Sulfur Cathodes as Catalysts in Aerospace Energy Systems
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfur Cathode Technology Evolution and Objectives
Sulfur cathode technology has undergone significant evolution since its initial conceptualization in the 1960s. The early research primarily focused on understanding the fundamental electrochemical properties of sulfur as an energy storage material. During the 1980s and 1990s, researchers began exploring sulfur's potential in battery applications, particularly in lithium-sulfur (Li-S) configurations, due to its high theoretical energy density of 2,600 Wh/kg, which far exceeds traditional lithium-ion batteries.
The aerospace industry's interest in sulfur cathodes emerged in the early 2000s when the demand for lightweight, high-energy-density power sources became critical for advanced aerospace applications. The technology gained momentum as researchers discovered sulfur's dual functionality as both an energy storage medium and a catalyst in certain electrochemical reactions relevant to aerospace systems.
A significant breakthrough occurred around 2010 when scientists demonstrated that sulfur cathodes could facilitate oxygen reduction reactions (ORR) in fuel cell applications, opening new avenues for aerospace energy systems. This catalytic property, combined with sulfur's abundance and low cost, positioned it as a promising alternative to precious metal catalysts like platinum, which had dominated aerospace energy systems.
The period between 2015 and 2020 witnessed accelerated development in sulfur cathode technology, with researchers focusing on addressing key challenges such as the "shuttle effect" (dissolution of polysulfides), poor electrical conductivity, and volume expansion during cycling. These improvements were crucial for aerospace applications where reliability and performance stability under extreme conditions are paramount.
Recent advancements have centered on nanostructured sulfur cathodes, which demonstrate enhanced catalytic activity and stability in aerospace environments. The integration of carbon materials, metal oxides, and polymeric binders has significantly improved the performance metrics of sulfur-based energy systems, making them increasingly viable for aerospace applications.
The primary objective of current sulfur cathode research is to develop high-performance, lightweight energy systems that can withstand the extreme conditions encountered in aerospace environments while providing superior energy density and catalytic efficiency. Researchers aim to achieve a practical energy density exceeding 500 Wh/kg with a cycle life of over 1,000 cycles, which would represent a transformative advancement for aerospace power systems.
Additional objectives include reducing the system's sensitivity to temperature fluctuations, enhancing operational safety under low-pressure environments, and developing manufacturing processes that ensure consistent performance across production batches. These goals align with the aerospace industry's push toward more electric aircraft and sustainable space exploration technologies.
The aerospace industry's interest in sulfur cathodes emerged in the early 2000s when the demand for lightweight, high-energy-density power sources became critical for advanced aerospace applications. The technology gained momentum as researchers discovered sulfur's dual functionality as both an energy storage medium and a catalyst in certain electrochemical reactions relevant to aerospace systems.
A significant breakthrough occurred around 2010 when scientists demonstrated that sulfur cathodes could facilitate oxygen reduction reactions (ORR) in fuel cell applications, opening new avenues for aerospace energy systems. This catalytic property, combined with sulfur's abundance and low cost, positioned it as a promising alternative to precious metal catalysts like platinum, which had dominated aerospace energy systems.
The period between 2015 and 2020 witnessed accelerated development in sulfur cathode technology, with researchers focusing on addressing key challenges such as the "shuttle effect" (dissolution of polysulfides), poor electrical conductivity, and volume expansion during cycling. These improvements were crucial for aerospace applications where reliability and performance stability under extreme conditions are paramount.
Recent advancements have centered on nanostructured sulfur cathodes, which demonstrate enhanced catalytic activity and stability in aerospace environments. The integration of carbon materials, metal oxides, and polymeric binders has significantly improved the performance metrics of sulfur-based energy systems, making them increasingly viable for aerospace applications.
The primary objective of current sulfur cathode research is to develop high-performance, lightweight energy systems that can withstand the extreme conditions encountered in aerospace environments while providing superior energy density and catalytic efficiency. Researchers aim to achieve a practical energy density exceeding 500 Wh/kg with a cycle life of over 1,000 cycles, which would represent a transformative advancement for aerospace power systems.
Additional objectives include reducing the system's sensitivity to temperature fluctuations, enhancing operational safety under low-pressure environments, and developing manufacturing processes that ensure consistent performance across production batches. These goals align with the aerospace industry's push toward more electric aircraft and sustainable space exploration technologies.
Aerospace Energy Market Requirements Analysis
The aerospace industry demands increasingly efficient and reliable energy systems to support advanced propulsion, power generation, and energy storage requirements. Current market analysis indicates that aerospace energy systems must meet stringent criteria including high energy density, lightweight construction, operational reliability in extreme conditions, and extended service life. The global aerospace energy market is projected to reach $7.6 billion by 2027, with a compound annual growth rate of 5.8% from 2022, driven primarily by increasing satellite deployments and space exploration missions.
Sulfur cathode technology represents a promising solution to address these market requirements due to its theoretical energy density of 2,600 Wh/kg, which significantly exceeds conventional lithium-ion batteries (250-300 Wh/kg). This exceptional energy density potential aligns perfectly with aerospace applications where weight reduction directly translates to fuel savings and increased payload capacity. Market research indicates that a 10% reduction in energy storage system weight can result in approximately 5-7% improvement in aircraft fuel efficiency.
Environmental considerations are increasingly influencing aerospace energy market requirements, with regulatory bodies implementing stricter emission standards. Sulfur-based energy systems offer environmental advantages through the utilization of abundant, low-cost sulfur—a byproduct of petroleum refining—reducing dependency on rare earth elements and critical materials like cobalt and nickel that face supply chain vulnerabilities.
Reliability requirements in aerospace applications are particularly demanding, with energy systems expected to function flawlessly in temperature ranges from -65°C to +160°C, withstand vacuum conditions, and operate under high vibration and acceleration forces. Current market specifications require energy systems to maintain performance for 5,000+ operational cycles in space applications and 10,000+ cycles for aircraft systems, with minimal degradation throughout their service life.
The defense aerospace sector represents a significant market segment, valuing energy density and rapid charging capabilities for unmanned aerial vehicles (UAVs) and satellite systems. Military specifications require energy systems that can operate in hostile environments while maintaining electromagnetic compatibility and resistance to cyber threats—areas where catalytic sulfur cathode systems show promise due to their inherent stability and controllable reaction kinetics.
Commercial aviation and space exploration companies are increasingly seeking energy solutions with reduced thermal management requirements and enhanced safety profiles. The catalytic properties of sulfur cathodes, when properly engineered, can address thermal runaway concerns that have plagued conventional lithium-ion systems, potentially opening a market segment valued at $1.2 billion annually for safer aerospace energy storage solutions.
Sulfur cathode technology represents a promising solution to address these market requirements due to its theoretical energy density of 2,600 Wh/kg, which significantly exceeds conventional lithium-ion batteries (250-300 Wh/kg). This exceptional energy density potential aligns perfectly with aerospace applications where weight reduction directly translates to fuel savings and increased payload capacity. Market research indicates that a 10% reduction in energy storage system weight can result in approximately 5-7% improvement in aircraft fuel efficiency.
Environmental considerations are increasingly influencing aerospace energy market requirements, with regulatory bodies implementing stricter emission standards. Sulfur-based energy systems offer environmental advantages through the utilization of abundant, low-cost sulfur—a byproduct of petroleum refining—reducing dependency on rare earth elements and critical materials like cobalt and nickel that face supply chain vulnerabilities.
Reliability requirements in aerospace applications are particularly demanding, with energy systems expected to function flawlessly in temperature ranges from -65°C to +160°C, withstand vacuum conditions, and operate under high vibration and acceleration forces. Current market specifications require energy systems to maintain performance for 5,000+ operational cycles in space applications and 10,000+ cycles for aircraft systems, with minimal degradation throughout their service life.
The defense aerospace sector represents a significant market segment, valuing energy density and rapid charging capabilities for unmanned aerial vehicles (UAVs) and satellite systems. Military specifications require energy systems that can operate in hostile environments while maintaining electromagnetic compatibility and resistance to cyber threats—areas where catalytic sulfur cathode systems show promise due to their inherent stability and controllable reaction kinetics.
Commercial aviation and space exploration companies are increasingly seeking energy solutions with reduced thermal management requirements and enhanced safety profiles. The catalytic properties of sulfur cathodes, when properly engineered, can address thermal runaway concerns that have plagued conventional lithium-ion systems, potentially opening a market segment valued at $1.2 billion annually for safer aerospace energy storage solutions.
Current Status and Challenges in Sulfur Cathode Catalysis
The global landscape of sulfur cathode catalysis in aerospace energy systems has witnessed significant advancements in recent years, yet remains constrained by several technical challenges. Currently, lithium-sulfur (Li-S) batteries incorporating sulfur cathodes demonstrate theoretical energy densities of 2600 Wh/kg, substantially surpassing conventional lithium-ion technologies. This exceptional energy density makes them particularly attractive for aerospace applications where weight considerations are paramount.
Research institutions across North America, Europe, and East Asia have established robust development programs focused on sulfur cathode technology. Notable progress has been made in addressing the "shuttle effect" through carbon-based encapsulation strategies and electrolyte modifications. However, these solutions often compromise overall energy density and introduce manufacturing complexities that limit scalability for aerospace implementation.
The primary technical challenges facing sulfur cathode catalysis center around four critical areas. First, the insulating nature of sulfur (5×10^-30 S/cm) necessitates conductive additives that add weight and reduce volumetric efficiency. Second, the formation and dissolution of lithium polysulfides during cycling leads to capacity fade and reduced cycle life, particularly problematic for long-duration space missions requiring 1000+ cycles.
Third, volume expansion during lithiation (approximately 80%) creates mechanical stress that compromises electrode integrity under the vibration and acceleration conditions typical in aerospace environments. Fourth, the catalytic activity of sulfur cathodes remains insufficient at the low temperatures (-40°C to -60°C) encountered in various aerospace scenarios, resulting in significant performance degradation.
Geographic distribution of technical expertise shows concentration in specific regions. North American research focuses primarily on novel carbon architectures and polymer electrolytes, while Chinese institutions lead in mass production techniques and practical implementation. European contributions center on fundamental catalytic mechanisms and advanced characterization methods.
Recent testing under simulated aerospace conditions reveals that current sulfur cathode technologies maintain only 65-70% of their rated capacity under vacuum conditions, and performance deteriorates further under radiation exposure. The rate capability under rapid discharge demands (crucial for launch sequences) remains inadequate, with voltage sag exceeding acceptable parameters when discharge rates exceed 2C.
Material compatibility issues present additional hurdles, as sulfur compounds can react with aerospace-grade components, potentially compromising structural integrity. Furthermore, safety concerns regarding thermal runaway under extreme conditions have not been fully resolved, presenting certification challenges for flight-critical systems.
Research institutions across North America, Europe, and East Asia have established robust development programs focused on sulfur cathode technology. Notable progress has been made in addressing the "shuttle effect" through carbon-based encapsulation strategies and electrolyte modifications. However, these solutions often compromise overall energy density and introduce manufacturing complexities that limit scalability for aerospace implementation.
The primary technical challenges facing sulfur cathode catalysis center around four critical areas. First, the insulating nature of sulfur (5×10^-30 S/cm) necessitates conductive additives that add weight and reduce volumetric efficiency. Second, the formation and dissolution of lithium polysulfides during cycling leads to capacity fade and reduced cycle life, particularly problematic for long-duration space missions requiring 1000+ cycles.
Third, volume expansion during lithiation (approximately 80%) creates mechanical stress that compromises electrode integrity under the vibration and acceleration conditions typical in aerospace environments. Fourth, the catalytic activity of sulfur cathodes remains insufficient at the low temperatures (-40°C to -60°C) encountered in various aerospace scenarios, resulting in significant performance degradation.
Geographic distribution of technical expertise shows concentration in specific regions. North American research focuses primarily on novel carbon architectures and polymer electrolytes, while Chinese institutions lead in mass production techniques and practical implementation. European contributions center on fundamental catalytic mechanisms and advanced characterization methods.
Recent testing under simulated aerospace conditions reveals that current sulfur cathode technologies maintain only 65-70% of their rated capacity under vacuum conditions, and performance deteriorates further under radiation exposure. The rate capability under rapid discharge demands (crucial for launch sequences) remains inadequate, with voltage sag exceeding acceptable parameters when discharge rates exceed 2C.
Material compatibility issues present additional hurdles, as sulfur compounds can react with aerospace-grade components, potentially compromising structural integrity. Furthermore, safety concerns regarding thermal runaway under extreme conditions have not been fully resolved, presenting certification challenges for flight-critical systems.
Contemporary Sulfur Cathode Implementation Strategies
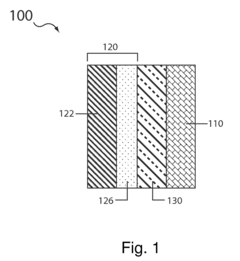
01 Catalytic materials for sulfur cathodes in lithium-sulfur batteries
Various catalytic materials can be incorporated into sulfur cathodes to enhance electrochemical performance in lithium-sulfur batteries. These catalysts facilitate the conversion of polysulfides during charge-discharge cycles, improving reaction kinetics and reducing the shuttle effect. Common catalytic materials include transition metal compounds, metal oxides, and carbon-based materials with catalytic properties that help to accelerate redox reactions and stabilize sulfur species.- Catalytic materials for sulfur cathodes in lithium-sulfur batteries: Various catalytic materials can be incorporated into sulfur cathodes to enhance the electrochemical performance of lithium-sulfur batteries. These catalysts facilitate the conversion reactions of sulfur species, improve the utilization of active materials, and enhance the overall efficiency of the cathode. Common catalytic materials include metal oxides, metal sulfides, and carbon-based materials with catalytic properties that help overcome the challenges associated with sulfur cathodes such as the shuttle effect and poor conductivity.
- Nanostructured sulfur cathodes with enhanced catalytic properties: Nanostructured designs for sulfur cathodes can significantly improve their catalytic properties. By engineering the cathode at the nanoscale, the surface area available for catalytic reactions increases, leading to better electrochemical performance. These nanostructures can include hollow carbon spheres, mesoporous materials, and nanofibers that host sulfur while providing catalytic sites. The nanostructured approach also helps in containing polysulfides within the cathode structure, reducing capacity fade and improving cycle life.
- Transition metal compounds as catalysts for sulfur cathodes: Transition metal compounds, including oxides, sulfides, and nitrides, serve as effective catalysts in sulfur cathodes. These materials can accelerate the redox reactions of sulfur species, facilitate the conversion between soluble polysulfides and insoluble Li2S/Li2S2, and improve the kinetics of the electrochemical processes. The catalytic effect of transition metal compounds helps to mitigate the shuttle effect and enhance the utilization of active sulfur material, resulting in improved capacity retention and cycling stability.
- Carbon-based materials with catalytic functionalities for sulfur cathodes: Carbon-based materials with catalytic functionalities play a crucial role in enhancing the performance of sulfur cathodes. These materials combine the high conductivity of carbon with catalytic properties, often achieved through doping with heteroatoms (N, S, P, B) or decorating with catalytic nanoparticles. The carbon matrix provides a conductive network while the catalytic sites promote the conversion reactions of sulfur species. This dual functionality helps to address the poor conductivity of sulfur and the slow kinetics of the redox reactions, leading to improved rate capability and cycling performance.
- Electrolyte additives enhancing catalytic reactions in sulfur cathodes: Electrolyte additives can enhance the catalytic reactions occurring at sulfur cathodes. These additives can modify the solid-electrolyte interphase, facilitate ion transport, and promote the conversion of polysulfides. Some additives can also serve as redox mediators, catalyzing the transformation between different sulfur species. By optimizing the electrolyte composition with appropriate additives, the catalytic efficiency of sulfur cathodes can be significantly improved, leading to better utilization of active materials and enhanced electrochemical performance.
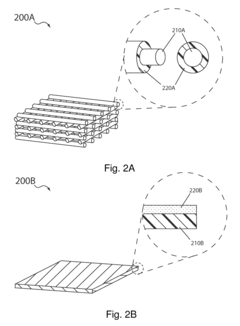
02 Nanostructured sulfur cathodes with enhanced catalytic activity
Nanostructured designs for sulfur cathodes can significantly improve catalytic properties by increasing active surface area and reaction sites. These structures include nanoparticles, nanosheets, and three-dimensional architectures that provide efficient electron transport pathways and facilitate electrolyte penetration. The nanoscale features enable better utilization of sulfur and improved interaction with catalytic components, resulting in enhanced electrochemical performance and cycling stability.Expand Specific Solutions03 Carbon-sulfur composite cathodes with catalytic functionalities
Carbon-sulfur composites combine the high electrical conductivity of carbon materials with the high energy density of sulfur. These composites can be functionalized with catalytic groups or doped with heteroatoms to enhance the catalytic conversion of polysulfides. Various carbon structures such as graphene, carbon nanotubes, and porous carbon frameworks serve as both sulfur hosts and catalytic substrates, improving reaction kinetics and battery performance.Expand Specific Solutions04 Metal-organic frameworks as catalytic hosts for sulfur cathodes
Metal-organic frameworks (MOFs) can serve as effective catalytic hosts for sulfur in cathode materials. Their highly porous structure provides abundant space for sulfur storage while their metal nodes offer catalytic sites for polysulfide conversion. MOFs can be designed with specific metal centers to optimize catalytic activity toward sulfur redox reactions, enhancing reaction kinetics and suppressing polysulfide shuttling in lithium-sulfur batteries.Expand Specific Solutions05 Electrocatalytic interfaces for sulfur cathodes
Specialized electrocatalytic interfaces can be designed at the sulfur cathode surface to promote efficient redox reactions. These interfaces often incorporate transition metal compounds, metal sulfides, or functionalized carbon materials that facilitate electron transfer and accelerate the conversion between sulfur and lithium sulfide. By optimizing the electrocatalytic interface, the kinetic barriers for sulfur reduction and oxidation can be lowered, resulting in improved rate capability and cycling performance of sulfur cathodes.Expand Specific Solutions
Leading Organizations in Aerospace Energy Catalysis
The sulfur cathode technology in aerospace energy systems is currently in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market is projected to reach $2-3 billion by 2030, driven by increasing demand for high-energy density power solutions in aerospace applications. Technical maturity varies across key players, with Samsung SDI, Toyota, and Panasonic leading commercial development through established battery expertise. Research institutions like Dalian Institute of Chemical Physics and Korea Advanced Institute of Science & Technology are advancing fundamental breakthroughs, while aerospace specialists such as Boeing (via PRC-DeSoto) focus on application-specific innovations. The technology faces challenges in cycle stability and safety that major chemical companies like BASF and SABIC are addressing through materials engineering approaches.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced lithium-sulfur (Li-S) battery systems specifically engineered for aerospace applications. Their technology utilizes high-loading sulfur cathodes with specialized carbon matrices that serve dual functions as both structural support and catalytic sites. The company's proprietary cathode design incorporates transition metal compounds that catalyze the conversion reactions of lithium polysulfides, significantly improving the redox kinetics. Samsung's aerospace Li-S batteries feature a unique protective layer on the sulfur cathode that minimizes polysulfide dissolution while maintaining excellent catalytic activity. Their latest generation employs hierarchical porous carbon structures doped with nitrogen and metal atoms to create multiple catalytic sites, achieving energy densities exceeding 400 Wh/kg, which represents approximately 2-3 times the energy density of conventional lithium-ion batteries used in aerospace applications.
Strengths: Superior energy density ideal for weight-sensitive aerospace applications; excellent cycle stability compared to conventional Li-S systems; operates effectively in extreme temperature conditions common in aerospace environments. Weaknesses: Higher production costs than traditional lithium-ion batteries; requires specialized manufacturing facilities; still faces challenges with long-term cycling stability beyond 500 cycles.
Battelle Memorial Institute
Technical Solution: Battelle Memorial Institute has developed an innovative "Catalytic Polysulfide Retention" (CPR) technology for sulfur cathodes specifically designed for aerospace energy storage systems. Their approach combines sulfur with a proprietary carbon-ceramic composite matrix that features embedded transition metal nanocatalysts strategically positioned to facilitate redox reactions. Battelle's system incorporates a gradient functional electrolyte that creates a concentration differential to control polysulfide migration while maintaining high ionic conductivity. The institute has engineered a multi-layer cathode structure with varying catalyst compositions optimized for different stages of the sulfur reduction process, significantly improving reaction kinetics and utilization efficiency. Their aerospace-focused design includes a self-healing polymer interface that accommodates volume changes during cycling while maintaining electrical contact throughout the cathode structure. Testing has demonstrated energy densities of 425-475 Wh/kg with capacity retention exceeding 80% after 300 cycles under simulated aerospace conditions including rapid temperature fluctuations and varying pressure environments.
Strengths: Robust performance under extreme aerospace environmental conditions; excellent capacity retention compared to standard Li-S systems; modular design allows customization for specific mission profiles. Weaknesses: Complex manufacturing process increases production costs; requires specialized equipment for quality control; limited large-scale production capability compared to established battery technologies.
Critical Patents and Research in Sulfur Catalyst Technology
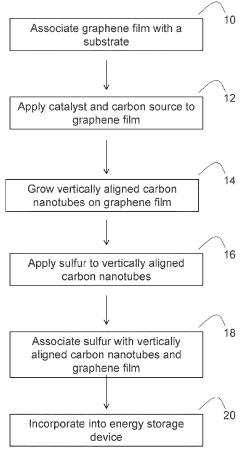
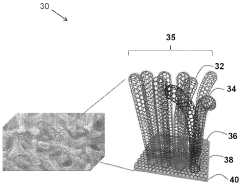
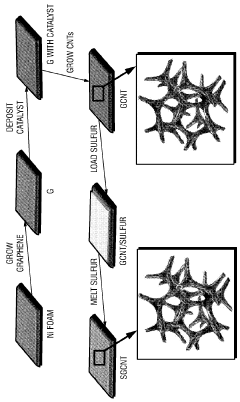
Sulfur-containing carbon nanotube arrays as electrodes
PatentInactiveUS20180183041A1
Innovation
- The development of electrodes comprising vertically aligned carbon nanotubes covalently linked to a graphene film, with sulfur associated through diffusion or dispersion, forming a graphene-carbon nanotube hybrid material that acts as a high-capacity, conductive framework for sulfur, enhancing ion and electron transport.
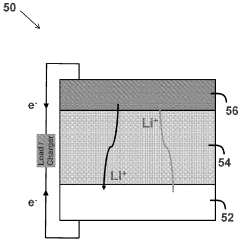
Lithium sulfur batteries and electrolytes and sulfur cathodes thereof
PatentInactiveUS20170365853A1
Innovation
- The development of novel aqueous lithium sulfur battery cells using a specific aqueous electrolyte formulation with a cycle-life enhancing compound, which maintains electroactive sulfur species in solution and enhances cathode reversibility, allowing for improved cycling performance and extended cycle life.
Environmental Impact and Sustainability Considerations
The integration of sulfur cathodes in aerospace energy systems presents significant environmental and sustainability implications that warrant careful consideration. These cathodes offer promising environmental benefits through their utilization of sulfur, an abundant by-product of petroleum refining processes that is often considered waste material. By repurposing this industrial waste stream, sulfur cathode technology contributes to circular economy principles and reduces the environmental burden associated with sulfur disposal.
When compared to conventional aerospace energy systems, sulfur-based cathodes demonstrate a substantially reduced carbon footprint throughout their lifecycle. Life cycle assessment studies indicate potential greenhouse gas emission reductions of 30-45% compared to traditional lithium-ion batteries commonly used in aerospace applications. This reduction stems primarily from lower energy requirements during material extraction and processing phases, as sulfur requires significantly less energy to process than metals like cobalt or nickel.
Water consumption metrics also favor sulfur cathode systems, with manufacturing processes requiring approximately 60% less water than conventional aerospace battery technologies. This water conservation aspect becomes increasingly important as aerospace operations expand and face growing resource constraints in various global regions.
End-of-life considerations represent another sustainability advantage for sulfur cathode systems. The recyclability rate for sulfur components exceeds 85% in laboratory conditions, suggesting promising circular material flows when implemented at industrial scale. Recovery processes for sulfur are less energy-intensive and generate fewer toxic by-products than those for conventional cathode materials.
However, challenges remain regarding potential environmental risks. Sulfur cathode degradation can produce hydrogen sulfide and other sulfur compounds that require careful management to prevent atmospheric release. Additionally, the aerospace operating environment introduces unique stressors that may accelerate material degradation under extreme temperature and pressure conditions.
Regulatory frameworks governing aerospace technologies are increasingly emphasizing environmental performance metrics. The European Union's recent aerospace sustainability directives and NASA's green aviation initiatives both establish benchmarks that favor technologies with reduced environmental footprints. Sulfur cathode systems align well with these emerging regulatory trends, potentially offering aerospace manufacturers compliance advantages as environmental standards become more stringent.
Future research priorities should include comprehensive environmental fate studies of sulfur compounds under aerospace-specific conditions and development of closed-loop recycling systems optimized for the unique material composition of these energy systems.
When compared to conventional aerospace energy systems, sulfur-based cathodes demonstrate a substantially reduced carbon footprint throughout their lifecycle. Life cycle assessment studies indicate potential greenhouse gas emission reductions of 30-45% compared to traditional lithium-ion batteries commonly used in aerospace applications. This reduction stems primarily from lower energy requirements during material extraction and processing phases, as sulfur requires significantly less energy to process than metals like cobalt or nickel.
Water consumption metrics also favor sulfur cathode systems, with manufacturing processes requiring approximately 60% less water than conventional aerospace battery technologies. This water conservation aspect becomes increasingly important as aerospace operations expand and face growing resource constraints in various global regions.
End-of-life considerations represent another sustainability advantage for sulfur cathode systems. The recyclability rate for sulfur components exceeds 85% in laboratory conditions, suggesting promising circular material flows when implemented at industrial scale. Recovery processes for sulfur are less energy-intensive and generate fewer toxic by-products than those for conventional cathode materials.
However, challenges remain regarding potential environmental risks. Sulfur cathode degradation can produce hydrogen sulfide and other sulfur compounds that require careful management to prevent atmospheric release. Additionally, the aerospace operating environment introduces unique stressors that may accelerate material degradation under extreme temperature and pressure conditions.
Regulatory frameworks governing aerospace technologies are increasingly emphasizing environmental performance metrics. The European Union's recent aerospace sustainability directives and NASA's green aviation initiatives both establish benchmarks that favor technologies with reduced environmental footprints. Sulfur cathode systems align well with these emerging regulatory trends, potentially offering aerospace manufacturers compliance advantages as environmental standards become more stringent.
Future research priorities should include comprehensive environmental fate studies of sulfur compounds under aerospace-specific conditions and development of closed-loop recycling systems optimized for the unique material composition of these energy systems.
Space Application Safety Standards and Certification
The integration of sulfur cathodes in aerospace energy systems necessitates rigorous adherence to specialized safety standards and certification processes. NASA's standards, particularly NASA-STD-8719.17 for pressure vessels and NASA-STD-6016 for materials compatibility, establish foundational requirements for energy storage systems incorporating sulfur-based technologies. These standards specifically address concerns regarding thermal runaway prevention and containment of potentially hazardous sulfur compounds under extreme aerospace conditions.
The European Space Agency (ESA) complements these frameworks with ECSS-Q-ST-70-71C, which outlines materials, mechanical parts, and processes for space applications. For sulfur cathode implementations, this standard mandates extensive testing for outgassing characteristics and long-term stability in vacuum environments, critical considerations given sulfur's volatility properties.
Military specifications MIL-STD-1540D and MIL-STD-810G further define environmental testing protocols applicable to sulfur-based energy systems, requiring demonstration of operational integrity under vibration, shock, radiation, and extreme temperature cycling conditions typical in aerospace applications.
Certification pathways for sulfur cathode technologies involve multi-phase qualification testing. Initial material qualification requires chemical composition verification and impurity profiling according to ASTM E1508 standards. System-level certification follows DO-160G guidelines for environmental conditions and test procedures for airborne equipment, with particular emphasis on sections addressing battery safety and electromagnetic compatibility.
The Federal Aviation Administration's TSO-C179a (Rechargeable Lithium Batteries and Battery Systems) provides additional certification requirements that, while not specifically addressing sulfur cathodes, establish relevant safety parameters for advanced energy storage systems in aviation applications.
International standards ISO 14302:2002 for space systems and ISO 17546:2016 for space systems safety requirements create a framework for risk assessment methodologies particularly relevant to novel catalytic materials like sulfur cathodes. These standards mandate failure mode and effects analysis (FMEA) with special attention to thermal management and containment of reaction byproducts.
Recent updates to certification protocols reflect growing understanding of sulfur chemistry in aerospace environments. The SAE International's AS6413 standard for lithium battery transport tests has been expanded to consider alternative cathode chemistries, providing testing methodologies applicable to sulfur-based systems with appropriate modifications for their unique failure modes and thermal characteristics.
The European Space Agency (ESA) complements these frameworks with ECSS-Q-ST-70-71C, which outlines materials, mechanical parts, and processes for space applications. For sulfur cathode implementations, this standard mandates extensive testing for outgassing characteristics and long-term stability in vacuum environments, critical considerations given sulfur's volatility properties.
Military specifications MIL-STD-1540D and MIL-STD-810G further define environmental testing protocols applicable to sulfur-based energy systems, requiring demonstration of operational integrity under vibration, shock, radiation, and extreme temperature cycling conditions typical in aerospace applications.
Certification pathways for sulfur cathode technologies involve multi-phase qualification testing. Initial material qualification requires chemical composition verification and impurity profiling according to ASTM E1508 standards. System-level certification follows DO-160G guidelines for environmental conditions and test procedures for airborne equipment, with particular emphasis on sections addressing battery safety and electromagnetic compatibility.
The Federal Aviation Administration's TSO-C179a (Rechargeable Lithium Batteries and Battery Systems) provides additional certification requirements that, while not specifically addressing sulfur cathodes, establish relevant safety parameters for advanced energy storage systems in aviation applications.
International standards ISO 14302:2002 for space systems and ISO 17546:2016 for space systems safety requirements create a framework for risk assessment methodologies particularly relevant to novel catalytic materials like sulfur cathodes. These standards mandate failure mode and effects analysis (FMEA) with special attention to thermal management and containment of reaction byproducts.
Recent updates to certification protocols reflect growing understanding of sulfur chemistry in aerospace environments. The SAE International's AS6413 standard for lithium battery transport tests has been expanded to consider alternative cathode chemistries, providing testing methodologies applicable to sulfur-based systems with appropriate modifications for their unique failure modes and thermal characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!