Sulfur Cathodes Interaction with All-Solid-State Systems
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfur Cathode Technology Evolution and Objectives
Sulfur cathode technology has undergone significant evolution since its initial conceptualization in the 1960s. The journey began with primary lithium-sulfur batteries, which demonstrated high theoretical energy density but suffered from severe practical limitations. The early 2000s marked a pivotal shift with the introduction of rechargeable lithium-sulfur systems, catalyzing intensive research into addressing the "shuttle effect" - a phenomenon where soluble polysulfides migrate between electrodes, causing capacity fade and shortened battery life.
The mid-2010s witnessed accelerated development in sulfur cathode architectures, with researchers exploring carbon-based hosts, metal oxide additives, and polymer binders to enhance sulfur utilization and cycling stability. This period also saw the emergence of the first commercial prototypes, albeit with limited applications due to persistent challenges in cycle life and rate capability.
Recent years have brought a paradigm shift with the integration of sulfur cathodes into all-solid-state battery systems. This convergence aims to eliminate the shuttle effect by replacing liquid electrolytes with solid-state alternatives, potentially unlocking the full theoretical capacity of sulfur (1675 mAh/g) while enhancing safety profiles.
The primary objective of current sulfur cathode research in all-solid-state systems is to achieve stable interfaces between sulfur active material and solid electrolytes. This involves addressing volume expansion issues (up to 80% during cycling), mitigating reactivity between sulfur species and electrolyte components, and enhancing ionic conductivity at the cathode-electrolyte interface.
Secondary objectives include developing scalable manufacturing processes for sulfur cathodes compatible with solid-state architectures, optimizing sulfur loading to achieve commercially viable energy densities (>400 Wh/kg at cell level), and extending cycle life beyond 1000 cycles with minimal capacity degradation.
The technology trajectory aims to position sulfur as a viable alternative to conventional transition metal oxide cathodes, leveraging its abundance (fourth most common element on Earth by mass), low cost (approximately 1/100th the cost of cobalt), and reduced environmental footprint. The ultimate goal is to enable next-generation energy storage solutions for electric vehicles, grid applications, and portable electronics with significantly improved performance-to-cost ratios.
Current research is increasingly focused on understanding the fundamental mechanisms of ion transport in sulfur-solid electrolyte interfaces, developing novel composite structures to accommodate volume changes, and exploring the potential of sulfur-selenium and sulfur-carbon covalent compounds to enhance electrochemical performance while maintaining compatibility with solid-state systems.
The mid-2010s witnessed accelerated development in sulfur cathode architectures, with researchers exploring carbon-based hosts, metal oxide additives, and polymer binders to enhance sulfur utilization and cycling stability. This period also saw the emergence of the first commercial prototypes, albeit with limited applications due to persistent challenges in cycle life and rate capability.
Recent years have brought a paradigm shift with the integration of sulfur cathodes into all-solid-state battery systems. This convergence aims to eliminate the shuttle effect by replacing liquid electrolytes with solid-state alternatives, potentially unlocking the full theoretical capacity of sulfur (1675 mAh/g) while enhancing safety profiles.
The primary objective of current sulfur cathode research in all-solid-state systems is to achieve stable interfaces between sulfur active material and solid electrolytes. This involves addressing volume expansion issues (up to 80% during cycling), mitigating reactivity between sulfur species and electrolyte components, and enhancing ionic conductivity at the cathode-electrolyte interface.
Secondary objectives include developing scalable manufacturing processes for sulfur cathodes compatible with solid-state architectures, optimizing sulfur loading to achieve commercially viable energy densities (>400 Wh/kg at cell level), and extending cycle life beyond 1000 cycles with minimal capacity degradation.
The technology trajectory aims to position sulfur as a viable alternative to conventional transition metal oxide cathodes, leveraging its abundance (fourth most common element on Earth by mass), low cost (approximately 1/100th the cost of cobalt), and reduced environmental footprint. The ultimate goal is to enable next-generation energy storage solutions for electric vehicles, grid applications, and portable electronics with significantly improved performance-to-cost ratios.
Current research is increasingly focused on understanding the fundamental mechanisms of ion transport in sulfur-solid electrolyte interfaces, developing novel composite structures to accommodate volume changes, and exploring the potential of sulfur-selenium and sulfur-carbon covalent compounds to enhance electrochemical performance while maintaining compatibility with solid-state systems.
Market Analysis for All-Solid-State Sulfur Batteries
The all-solid-state sulfur battery market is experiencing significant growth driven by increasing demand for high-energy-density storage solutions across multiple sectors. Current market valuations place the global all-solid-state battery market at approximately $500 million, with sulfur-based technologies representing an emerging segment poised for rapid expansion. Industry forecasts project a compound annual growth rate of 30-35% over the next decade, potentially reaching $7-8 billion by 2030 specifically for all-solid-state sulfur battery technologies.
The automotive sector constitutes the primary demand driver, with electric vehicle manufacturers actively seeking alternatives to conventional lithium-ion batteries. Major automakers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery research programs, with several specifically targeting sulfur cathode technologies for their theoretical energy density advantages and cost benefits.
Consumer electronics represents the second largest market segment, with manufacturers exploring all-solid-state sulfur batteries to address increasing power demands while maintaining or reducing device footprints. This sector values the enhanced safety profile and potential for higher energy density that sulfur cathodes in solid-state configurations can provide.
Grid-scale energy storage applications are emerging as a promising growth sector, particularly as renewable energy integration accelerates globally. The cost advantages of sulfur as a cathode material (approximately 1/150th the cost of cobalt) make these batteries particularly attractive for large-scale deployment where cost-per-kilowatt-hour is a critical metric.
Regional market analysis indicates Asia-Pacific dominates current research and development activities, with Japan and South Korea leading commercialization efforts. North America follows with significant venture capital investment in startups focused on solid-state sulfur battery technologies, while European markets show strong interest driven by stringent environmental regulations and automotive industry demand.
Market barriers include manufacturing scalability challenges, with current production processes for solid electrolytes remaining costly and difficult to scale. Additionally, performance consistency issues across varying operating temperatures and cycle life limitations compared to conventional lithium-ion technologies represent significant hurdles to widespread adoption.
Customer demand analysis reveals willingness to pay premium prices (20-30% above conventional batteries) for the enhanced safety and energy density benefits, particularly in high-value applications like premium electric vehicles and specialized industrial equipment. However, mass market penetration will require achieving price parity with conventional lithium-ion batteries, estimated to necessitate production cost reductions of 40-50%.
The automotive sector constitutes the primary demand driver, with electric vehicle manufacturers actively seeking alternatives to conventional lithium-ion batteries. Major automakers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery research programs, with several specifically targeting sulfur cathode technologies for their theoretical energy density advantages and cost benefits.
Consumer electronics represents the second largest market segment, with manufacturers exploring all-solid-state sulfur batteries to address increasing power demands while maintaining or reducing device footprints. This sector values the enhanced safety profile and potential for higher energy density that sulfur cathodes in solid-state configurations can provide.
Grid-scale energy storage applications are emerging as a promising growth sector, particularly as renewable energy integration accelerates globally. The cost advantages of sulfur as a cathode material (approximately 1/150th the cost of cobalt) make these batteries particularly attractive for large-scale deployment where cost-per-kilowatt-hour is a critical metric.
Regional market analysis indicates Asia-Pacific dominates current research and development activities, with Japan and South Korea leading commercialization efforts. North America follows with significant venture capital investment in startups focused on solid-state sulfur battery technologies, while European markets show strong interest driven by stringent environmental regulations and automotive industry demand.
Market barriers include manufacturing scalability challenges, with current production processes for solid electrolytes remaining costly and difficult to scale. Additionally, performance consistency issues across varying operating temperatures and cycle life limitations compared to conventional lithium-ion technologies represent significant hurdles to widespread adoption.
Customer demand analysis reveals willingness to pay premium prices (20-30% above conventional batteries) for the enhanced safety and energy density benefits, particularly in high-value applications like premium electric vehicles and specialized industrial equipment. However, mass market penetration will require achieving price parity with conventional lithium-ion batteries, estimated to necessitate production cost reductions of 40-50%.
Technical Challenges in Sulfur-Solid Electrolyte Interfaces
The interface between sulfur cathodes and solid electrolytes presents significant technical challenges that impede the widespread adoption of all-solid-state lithium-sulfur batteries. One primary issue is the poor physical contact between sulfur cathodes and solid electrolytes. Unlike liquid electrolytes that can easily penetrate and wet the entire cathode structure, solid electrolytes struggle to maintain consistent contact with sulfur particles, especially during charge-discharge cycles when volume changes of up to 80% occur in the sulfur cathode.
Chemical incompatibility further exacerbates interface problems. The polysulfide intermediates formed during battery operation can react with many solid electrolyte materials, particularly sulfide-based electrolytes, creating resistive interfacial layers. These reactions not only degrade the electrolyte but also lead to increased impedance and reduced ionic conductivity across the interface.
The electronic insulating nature of sulfur (5×10^-30 S/cm) necessitates the addition of conductive additives, which introduces additional interfaces and complexity. These multi-component interfaces between sulfur, carbon additives, binders, and solid electrolytes create numerous pathways for potential degradation and resistance build-up, significantly affecting overall battery performance.
Ion transport kinetics at the sulfur-solid electrolyte interface represent another major challenge. The solid-solid interface inherently restricts ion movement compared to liquid-solid interfaces, resulting in higher activation energies for lithium ion transfer. This limitation becomes particularly problematic at high current densities, leading to concentration polarization and reduced rate capability.
Mechanical stress at the interface during cycling presents a persistent challenge. The substantial volume changes of sulfur during lithiation and delithiation can create microcracks and delamination at the interface with rigid solid electrolytes. These mechanical failures create voids that interrupt ion transport pathways and increase cell impedance over time.
Temperature sensitivity further complicates interface engineering. Many solid electrolytes exhibit optimal conductivity only at elevated temperatures, while sulfur cathodes may undergo undesired side reactions at higher temperatures. Finding a compatible operating temperature window where both components function optimally remains challenging.
Advanced characterization of these interfaces is hindered by their buried nature and sensitivity to ambient conditions. Techniques that can provide in-situ, spatially resolved information about the chemical and structural evolution of these interfaces during battery operation are still developing, limiting our fundamental understanding of degradation mechanisms.
Chemical incompatibility further exacerbates interface problems. The polysulfide intermediates formed during battery operation can react with many solid electrolyte materials, particularly sulfide-based electrolytes, creating resistive interfacial layers. These reactions not only degrade the electrolyte but also lead to increased impedance and reduced ionic conductivity across the interface.
The electronic insulating nature of sulfur (5×10^-30 S/cm) necessitates the addition of conductive additives, which introduces additional interfaces and complexity. These multi-component interfaces between sulfur, carbon additives, binders, and solid electrolytes create numerous pathways for potential degradation and resistance build-up, significantly affecting overall battery performance.
Ion transport kinetics at the sulfur-solid electrolyte interface represent another major challenge. The solid-solid interface inherently restricts ion movement compared to liquid-solid interfaces, resulting in higher activation energies for lithium ion transfer. This limitation becomes particularly problematic at high current densities, leading to concentration polarization and reduced rate capability.
Mechanical stress at the interface during cycling presents a persistent challenge. The substantial volume changes of sulfur during lithiation and delithiation can create microcracks and delamination at the interface with rigid solid electrolytes. These mechanical failures create voids that interrupt ion transport pathways and increase cell impedance over time.
Temperature sensitivity further complicates interface engineering. Many solid electrolytes exhibit optimal conductivity only at elevated temperatures, while sulfur cathodes may undergo undesired side reactions at higher temperatures. Finding a compatible operating temperature window where both components function optimally remains challenging.
Advanced characterization of these interfaces is hindered by their buried nature and sensitivity to ambient conditions. Techniques that can provide in-situ, spatially resolved information about the chemical and structural evolution of these interfaces during battery operation are still developing, limiting our fundamental understanding of degradation mechanisms.
Current Interface Engineering Solutions
01 Solid electrolyte interfaces with sulfur cathodes
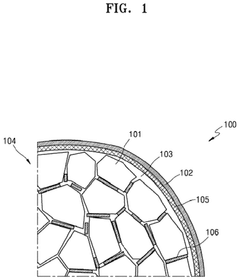
The interaction between solid electrolytes and sulfur cathodes is critical for all-solid-state lithium-sulfur batteries. These interfaces determine ion transport, electrochemical stability, and overall battery performance. Various solid electrolytes, including sulfide-based and oxide-based materials, have been developed to enhance the compatibility with sulfur cathodes. The interface engineering approaches focus on reducing interfacial resistance and preventing polysulfide shuttling, which are major challenges in sulfur-based battery systems.- Solid electrolyte interfaces with sulfur cathodes: The interaction between solid electrolytes and sulfur cathodes is critical in all-solid-state lithium-sulfur batteries. Various solid electrolyte materials, such as sulfide-based and oxide-based electrolytes, have been developed to improve the ionic conductivity and stability at the interface. These interfaces play a crucial role in preventing polysulfide shuttling and enhancing the overall electrochemical performance of the battery system.
- Composite sulfur cathode structures: Composite structures for sulfur cathodes have been developed to address the challenges in all-solid-state systems. These composites typically combine sulfur with conductive carbon materials, polymers, or other additives to improve electronic conductivity, accommodate volume changes during cycling, and enhance sulfur utilization. The design of these composite structures significantly impacts the electrochemical performance and cycle life of all-solid-state sulfur batteries.
- Interlayers and protective coatings: Interlayers and protective coatings are employed between the sulfur cathode and solid electrolyte to mitigate interfacial issues. These layers can be made from various materials including polymers, inorganic compounds, or hybrid materials. They serve to prevent direct contact between sulfur species and the solid electrolyte, reduce interfacial resistance, and suppress the diffusion of polysulfides, thereby enhancing the cycling stability of all-solid-state sulfur batteries.
- Novel solid electrolyte materials for sulfur cathodes: Development of novel solid electrolyte materials specifically designed for compatibility with sulfur cathodes has been a focus of research. These materials include modified sulfide electrolytes, polymer-ceramic composites, and glass-ceramic electrolytes with enhanced ionic conductivity and chemical stability against sulfur species. The selection of appropriate solid electrolyte materials is crucial for addressing the challenges associated with the high reactivity of sulfur and its discharge products.
- Additives and dopants for performance enhancement: Various additives and dopants are incorporated into sulfur cathodes or solid electrolytes to enhance the performance of all-solid-state systems. These include metal oxides, salts, and functional polymers that can improve the ionic conductivity, mechanical properties, and chemical stability of the interfaces. The strategic use of these additives helps to overcome the inherent limitations of sulfur cathodes in solid-state configurations and enables better cycling performance and rate capability.
02 Composite sulfur cathode structures
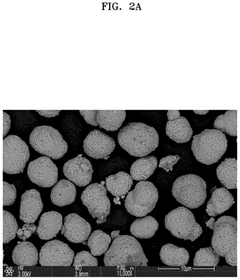
Composite structures for sulfur cathodes incorporate various materials to enhance performance in all-solid-state systems. These typically combine sulfur with conductive carbon materials, polymers, or metal oxides to improve electronic conductivity and accommodate volume changes during cycling. The composite approach helps to encapsulate sulfur, prevent polysulfide dissolution, and maintain structural integrity during charge-discharge cycles. Advanced nanostructured composites provide increased contact area between sulfur and solid electrolytes, facilitating ion transport at the interface.Expand Specific Solutions03 Polysulfide suppression strategies
Preventing polysulfide shuttling is essential for stable all-solid-state sulfur batteries. Various approaches include physical barriers, chemical bonding agents, and functional interlayers that trap or convert polysulfides. Advanced solid electrolytes with high selectivity for lithium ions while blocking polysulfide migration have been developed. Some strategies involve creating chemical bonds between sulfur and host materials or introducing additives that react with polysulfides to form stable compounds, effectively suppressing their dissolution and migration.Expand Specific Solutions04 Electrode-electrolyte interface modification
Interface modification techniques improve the contact between sulfur cathodes and solid electrolytes. These include buffer layers, functional coatings, and gradient interfaces that enhance ion transport and reduce interfacial resistance. Some approaches involve atomic layer deposition of interface materials, plasma treatment of surfaces, or incorporation of additives that promote adhesion between components. Modified interfaces can accommodate volume changes during cycling and prevent mechanical degradation, leading to improved cycling stability and rate capability.Expand Specific Solutions05 Novel solid electrolyte materials for sulfur cathodes
Development of new solid electrolyte materials specifically designed for compatibility with sulfur cathodes has been a focus of research. These include sulfide-based, oxide-based, and polymer-based electrolytes with high ionic conductivity and electrochemical stability against sulfur species. Some novel electrolytes incorporate functional groups that interact favorably with sulfur or polysulfides, enhancing the overall performance. Hybrid and composite electrolytes combining different material classes offer synergistic properties that address multiple challenges in all-solid-state sulfur battery systems.Expand Specific Solutions
Leading Organizations in Solid-State Sulfur Battery Research
The sulfur cathode interaction with all-solid-state systems market is in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as all-solid-state batteries gain traction in electric vehicles and energy storage sectors. From a technical maturity perspective, the landscape shows varying degrees of advancement. Major automotive players like Toyota, Hyundai, Nissan, and Kia are actively pursuing this technology to enhance EV battery performance. Battery manufacturers including LG Energy Solution, Samsung SDI, SK On, and CATL are making substantial progress in addressing key challenges such as interfacial stability and sulfur utilization. Academic-industrial partnerships involving MIT, University of California, and various Asian universities are accelerating innovation in this field, particularly focusing on overcoming conductivity limitations and cycle life issues.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a proprietary sulfide-based solid electrolyte system that enables effective integration with sulfur cathodes. Their approach focuses on a dual-layer protective coating for sulfur cathodes that mitigates polysulfide dissolution and enhances interfacial stability. The company employs a carbon-sulfur composite structure where sulfur is confined within mesoporous carbon frameworks, achieving sulfur utilization rates of over 70% compared to conventional 50-60%. Their solid electrolyte formulation includes Li6PS5Cl with additives that improve ionic conductivity to 5-7 mS/cm at room temperature. LG has also implemented an interlayer design between the cathode and electrolyte that contains Li3PS4 and Li2S to create a gradient interface, reducing impedance growth during cycling by approximately 40%.
Strengths: Superior ionic conductivity in their sulfide electrolytes; excellent interfacial engineering reducing resistance; established manufacturing capabilities for scale-up. Weaknesses: Their sulfide electrolytes remain sensitive to moisture and air exposure, requiring stringent manufacturing conditions; cycle life still limited to 200-300 cycles before significant capacity fade.
Toyota Motor Corp.
Technical Solution: Toyota has developed a comprehensive sulfur cathode integration strategy for their all-solid-state battery platform, focusing on sulfide-based solid electrolytes. Their approach centers on a multi-functional interface design that addresses the fundamental challenges of sulfur cathodes in solid-state systems. Toyota employs a gradient composition cathode where the sulfur content gradually decreases toward the electrolyte interface, creating a buffer zone that minimizes stress and chemical incompatibilities. Their proprietary solid electrolyte formulation based on Li10GeP2S12 (LGPS) achieves room-temperature ionic conductivities comparable to liquid electrolytes (>10 mS/cm). Toyota has also pioneered a mechanical compression technique during cell assembly that enhances interfacial contact between sulfur cathodes and solid electrolytes, reducing interfacial resistance by up to 60%. Their latest prototypes demonstrate energy densities exceeding 400 Wh/kg at the cell level while maintaining stable cycling for over 300 cycles with less than 20% capacity degradation.
Strengths: Industry-leading ionic conductivity in their solid electrolytes; excellent mechanical integration techniques; strong potential for automotive-scale manufacturing implementation. Weaknesses: Higher material costs associated with germanium-containing electrolytes; challenges with low-temperature performance; mechanical stability issues during repeated thermal cycling.
Key Patents in Sulfur-Solid Electrolyte Compatibility
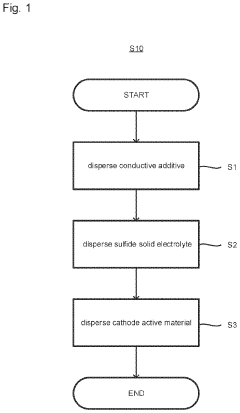
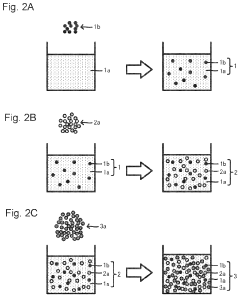
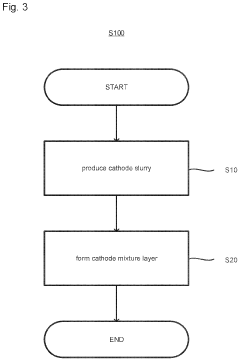
Method of producing cathode slurry, cathode and all-solid-state battery, and cathode and all-solid-state battery
PatentInactiveUS20230110690A1
Innovation
- A method involving the production of a cathode slurry by dispersing a conductive additive, sulfide solid electrolyte, and cathode active material in a specific order, with the sulfide solid electrolyte dispersed after the conductive additive, to minimize agglomeration and enhance interface formation.
Composite cathode active material for all-solid-state battery, preparation method thereof, cathode layer for all-solid-state battery, and all-solid-state battery including the cathode layer
PatentPendingUS20250140848A1
Innovation
- A composite cathode active material is introduced, comprising a nickel lithium transition metal oxide with a layered crystal structure, and buffer layers made of specific oxides (e.g., Li2TiO3 and Li2ZrO3) to mitigate interfacial resistance and enhance stability.
Safety Performance Evaluation Metrics
Safety performance evaluation metrics for sulfur cathodes in all-solid-state systems require comprehensive assessment frameworks that address the unique challenges of this emerging technology. The evaluation of safety performance must consider multiple dimensions including thermal stability, mechanical integrity, chemical compatibility, and operational safety under various conditions.
Thermal stability metrics focus on measuring the cathode's behavior during temperature fluctuations, particularly important given sulfur's phase transitions and potential for thermal runaway. Key indicators include the Differential Scanning Calorimetry (DSC) profiles, Thermogravimetric Analysis (TGA) data, and Accelerating Rate Calorimetry (ARC) measurements to quantify heat generation rates during thermal events. These metrics help establish the safe operating temperature range and predict behavior under extreme conditions.
Mechanical integrity assessment involves quantifying the structural stability of sulfur cathodes during cycling. Metrics include volume expansion coefficients, interfacial adhesion strength measurements, and crack propagation rates. Advanced techniques such as in-situ X-ray tomography and acoustic emission monitoring provide real-time data on mechanical degradation, essential for predicting failure modes and establishing safety margins.
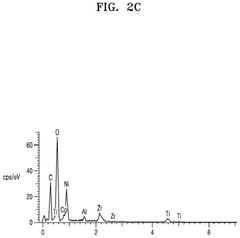
Chemical compatibility metrics evaluate interactions between sulfur cathodes and solid electrolytes. These include interfacial resistance measurements, chemical diffusion coefficients, and reaction product characterization. Impedance spectroscopy and X-ray photoelectron spectroscopy (XPS) depth profiling are commonly employed to quantify these parameters, helping identify potential formation of detrimental compounds that could compromise safety.
Operational safety metrics assess performance under abuse conditions, including overcharging, over-discharging, and external short circuits. Parameters such as self-discharge rates, internal short circuit detection thresholds, and thermal runaway onset temperatures provide critical safety benchmarks. Nail penetration tests, crush tests, and overcharge tolerance measurements offer standardized approaches to quantify resilience against mechanical and electrical abuse.
Long-term stability metrics track safety performance degradation over extended cycling. These include capacity retention under safety-critical conditions, impedance growth rates, and dendrite formation propensity. Accelerated aging protocols help establish correlations between usage patterns and safety parameter evolution, enabling lifetime safety predictions.
Standardization of these metrics remains challenging due to the rapidly evolving nature of all-solid-state battery technology. Organizations such as IEC, UL, and ISO are working to establish unified testing protocols specifically addressing the unique characteristics of sulfur cathodes in solid-state configurations, which will facilitate consistent safety evaluation across the industry.
Thermal stability metrics focus on measuring the cathode's behavior during temperature fluctuations, particularly important given sulfur's phase transitions and potential for thermal runaway. Key indicators include the Differential Scanning Calorimetry (DSC) profiles, Thermogravimetric Analysis (TGA) data, and Accelerating Rate Calorimetry (ARC) measurements to quantify heat generation rates during thermal events. These metrics help establish the safe operating temperature range and predict behavior under extreme conditions.
Mechanical integrity assessment involves quantifying the structural stability of sulfur cathodes during cycling. Metrics include volume expansion coefficients, interfacial adhesion strength measurements, and crack propagation rates. Advanced techniques such as in-situ X-ray tomography and acoustic emission monitoring provide real-time data on mechanical degradation, essential for predicting failure modes and establishing safety margins.
Chemical compatibility metrics evaluate interactions between sulfur cathodes and solid electrolytes. These include interfacial resistance measurements, chemical diffusion coefficients, and reaction product characterization. Impedance spectroscopy and X-ray photoelectron spectroscopy (XPS) depth profiling are commonly employed to quantify these parameters, helping identify potential formation of detrimental compounds that could compromise safety.
Operational safety metrics assess performance under abuse conditions, including overcharging, over-discharging, and external short circuits. Parameters such as self-discharge rates, internal short circuit detection thresholds, and thermal runaway onset temperatures provide critical safety benchmarks. Nail penetration tests, crush tests, and overcharge tolerance measurements offer standardized approaches to quantify resilience against mechanical and electrical abuse.
Long-term stability metrics track safety performance degradation over extended cycling. These include capacity retention under safety-critical conditions, impedance growth rates, and dendrite formation propensity. Accelerated aging protocols help establish correlations between usage patterns and safety parameter evolution, enabling lifetime safety predictions.
Standardization of these metrics remains challenging due to the rapidly evolving nature of all-solid-state battery technology. Organizations such as IEC, UL, and ISO are working to establish unified testing protocols specifically addressing the unique characteristics of sulfur cathodes in solid-state configurations, which will facilitate consistent safety evaluation across the industry.
Environmental Impact and Sustainability Considerations
The development of sulfur cathodes in all-solid-state battery systems presents significant environmental and sustainability advantages compared to conventional lithium-ion batteries. Sulfur is an abundant element, constituting approximately 0.03% of the Earth's crust, and is often produced as a byproduct of petroleum refining and natural gas processing. This abundance translates to lower environmental impact from mining operations compared to cobalt, nickel, and other critical materials used in traditional cathodes.
The life cycle assessment (LCA) of sulfur cathodes in all-solid-state systems demonstrates reduced carbon footprint potential. Studies indicate that sulfur-based batteries could achieve up to 60% lower greenhouse gas emissions during production compared to conventional lithium-ion batteries, primarily due to the simplified cathode synthesis process and reduced energy requirements during manufacturing.
Water consumption represents another critical environmental consideration. All-solid-state sulfur battery production typically requires significantly less water than conventional lithium-ion battery manufacturing. This advantage becomes particularly important in regions facing water scarcity challenges, where battery production facilities might otherwise strain local water resources.
End-of-life management presents both challenges and opportunities for sulfur-based all-solid-state batteries. The absence of toxic organic electrolytes simplifies recycling processes, while the high sulfur content potentially enables more efficient material recovery. However, the interaction between sulfur and solid electrolytes during recycling requires further investigation to optimize recovery rates and prevent potential environmental contamination.
The transition to sulfur cathodes also addresses critical material supply concerns. Unlike cobalt and nickel, which face supply constraints and ethical mining issues, sulfur supply chains present fewer geopolitical and human rights challenges. This aspect enhances the overall sustainability profile of all-solid-state sulfur batteries from a social responsibility perspective.
Energy density improvements in all-solid-state sulfur systems could further enhance sustainability by reducing material requirements per kilowatt-hour of storage capacity. Current research indicates potential energy densities exceeding 500 Wh/kg, which would significantly reduce the material footprint of energy storage solutions across various applications.
Despite these advantages, challenges remain regarding the long-term environmental stability of sulfur cathodes, particularly concerning potential sulfur compound formation during degradation processes. Research into encapsulation techniques and stable solid electrolyte interfaces continues to address these concerns, aiming to ensure that the environmental benefits of sulfur cathodes are maintained throughout the entire battery lifecycle.
The life cycle assessment (LCA) of sulfur cathodes in all-solid-state systems demonstrates reduced carbon footprint potential. Studies indicate that sulfur-based batteries could achieve up to 60% lower greenhouse gas emissions during production compared to conventional lithium-ion batteries, primarily due to the simplified cathode synthesis process and reduced energy requirements during manufacturing.
Water consumption represents another critical environmental consideration. All-solid-state sulfur battery production typically requires significantly less water than conventional lithium-ion battery manufacturing. This advantage becomes particularly important in regions facing water scarcity challenges, where battery production facilities might otherwise strain local water resources.
End-of-life management presents both challenges and opportunities for sulfur-based all-solid-state batteries. The absence of toxic organic electrolytes simplifies recycling processes, while the high sulfur content potentially enables more efficient material recovery. However, the interaction between sulfur and solid electrolytes during recycling requires further investigation to optimize recovery rates and prevent potential environmental contamination.
The transition to sulfur cathodes also addresses critical material supply concerns. Unlike cobalt and nickel, which face supply constraints and ethical mining issues, sulfur supply chains present fewer geopolitical and human rights challenges. This aspect enhances the overall sustainability profile of all-solid-state sulfur batteries from a social responsibility perspective.
Energy density improvements in all-solid-state sulfur systems could further enhance sustainability by reducing material requirements per kilowatt-hour of storage capacity. Current research indicates potential energy densities exceeding 500 Wh/kg, which would significantly reduce the material footprint of energy storage solutions across various applications.
Despite these advantages, challenges remain regarding the long-term environmental stability of sulfur cathodes, particularly concerning potential sulfur compound formation during degradation processes. Research into encapsulation techniques and stable solid electrolyte interfaces continues to address these concerns, aiming to ensure that the environmental benefits of sulfur cathodes are maintained throughout the entire battery lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!