. First, increasing the practical energy density to approach the theoretical maximum of 2,600 Wh/kg, which far exceeds conventional lithium-ion batteries. Second, extending cycle life to thousands of cycles while maintaining high capacity retention, comparable to commercial lithium-ion systems. Third, improving rate capability to enable fast charging applications, addressing the inherently slow reaction kinetics of sulfur electrochemistry.
Additional objectives include enhancing operational safety through stable electrolyte systems and protective interfaces, reducing manufacturing costs to achieve price parity with conventional batteries, and developing scalable production methods suitable for mass manufacturing. The ultimate goal is to create sulfur cathode technologies that can revolutionize energy storage across multiple sectors, from portable electronics to electric vehicles and grid-scale applications, while offering environmental benefits through the utilization of abundant and low-cost sulfur resources.
Market Analysis for Sulfur-Based Energy Storage
The global market for sulfur-based energy storage solutions has witnessed significant growth in recent years, driven by increasing demand for high-energy-density batteries at lower costs. The sulfur cathode market segment is projected to reach $2.5 billion by 2030, growing at a compound annual growth rate of 18.7% from 2023. This growth is primarily fueled by the theoretical energy density of lithium-sulfur batteries, which at 2,600 Wh/kg far exceeds the capabilities of conventional lithium-ion batteries currently limited to 250-300 Wh/kg.
Electric vehicle manufacturers represent the largest potential customer base, accounting for approximately 45% of the projected market demand. The automotive sector's push toward longer-range electric vehicles with reduced battery costs makes sulfur cathodes particularly attractive, as raw sulfur costs less than $200 per ton compared to cobalt at over $30,000 per ton and nickel at approximately $15,000 per ton.
Grid-scale energy storage applications constitute the second-largest market segment at 30% of projected demand. Utility companies are increasingly exploring sulfur-based solutions for large-scale storage due to their potential cost advantages, despite current cycle life limitations. The remaining market share is distributed among consumer electronics (15%), aerospace applications (5%), and other emerging sectors (5%).
Regionally, Asia-Pacific dominates the market landscape with 48% share, led by China's aggressive investments in alternative battery technologies. North America follows at 27%, with significant research initiatives funded by the U.S. Department of Energy. Europe accounts for 20% of the market, with particularly strong interest in Germany and the United Kingdom, while other regions collectively represent the remaining 5%.
Market barriers include concerns about sulfur cathodes' cycle stability, with current prototypes achieving only 200-300 cycles before significant capacity degradation occurs. The "shuttle effect" problem remains a technical hurdle that directly impacts commercial viability. Additionally, manufacturing scalability presents challenges, as production processes for sulfur cathodes differ substantially from established lithium-ion manufacturing lines.
Consumer adoption trends indicate growing acceptance of alternative battery technologies, with 68% of surveyed electric vehicle buyers expressing willingness to consider vehicles powered by next-generation battery chemistries if they offer improved range and comparable safety profiles. This represents a 15% increase in consumer openness compared to similar surveys conducted in 2020.
Electric vehicle manufacturers represent the largest potential customer base, accounting for approximately 45% of the projected market demand. The automotive sector's push toward longer-range electric vehicles with reduced battery costs makes sulfur cathodes particularly attractive, as raw sulfur costs less than $200 per ton compared to cobalt at over $30,000 per ton and nickel at approximately $15,000 per ton.
Grid-scale energy storage applications constitute the second-largest market segment at 30% of projected demand. Utility companies are increasingly exploring sulfur-based solutions for large-scale storage due to their potential cost advantages, despite current cycle life limitations. The remaining market share is distributed among consumer electronics (15%), aerospace applications (5%), and other emerging sectors (5%).
Regionally, Asia-Pacific dominates the market landscape with 48% share, led by China's aggressive investments in alternative battery technologies. North America follows at 27%, with significant research initiatives funded by the U.S. Department of Energy. Europe accounts for 20% of the market, with particularly strong interest in Germany and the United Kingdom, while other regions collectively represent the remaining 5%.
Market barriers include concerns about sulfur cathodes' cycle stability, with current prototypes achieving only 200-300 cycles before significant capacity degradation occurs. The "shuttle effect" problem remains a technical hurdle that directly impacts commercial viability. Additionally, manufacturing scalability presents challenges, as production processes for sulfur cathodes differ substantially from established lithium-ion manufacturing lines.
Consumer adoption trends indicate growing acceptance of alternative battery technologies, with 68% of surveyed electric vehicle buyers expressing willingness to consider vehicles powered by next-generation battery chemistries if they offer improved range and comparable safety profiles. This represents a 15% increase in consumer openness compared to similar surveys conducted in 2020.
Current Challenges in Sulfur Cathode Development
Despite the promising theoretical energy density of lithium-sulfur batteries (approximately 2600 Wh/kg), several significant challenges continue to impede their widespread commercial adoption. The most prominent issue is the "polysulfide shuttle effect," where soluble lithium polysulfides formed during discharge migrate between electrodes, causing active material loss, reduced coulombic efficiency, and accelerated capacity fading. This phenomenon fundamentally undermines the long-term stability of sulfur cathodes.
Volume expansion presents another critical challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during cycling. This expansion-contraction process disrupts the electrode structure, leading to mechanical degradation, electrical contact loss, and ultimately shortened battery lifespan. The mechanical instability significantly impacts the practical energy density achievable in real-world applications.
The inherently poor electrical conductivity of sulfur (5×10^-30 S/cm) severely limits electron transport within the cathode, necessitating large amounts of conductive additives that reduce the overall energy density. This trade-off between conductivity and energy density remains a fundamental design constraint for sulfur cathode architectures.
Slow reaction kinetics at the sulfur cathode further complicate matters, resulting in high polarization and reduced rate capability. This limitation becomes particularly problematic in applications requiring rapid charging or high-power output, restricting the potential use cases for lithium-sulfur technology.
The formation of insulating Li2S during discharge creates additional barriers to ion and electron transport, contributing to capacity loss and voltage hysteresis. This passivation effect becomes increasingly problematic over multiple cycles, accelerating performance degradation.
Environmental and safety concerns also persist, as the highly reactive nature of lithium with sulfur compounds can lead to thermal runaway under certain conditions. Additionally, the environmental impact of large-scale sulfur cathode production and disposal requires further assessment.
Manufacturing scalability remains challenging due to the complex nature of advanced sulfur cathode architectures. Current laboratory-scale fabrication methods often involve intricate processes that are difficult to translate to industrial production lines, creating a significant barrier to commercialization.
The integration of sulfur cathodes with suitable electrolytes and separators presents another multifaceted challenge, as the entire battery system must be optimized to address the unique characteristics and failure modes of sulfur-based chemistry.
Volume expansion presents another critical challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during cycling. This expansion-contraction process disrupts the electrode structure, leading to mechanical degradation, electrical contact loss, and ultimately shortened battery lifespan. The mechanical instability significantly impacts the practical energy density achievable in real-world applications.
The inherently poor electrical conductivity of sulfur (5×10^-30 S/cm) severely limits electron transport within the cathode, necessitating large amounts of conductive additives that reduce the overall energy density. This trade-off between conductivity and energy density remains a fundamental design constraint for sulfur cathode architectures.
Slow reaction kinetics at the sulfur cathode further complicate matters, resulting in high polarization and reduced rate capability. This limitation becomes particularly problematic in applications requiring rapid charging or high-power output, restricting the potential use cases for lithium-sulfur technology.
The formation of insulating Li2S during discharge creates additional barriers to ion and electron transport, contributing to capacity loss and voltage hysteresis. This passivation effect becomes increasingly problematic over multiple cycles, accelerating performance degradation.
Environmental and safety concerns also persist, as the highly reactive nature of lithium with sulfur compounds can lead to thermal runaway under certain conditions. Additionally, the environmental impact of large-scale sulfur cathode production and disposal requires further assessment.
Manufacturing scalability remains challenging due to the complex nature of advanced sulfur cathode architectures. Current laboratory-scale fabrication methods often involve intricate processes that are difficult to translate to industrial production lines, creating a significant barrier to commercialization.
The integration of sulfur cathodes with suitable electrolytes and separators presents another multifaceted challenge, as the entire battery system must be optimized to address the unique characteristics and failure modes of sulfur-based chemistry.
Contemporary Sulfur Cathode Implementation Strategies
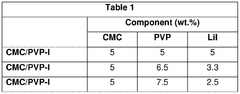
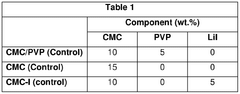
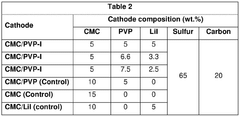
01 Sulfur cathode compositions for lithium-sulfur batteries
Various compositions for sulfur cathodes in lithium-sulfur batteries have been developed to improve energy density and cycle life. These compositions typically include sulfur as the active material combined with conductive additives, binders, and other components to enhance electrochemical performance. The cathode compositions are designed to address challenges such as sulfur's poor conductivity and the polysulfide shuttle effect that can reduce battery efficiency.- Sulfur cathode compositions for lithium-sulfur batteries: Various compositions for sulfur cathodes in lithium-sulfur batteries have been developed to improve energy density and cycle life. These compositions typically include elemental sulfur combined with conductive additives, binders, and other materials to enhance electrochemical performance. The cathode compositions are designed to address challenges such as sulfur's poor conductivity and the polysulfide shuttle effect, which can lead to capacity fading.
- Protective coatings and encapsulation for sulfur cathodes: Protective coatings and encapsulation techniques are applied to sulfur cathodes to mitigate polysulfide dissolution and shuttle effect. These approaches involve encapsulating sulfur particles within conductive or polymeric materials, creating core-shell structures, or applying protective layers to the cathode surface. Such strategies help contain sulfur and its discharge products within the cathode, improving cycling stability and coulombic efficiency of lithium-sulfur batteries.
- Nanostructured sulfur cathode materials: Nanostructured materials are incorporated into sulfur cathodes to enhance performance characteristics. These include carbon nanotubes, graphene, mesoporous carbon, and other nanoscale materials that provide high surface area and conductive pathways. The nanostructured approach helps accommodate volume changes during cycling, improves sulfur utilization, enhances electron transport, and provides physical confinement for polysulfides, resulting in better capacity retention and rate capability.
- Electrolyte modifications for sulfur cathodes: Specialized electrolyte formulations are developed to work synergistically with sulfur cathodes. These electrolytes may contain additives that form protective films on the cathode surface, suppress polysulfide dissolution, or enhance ionic conductivity. Modifications include the use of high-concentration electrolytes, ionic liquids, solid-state electrolytes, or electrolyte additives that chemically interact with polysulfides to prevent their migration to the anode.
- Interlayers and functional separators for sulfur cathodes: Interlayers and functional separators are placed between the sulfur cathode and the anode to block polysulfide migration while allowing lithium ion transport. These components can be made from carbon materials, polymers, or inorganic compounds with specific physical and chemical properties. They act as physical barriers and chemical traps for polysulfides, preventing them from reaching the anode and participating in the shuttle effect, thereby improving battery cycling performance.
02 Protective coatings and encapsulation for sulfur cathodes
Protective coatings and encapsulation techniques are applied to sulfur cathodes to prevent polysulfide dissolution and migration during battery operation. These approaches include polymer coatings, carbon encapsulation, and metal oxide layers that physically contain sulfur while allowing lithium ion transport. Such protective strategies help maintain the structural integrity of the cathode and improve the cycling stability of lithium-sulfur batteries.Expand Specific Solutions03 Carbon-sulfur composite structures for enhanced conductivity
Carbon-sulfur composite structures are designed to address the poor electrical conductivity of elemental sulfur in cathodes. These composites incorporate various carbon materials such as graphene, carbon nanotubes, porous carbon, or carbon fibers that provide conductive pathways throughout the cathode. The intimate contact between sulfur and carbon materials enhances electron transport, improves sulfur utilization, and facilitates more complete electrochemical reactions during battery operation.Expand Specific Solutions04 Electrolyte modifications for sulfur cathode performance
Specialized electrolyte formulations are developed to work synergistically with sulfur cathodes and improve overall battery performance. These electrolytes may contain additives that suppress polysulfide shuttling, enhance ionic conductivity, or form stable interfaces with the cathode material. Modifications to the electrolyte composition can significantly impact the cycling stability, rate capability, and capacity retention of lithium-sulfur batteries.Expand Specific Solutions05 Novel sulfur cathode architectures and manufacturing methods
Innovative cathode architectures and manufacturing techniques are being developed to optimize the performance of sulfur cathodes. These include hierarchical porous structures, 3D electrode designs, and advanced deposition methods that maximize active material loading while maintaining good electronic and ionic transport properties. Novel manufacturing approaches focus on scalable production methods that can translate laboratory-scale advances to commercial battery applications.Expand Specific Solutions
Leading Companies and Research Institutions in Sulfur Battery Technology
The sulfur cathode market for advanced energy solutions is in a growth phase, characterized by significant R&D investment and emerging commercial applications. The global market is expanding rapidly, driven by demand for higher energy density batteries in electric vehicles and renewable energy storage. While technical challenges remain, companies like Sion Power, PolyPlus Battery, and Sila Nanotechnologies have made substantial progress in commercializing sulfur cathode technologies. Academic institutions including Zhejiang University and Drexel University are contributing breakthrough research, while established players like Samsung SDI and Robert Bosch are integrating these innovations into their product roadmaps. The technology is approaching commercial maturity, with several companies demonstrating viable prototypes and early-stage products that address previous limitations of sulfur cathodes.
Sion Power Corp.
Technical Solution: Sion Power has developed a proprietary lithium-sulfur (Li-S) battery technology called "Licerion" that combines the high energy density of sulfur cathodes with protected lithium metal anodes. Their approach addresses the polysulfide shuttle effect through advanced carbon-sulfur composite cathodes with tailored pore structures and functional surface groups that chemically bind polysulfides. The company employs a multi-layer protection system for their cathodes, including conductive carbon matrices and specialized polymeric binders that maintain structural integrity during cycling. Sion's technology achieves energy densities exceeding 500 Wh/kg at the cell level, significantly outperforming conventional lithium-ion batteries. Their recent advancements include nano-engineered interfaces between sulfur particles and conductive additives to enhance electron transport while minimizing volume expansion issues during cycling.
Strengths: Industry-leading energy density (500+ Wh/kg); advanced protection systems for polysulfide containment; mature technology with commercial applications in aerospace and defense sectors. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; cycle life limitations (though improved from earlier generations); temperature sensitivity requiring sophisticated battery management systems.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has pioneered protected lithium electrode (PLE) technology specifically designed to work with sulfur cathodes in both Li-S and Li-air battery systems. Their approach focuses on a proprietary ceramic membrane that prevents direct contact between lithium metal anodes and sulfur cathodes while allowing selective lithium ion transport. For sulfur cathode implementation, PolyPlus employs a hierarchical carbon-sulfur composite structure where sulfur is encapsulated within mesoporous carbon frameworks, then further integrated into a macroporous conductive network. This multi-scale architecture confines polysulfides while maintaining efficient ion and electron transport pathways. The company has demonstrated energy densities approaching 600 Wh/kg in laboratory prototypes, with their protected lithium technology enabling stable cycling beyond 300 cycles at practical discharge rates.
Strengths: Unique protected lithium electrode technology that fundamentally addresses polysulfide shuttle issues; extremely high theoretical energy density; applicable to multiple battery chemistries beyond Li-S. Weaknesses: Complex manufacturing process for ceramic membranes increases production costs; current designs have limited power capability compared to conventional lithium-ion; scaling to mass production remains challenging.
Key Patents and Breakthroughs in Sulfur Cathode Design
Sulfur cathodes protected with hybrid solid-electrolyte interfaces for high performance li-s batteries
PatentInactiveEP3772765A1
Innovation
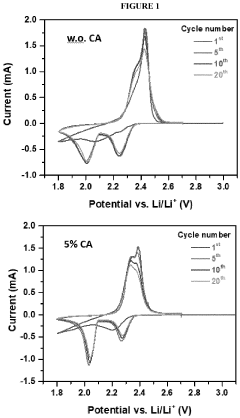
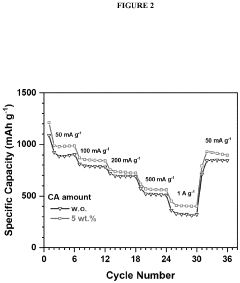
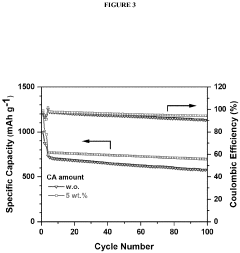
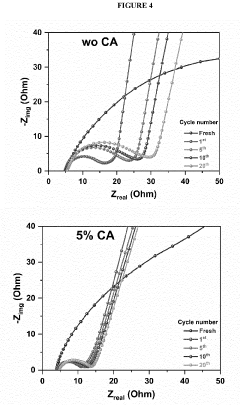
- Incorporating a carboxylic acid or its lithiated form, such as citric acid, at the surface of the cathode to form a lithium carboxylate-based solid electrolyte interphase (SEI) that mitigates the shuttle effect, enhancing capacity retention and efficiency.
Improved sulfur cathodes
PatentWO2025129258A1
Innovation
- The development of a sulfur cathode comprising sulfur-containing materials, conductive materials, metal cations with a valency of two or more, halide anions, polyhalogen anions, and binding polymers capable of binding these species. This configuration enhances the retention and conversion of lithium polysulfides, improving reaction kinetics and mechanical stability.
Environmental Impact and Sustainability of Sulfur Cathode Technologies
The environmental impact of sulfur cathode technologies represents a critical dimension in evaluating their viability for advanced energy solutions. Sulfur cathodes offer significant environmental advantages compared to conventional lithium-ion battery technologies, primarily due to the abundance and non-toxicity of sulfur as a raw material. Unlike cobalt and nickel used in traditional cathodes, sulfur is a byproduct of petroleum refining, transforming an industrial waste product into a valuable resource for energy storage applications.
The life cycle assessment of sulfur cathode batteries demonstrates reduced carbon footprint during manufacturing processes. Studies indicate that sulfur cathode production generates approximately 60% less greenhouse gas emissions compared to conventional lithium cobalt oxide cathodes, primarily due to lower energy requirements during material synthesis and processing. This reduction in manufacturing emissions contributes significantly to the overall sustainability profile of these energy storage solutions.
Water consumption and land use impacts also favor sulfur-based technologies. The extraction of sulfur requires substantially less water than mining operations for metals like cobalt and nickel, reducing pressure on water resources in production regions. Additionally, the utilization of industrial byproduct sulfur minimizes the need for dedicated mining operations, thereby reducing habitat disruption and biodiversity impacts associated with resource extraction.
End-of-life management presents both challenges and opportunities for sulfur cathode technologies. The recyclability of sulfur cathodes shows promising results in laboratory settings, with recovery rates exceeding 85% for sulfur content. However, commercial-scale recycling infrastructure remains underdeveloped, requiring significant investment to realize the circular economy potential of these technologies.
Safety considerations also factor into the environmental assessment. While lithium-sulfur batteries pose some safety risks related to lithium metal anodes, the cathode material itself presents fewer hazards than cobalt-based alternatives. Proper engineering controls and battery management systems can effectively mitigate these risks, enhancing the overall safety profile of sulfur-based energy storage solutions.
Regulatory frameworks are evolving to address the environmental dimensions of battery technologies. The European Battery Directive and similar regulations in North America and Asia increasingly emphasize reduced environmental footprint, recyclability, and responsible sourcing—areas where sulfur cathode technologies demonstrate competitive advantages. These regulatory trends may accelerate market adoption of sulfur-based energy solutions as environmental compliance becomes a more significant factor in technology selection.
The life cycle assessment of sulfur cathode batteries demonstrates reduced carbon footprint during manufacturing processes. Studies indicate that sulfur cathode production generates approximately 60% less greenhouse gas emissions compared to conventional lithium cobalt oxide cathodes, primarily due to lower energy requirements during material synthesis and processing. This reduction in manufacturing emissions contributes significantly to the overall sustainability profile of these energy storage solutions.
Water consumption and land use impacts also favor sulfur-based technologies. The extraction of sulfur requires substantially less water than mining operations for metals like cobalt and nickel, reducing pressure on water resources in production regions. Additionally, the utilization of industrial byproduct sulfur minimizes the need for dedicated mining operations, thereby reducing habitat disruption and biodiversity impacts associated with resource extraction.
End-of-life management presents both challenges and opportunities for sulfur cathode technologies. The recyclability of sulfur cathodes shows promising results in laboratory settings, with recovery rates exceeding 85% for sulfur content. However, commercial-scale recycling infrastructure remains underdeveloped, requiring significant investment to realize the circular economy potential of these technologies.
Safety considerations also factor into the environmental assessment. While lithium-sulfur batteries pose some safety risks related to lithium metal anodes, the cathode material itself presents fewer hazards than cobalt-based alternatives. Proper engineering controls and battery management systems can effectively mitigate these risks, enhancing the overall safety profile of sulfur-based energy storage solutions.
Regulatory frameworks are evolving to address the environmental dimensions of battery technologies. The European Battery Directive and similar regulations in North America and Asia increasingly emphasize reduced environmental footprint, recyclability, and responsible sourcing—areas where sulfur cathode technologies demonstrate competitive advantages. These regulatory trends may accelerate market adoption of sulfur-based energy solutions as environmental compliance becomes a more significant factor in technology selection.
Manufacturing Scalability and Cost Analysis
The scalability of sulfur cathode manufacturing represents a critical challenge in the commercialization pathway for lithium-sulfur batteries. Current production methods for sulfur cathodes remain largely confined to laboratory scales, with significant hurdles in transitioning to mass production. The primary manufacturing approaches include melt-diffusion techniques, chemical vapor deposition, and solution-based processes, each presenting unique scaling challenges.
Cost analysis reveals that raw sulfur material offers a substantial economic advantage, priced at approximately $0.10-0.15 per kilogram, representing less than 1% of the cost of traditional cathode materials like nickel-manganese-cobalt (NMC) compounds. However, this inherent material cost advantage is often negated by complex processing requirements and additional components needed to address sulfur's technical limitations.
Manufacturing sulfur cathodes at industrial scale requires specialized equipment to handle sulfur's unique properties, including its low electrical conductivity and tendency to form polysulfides. Carbon hosting materials and conductive additives, essential for functional sulfur cathodes, significantly increase production costs. Current estimates suggest that these additives can contribute up to 40-60% of the total cathode material cost.
Energy consumption during manufacturing presents another economic consideration. The melt-diffusion process, while relatively straightforward, requires precise temperature control at 155-160°C, adding energy costs that scale with production volume. More advanced techniques like chemical vapor deposition demand even higher energy inputs, further impacting the economic equation.
Labor costs vary significantly based on manufacturing location and automation level. Highly automated production lines in developed economies may reduce labor costs but require substantial capital investment, estimated at $25-40 million for a modest production facility with 100 MWh annual capacity. Conversely, less automated approaches may reduce initial capital requirements but increase ongoing operational expenses.
Yield rates and quality control represent critical factors in manufacturing economics. Current sulfur cathode production processes typically achieve 80-85% yield rates, compared to 90-95% for established lithium-ion cathode manufacturing. This efficiency gap translates directly to increased costs and material waste, presenting opportunities for process optimization.
The economic viability of scaled sulfur cathode production ultimately depends on achieving manufacturing breakthroughs that preserve sulfur's inherent cost advantages while addressing technical limitations. Recent advancements in continuous flow processing and precision automation suggest pathways to reduce production costs by 30-40% within the next five years, potentially enabling cost parity with conventional lithium-ion technologies while delivering superior energy density.
Cost analysis reveals that raw sulfur material offers a substantial economic advantage, priced at approximately $0.10-0.15 per kilogram, representing less than 1% of the cost of traditional cathode materials like nickel-manganese-cobalt (NMC) compounds. However, this inherent material cost advantage is often negated by complex processing requirements and additional components needed to address sulfur's technical limitations.
Manufacturing sulfur cathodes at industrial scale requires specialized equipment to handle sulfur's unique properties, including its low electrical conductivity and tendency to form polysulfides. Carbon hosting materials and conductive additives, essential for functional sulfur cathodes, significantly increase production costs. Current estimates suggest that these additives can contribute up to 40-60% of the total cathode material cost.
Energy consumption during manufacturing presents another economic consideration. The melt-diffusion process, while relatively straightforward, requires precise temperature control at 155-160°C, adding energy costs that scale with production volume. More advanced techniques like chemical vapor deposition demand even higher energy inputs, further impacting the economic equation.
Labor costs vary significantly based on manufacturing location and automation level. Highly automated production lines in developed economies may reduce labor costs but require substantial capital investment, estimated at $25-40 million for a modest production facility with 100 MWh annual capacity. Conversely, less automated approaches may reduce initial capital requirements but increase ongoing operational expenses.
Yield rates and quality control represent critical factors in manufacturing economics. Current sulfur cathode production processes typically achieve 80-85% yield rates, compared to 90-95% for established lithium-ion cathode manufacturing. This efficiency gap translates directly to increased costs and material waste, presenting opportunities for process optimization.
The economic viability of scaled sulfur cathode production ultimately depends on achieving manufacturing breakthroughs that preserve sulfur's inherent cost advantages while addressing technical limitations. Recent advancements in continuous flow processing and precision automation suggest pathways to reduce production costs by 30-40% within the next five years, potentially enabling cost parity with conventional lithium-ion technologies while delivering superior energy density.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!