Comprehensive Guide to Ethyl Acetate Reactivity and Use
JUN 27, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the molecular formula CH3COOC2H5, is widely used in various industries due to its unique properties and reactivity. This colorless liquid ester is characterized by its fruity odor and low toxicity, making it a popular choice in many applications. Ethyl acetate is primarily produced through the esterification of ethanol and acetic acid, a process that involves the reaction of these two compounds in the presence of an acid catalyst.
The chemical structure of ethyl acetate consists of an ethyl group (C2H5) bonded to an acetate group (CH3COO). This arrangement gives the molecule its distinctive reactivity and physical properties. With a boiling point of 77.1°C (170.8°F) and a melting point of -83.6°C (-118.5°F), ethyl acetate exists as a liquid at room temperature, contributing to its ease of handling and use in various processes.
One of the key features of ethyl acetate is its excellent solvency power. It can dissolve a wide range of organic compounds, including many polymers, resins, and cellulose-based materials. This property makes it an invaluable solvent in industries such as paints and coatings, adhesives, and pharmaceuticals. Additionally, ethyl acetate's low boiling point and high vapor pressure facilitate its use in applications where rapid evaporation is desired, such as in nail polish removers and certain cleaning products.
In terms of reactivity, ethyl acetate exhibits behavior typical of esters. It can undergo hydrolysis in the presence of water and a catalyst, reversing the esterification process to yield ethanol and acetic acid. This reaction is particularly important in the context of biodegradation, as it allows ethyl acetate to break down into environmentally benign components. The compound can also participate in transesterification reactions, where the ethyl group is exchanged with another alcohol, leading to the formation of different esters.
From an industrial perspective, ethyl acetate plays a crucial role in various manufacturing processes. Its use as a solvent in the production of pharmaceuticals, flavorings, and perfumes is well-established. In the electronics industry, it serves as a cleaning agent for circuit boards and components. The food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. Its low toxicity and biodegradability make it an attractive option in many applications where environmental concerns are paramount.
Understanding the comprehensive properties and reactivity of ethyl acetate is essential for its effective and safe use across different sectors. As industries continue to seek more sustainable and versatile chemicals, ethyl acetate's balanced profile of reactivity, solvency, and environmental impact ensures its ongoing importance in chemical processes and product formulations.
The chemical structure of ethyl acetate consists of an ethyl group (C2H5) bonded to an acetate group (CH3COO). This arrangement gives the molecule its distinctive reactivity and physical properties. With a boiling point of 77.1°C (170.8°F) and a melting point of -83.6°C (-118.5°F), ethyl acetate exists as a liquid at room temperature, contributing to its ease of handling and use in various processes.
One of the key features of ethyl acetate is its excellent solvency power. It can dissolve a wide range of organic compounds, including many polymers, resins, and cellulose-based materials. This property makes it an invaluable solvent in industries such as paints and coatings, adhesives, and pharmaceuticals. Additionally, ethyl acetate's low boiling point and high vapor pressure facilitate its use in applications where rapid evaporation is desired, such as in nail polish removers and certain cleaning products.
In terms of reactivity, ethyl acetate exhibits behavior typical of esters. It can undergo hydrolysis in the presence of water and a catalyst, reversing the esterification process to yield ethanol and acetic acid. This reaction is particularly important in the context of biodegradation, as it allows ethyl acetate to break down into environmentally benign components. The compound can also participate in transesterification reactions, where the ethyl group is exchanged with another alcohol, leading to the formation of different esters.
From an industrial perspective, ethyl acetate plays a crucial role in various manufacturing processes. Its use as a solvent in the production of pharmaceuticals, flavorings, and perfumes is well-established. In the electronics industry, it serves as a cleaning agent for circuit boards and components. The food industry utilizes ethyl acetate as a flavoring agent and in the decaffeination of coffee and tea. Its low toxicity and biodegradability make it an attractive option in many applications where environmental concerns are paramount.
Understanding the comprehensive properties and reactivity of ethyl acetate is essential for its effective and safe use across different sectors. As industries continue to seek more sustainable and versatile chemicals, ethyl acetate's balanced profile of reactivity, solvency, and environmental impact ensures its ongoing importance in chemical processes and product formulations.
Industrial Applications
Ethyl acetate finds extensive use across various industrial sectors due to its versatile properties and relatively low cost. In the coatings industry, it serves as a crucial solvent for paints, varnishes, and lacquers. Its rapid evaporation rate and ability to dissolve a wide range of resins make it ideal for creating smooth, glossy finishes in automotive and furniture coatings. The electronics industry relies on ethyl acetate for cleaning circuit boards and removing flux residues, taking advantage of its excellent solvency and low toxicity.
The pharmaceutical sector employs ethyl acetate in the production of various medications and as an extraction solvent in drug manufacturing processes. Its low boiling point and high selectivity make it valuable for isolating and purifying pharmaceutical compounds. In the food industry, ethyl acetate is utilized as a flavoring agent and in the decaffeination of coffee and tea. Its natural occurrence in fruits and wines allows for its use as a food additive within regulatory limits.
Ethyl acetate plays a significant role in the adhesives industry, particularly in the production of flexible packaging materials. Its compatibility with cellulose-based adhesives and quick-drying properties make it essential for laminating films and creating multi-layer packaging. The printing industry also benefits from ethyl acetate's use in flexographic and rotogravure inks, where it acts as a fast-evaporating solvent that ensures rapid drying and high-quality print results.
In the fragrance and cosmetics industry, ethyl acetate serves as a solvent for perfumes and nail polish removers. Its pleasant, fruity odor and ability to blend well with other fragrances contribute to its popularity in these applications. The textile industry utilizes ethyl acetate in dyeing processes and as a carrier solvent for fabric finishes, taking advantage of its ability to penetrate fibers effectively.
The petrochemical industry employs ethyl acetate as a process solvent in various refining and separation processes. Its selective solubility properties make it useful in extracting specific compounds from complex mixtures. In the agrochemical sector, ethyl acetate finds application as a solvent for pesticides and herbicides, facilitating their formulation and application.
As environmental regulations become stricter, industries are increasingly turning to ethyl acetate as a more environmentally friendly alternative to traditional solvents. Its lower toxicity, biodegradability, and reduced VOC emissions compared to some other solvents contribute to its growing adoption across various industrial applications. This trend is likely to continue, driving further innovation in ethyl acetate-based products and processes across multiple sectors.
The pharmaceutical sector employs ethyl acetate in the production of various medications and as an extraction solvent in drug manufacturing processes. Its low boiling point and high selectivity make it valuable for isolating and purifying pharmaceutical compounds. In the food industry, ethyl acetate is utilized as a flavoring agent and in the decaffeination of coffee and tea. Its natural occurrence in fruits and wines allows for its use as a food additive within regulatory limits.
Ethyl acetate plays a significant role in the adhesives industry, particularly in the production of flexible packaging materials. Its compatibility with cellulose-based adhesives and quick-drying properties make it essential for laminating films and creating multi-layer packaging. The printing industry also benefits from ethyl acetate's use in flexographic and rotogravure inks, where it acts as a fast-evaporating solvent that ensures rapid drying and high-quality print results.
In the fragrance and cosmetics industry, ethyl acetate serves as a solvent for perfumes and nail polish removers. Its pleasant, fruity odor and ability to blend well with other fragrances contribute to its popularity in these applications. The textile industry utilizes ethyl acetate in dyeing processes and as a carrier solvent for fabric finishes, taking advantage of its ability to penetrate fibers effectively.
The petrochemical industry employs ethyl acetate as a process solvent in various refining and separation processes. Its selective solubility properties make it useful in extracting specific compounds from complex mixtures. In the agrochemical sector, ethyl acetate finds application as a solvent for pesticides and herbicides, facilitating their formulation and application.
As environmental regulations become stricter, industries are increasingly turning to ethyl acetate as a more environmentally friendly alternative to traditional solvents. Its lower toxicity, biodegradability, and reduced VOC emissions compared to some other solvents contribute to its growing adoption across various industrial applications. This trend is likely to continue, driving further innovation in ethyl acetate-based products and processes across multiple sectors.
Chemical Properties
Ethyl acetate, a versatile organic compound, exhibits a range of chemical properties that contribute to its widespread use in various industries. Its molecular formula, C4H8O2, reveals a structure consisting of an ethyl group attached to an acetate group. This ester has a relatively low molecular weight of 88.11 g/mol, which contributes to its high volatility.
One of the most notable properties of ethyl acetate is its low boiling point of 77.1°C (170.8°F) at standard atmospheric pressure. This characteristic makes it an excellent solvent for many organic compounds and facilitates its use in various applications, including as a component in nail polish removers and in the production of perfumes and flavorings.
Ethyl acetate is a colorless liquid at room temperature, with a characteristic sweet, fruity odor often described as similar to pear drops. Its pleasant scent contributes to its use in the food and fragrance industries. The compound has a density of 0.902 g/cm³ at 20°C, slightly less dense than water, which explains its tendency to float on water surfaces.
In terms of solubility, ethyl acetate demonstrates moderate solubility in water, with approximately 8.3 g dissolving in 100 mL of water at 20°C. However, it is highly soluble in most organic solvents, including alcohols, ketones, and other esters. This property makes it an excellent solvent for a wide range of organic compounds and polymers.
The reactivity of ethyl acetate is primarily due to its ester functional group. It undergoes hydrolysis in the presence of water, especially under acidic or basic conditions, yielding ethanol and acetic acid. This reaction is reversible and forms the basis for the industrial production of ethyl acetate through esterification of ethanol and acetic acid.
Ethyl acetate also participates in transesterification reactions, where the ethyl group can be exchanged with other alcohols. This property is utilized in various organic syntheses and in the production of other esters. Additionally, the compound can undergo oxidation reactions, particularly in the presence of strong oxidizing agents.
From a safety perspective, ethyl acetate is highly flammable with a flash point of -4°C (25°F), necessitating careful handling and storage. It has a relatively low toxicity compared to many other organic solvents, but prolonged exposure can cause irritation to the eyes, skin, and respiratory system. Its low odor threshold allows for early detection of potential leaks or spills, enhancing workplace safety in industrial settings.
One of the most notable properties of ethyl acetate is its low boiling point of 77.1°C (170.8°F) at standard atmospheric pressure. This characteristic makes it an excellent solvent for many organic compounds and facilitates its use in various applications, including as a component in nail polish removers and in the production of perfumes and flavorings.
Ethyl acetate is a colorless liquid at room temperature, with a characteristic sweet, fruity odor often described as similar to pear drops. Its pleasant scent contributes to its use in the food and fragrance industries. The compound has a density of 0.902 g/cm³ at 20°C, slightly less dense than water, which explains its tendency to float on water surfaces.
In terms of solubility, ethyl acetate demonstrates moderate solubility in water, with approximately 8.3 g dissolving in 100 mL of water at 20°C. However, it is highly soluble in most organic solvents, including alcohols, ketones, and other esters. This property makes it an excellent solvent for a wide range of organic compounds and polymers.
The reactivity of ethyl acetate is primarily due to its ester functional group. It undergoes hydrolysis in the presence of water, especially under acidic or basic conditions, yielding ethanol and acetic acid. This reaction is reversible and forms the basis for the industrial production of ethyl acetate through esterification of ethanol and acetic acid.
Ethyl acetate also participates in transesterification reactions, where the ethyl group can be exchanged with other alcohols. This property is utilized in various organic syntheses and in the production of other esters. Additionally, the compound can undergo oxidation reactions, particularly in the presence of strong oxidizing agents.
From a safety perspective, ethyl acetate is highly flammable with a flash point of -4°C (25°F), necessitating careful handling and storage. It has a relatively low toxicity compared to many other organic solvents, but prolonged exposure can cause irritation to the eyes, skin, and respiratory system. Its low odor threshold allows for early detection of potential leaks or spills, enhancing workplace safety in industrial settings.
Reaction Mechanisms
01 Esterification reactions
Ethyl acetate is commonly produced through esterification reactions, typically involving the reaction of ethanol with acetic acid or acetic anhydride. This process can be catalyzed by various acids or enzymes to improve yield and efficiency. The reactivity of ethyl acetate in esterification is crucial for its industrial production and applications in organic synthesis.- Esterification reactions: Ethyl acetate is commonly produced through esterification reactions, typically involving the reaction of ethanol with acetic acid or acetic anhydride. This process can be catalyzed by various acids or enzymes to improve yield and efficiency. The reactivity of ethyl acetate in esterification is crucial for its industrial production and applications in organic synthesis.
- Hydrolysis of ethyl acetate: Ethyl acetate undergoes hydrolysis in the presence of water, especially under acidic or basic conditions. This reaction results in the formation of ethanol and acetic acid. The hydrolysis of ethyl acetate is reversible and plays a significant role in various industrial processes and chemical equilibrium studies.
- Use as a solvent in chemical reactions: Ethyl acetate is widely used as a solvent in various chemical reactions due to its moderate polarity and low boiling point. It can dissolve a wide range of organic compounds and is often employed in extractions, purifications, and as a reaction medium. Its reactivity as a solvent can influence the outcome of chemical processes and syntheses.
- Transesterification reactions: Ethyl acetate can participate in transesterification reactions, where the ethoxy group is exchanged with another alcohol. This reactivity is important in the synthesis of various esters and in biodiesel production. The process can be catalyzed by acids, bases, or enzymes, and the reactivity of ethyl acetate in these reactions is influenced by reaction conditions and catalysts.
- Decomposition and pyrolysis: Under high temperatures or in the presence of certain catalysts, ethyl acetate can undergo decomposition or pyrolysis. These processes can lead to the formation of various products such as ethylene, acetic acid, and carbon oxides. Understanding the reactivity of ethyl acetate under these conditions is crucial for industrial processes and safety considerations in chemical handling and storage.
02 Hydrolysis of ethyl acetate
Ethyl acetate undergoes hydrolysis in the presence of water, especially under acidic or basic conditions. This reaction results in the formation of ethanol and acetic acid. The hydrolysis of ethyl acetate is reversible and plays a significant role in various industrial processes and chemical equilibrium studies.Expand Specific Solutions03 Use as a solvent in chemical reactions
Ethyl acetate is widely used as a solvent in various chemical reactions due to its moderate polarity and low boiling point. Its reactivity as a solvent can affect the outcome of reactions, particularly in organic synthesis and extraction processes. The solvent properties of ethyl acetate make it valuable in industries such as pharmaceuticals and coatings.Expand Specific Solutions04 Transesterification reactions
Ethyl acetate participates in transesterification reactions, where the ethoxy group is exchanged with another alcohol. This reactivity is important in the synthesis of various esters and in biodiesel production. The transesterification of ethyl acetate can be catalyzed by acids, bases, or enzymes, depending on the specific application and desired products.Expand Specific Solutions05 Decomposition and thermal reactivity
Under certain conditions, ethyl acetate can undergo decomposition or exhibit thermal reactivity. This includes pyrolysis at high temperatures or reactions in the presence of strong oxidizing agents. Understanding the decomposition and thermal behavior of ethyl acetate is crucial for safety considerations in storage, handling, and industrial processes involving this compound.Expand Specific Solutions
Key Manufacturers
The ethyl acetate market is in a mature stage, with a global market size estimated to reach $4.3 billion by 2027. The technology for producing ethyl acetate is well-established, with major players like Celanese, BASF, and Wacker Chemie dominating the market. However, there is growing interest in developing more sustainable production methods, as evidenced by companies like Viridis Chemical and Greenyug focusing on bio-based ethyl acetate. The competitive landscape is characterized by a mix of large chemical conglomerates and specialized manufacturers, with increasing emphasis on eco-friendly processes and applications in various industries such as pharmaceuticals, food, and electronics.
Celanese International Corp.
Technical Solution: Celanese has developed advanced catalytic processes for ethyl acetate production, focusing on improving yield and selectivity. Their technology utilizes a novel heterogeneous catalyst system that enhances the esterification reaction between ethanol and acetic acid[1]. This process operates at lower temperatures and pressures compared to traditional methods, resulting in reduced energy consumption and improved process economics[3]. Celanese has also implemented innovative purification techniques, such as reactive distillation, to achieve high-purity ethyl acetate with minimal byproduct formation[5].
Strengths: High yield and selectivity, energy-efficient process, reduced byproduct formation. Weaknesses: Potential higher initial capital costs for catalyst development and specialized equipment.
BASF Corp.
Technical Solution: BASF has pioneered a green chemistry approach to ethyl acetate production, focusing on sustainable feedstocks and process intensification. Their technology utilizes bio-based ethanol and acetic acid derived from renewable resources[2]. The company has developed a highly efficient continuous flow reactor system that maximizes conversion rates while minimizing residence time[4]. BASF's process incorporates advanced process control systems and real-time monitoring to optimize reaction conditions and maintain product quality. Additionally, they have implemented heat integration strategies to recover and reuse energy throughout the production process, significantly reducing overall energy consumption[6].
Strengths: Sustainable feedstock utilization, high efficiency continuous process, advanced process control. Weaknesses: Potential higher costs associated with bio-based raw materials, dependency on renewable resource availability.
Safety Considerations
A process for reactive distillation of a carboxylic acid
PatentInactiveEP1989168B1
Innovation
- Supplying an inert entrainer to the bottom part of the reactive distillation column, where it acts as a mass separation agent to separate water from alcohol, allowing for higher carboxylic acid conversion, reduced ester contamination, and lower energy requirements.
Ethyl Acetate Production
PatentActiveUS20120178962A1
Innovation
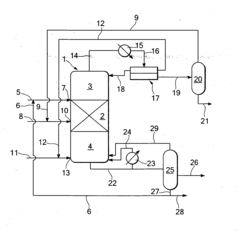
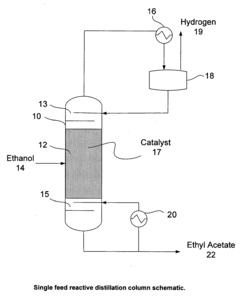
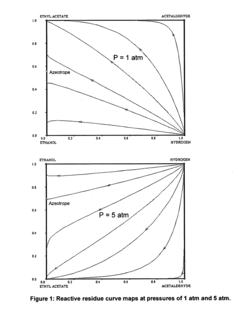
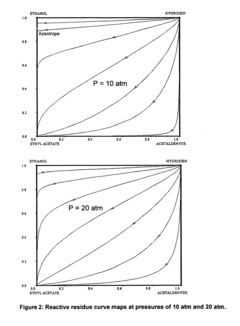
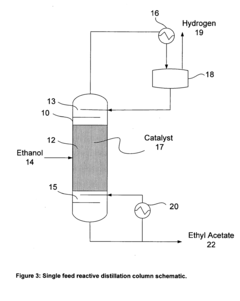
- A reactive distillation process using a single reactive distillation column where ethanol is dehydrogenated over a catalyst to produce ethyl acetate and hydrogen, with optional hydrogenation of byproducts to simplify separation and achieve high purity ethyl acetate, utilizing catalysts like copper, ruthenium, and platinum supported on materials like carbon or alumina.
Emerging Technologies
Ethyl acetate, a versatile organic compound, is at the forefront of several emerging technologies due to its unique properties and reactivity. One of the most promising areas of development is in the field of green chemistry, where ethyl acetate is being explored as a more environmentally friendly solvent alternative to traditional petrochemical-based solvents. Its low toxicity and biodegradability make it an attractive option for sustainable industrial processes.
In the realm of advanced materials, ethyl acetate is playing a crucial role in the development of novel polymer composites. Researchers are utilizing its solvent properties to create innovative manufacturing techniques for high-performance materials with enhanced mechanical and thermal properties. These materials have potential applications in aerospace, automotive, and construction industries, offering lighter and more durable alternatives to conventional materials.
Nanotechnology is another field where ethyl acetate is making significant strides. Its ability to dissolve a wide range of organic compounds is being harnessed in the synthesis of nanoparticles and nanostructures. These nanomaterials have potential applications in drug delivery systems, advanced electronics, and energy storage devices. The controlled reactivity of ethyl acetate allows for precise manipulation of nanostructure formation, leading to tailored properties for specific applications.
In the pharmaceutical industry, ethyl acetate is being explored for its potential in continuous flow chemistry. This emerging technology allows for more efficient and safer production of pharmaceutical compounds. The use of ethyl acetate in these systems can lead to improved reaction kinetics, higher yields, and reduced environmental impact compared to traditional batch processes.
The food and beverage industry is also benefiting from emerging technologies involving ethyl acetate. Advanced extraction techniques using supercritical ethyl acetate are being developed for the isolation of natural compounds from plant materials. This method offers advantages over traditional extraction methods, including higher selectivity and purity of extracted compounds, with potential applications in the production of nutraceuticals and functional foods.
Lastly, in the field of energy storage, ethyl acetate is being investigated as a component in next-generation electrolytes for lithium-ion batteries. Its unique combination of properties, including high dielectric constant and low viscosity, makes it a promising candidate for improving battery performance and safety. Research in this area could lead to the development of more efficient and longer-lasting energy storage solutions for electric vehicles and renewable energy systems.
In the realm of advanced materials, ethyl acetate is playing a crucial role in the development of novel polymer composites. Researchers are utilizing its solvent properties to create innovative manufacturing techniques for high-performance materials with enhanced mechanical and thermal properties. These materials have potential applications in aerospace, automotive, and construction industries, offering lighter and more durable alternatives to conventional materials.
Nanotechnology is another field where ethyl acetate is making significant strides. Its ability to dissolve a wide range of organic compounds is being harnessed in the synthesis of nanoparticles and nanostructures. These nanomaterials have potential applications in drug delivery systems, advanced electronics, and energy storage devices. The controlled reactivity of ethyl acetate allows for precise manipulation of nanostructure formation, leading to tailored properties for specific applications.
In the pharmaceutical industry, ethyl acetate is being explored for its potential in continuous flow chemistry. This emerging technology allows for more efficient and safer production of pharmaceutical compounds. The use of ethyl acetate in these systems can lead to improved reaction kinetics, higher yields, and reduced environmental impact compared to traditional batch processes.
The food and beverage industry is also benefiting from emerging technologies involving ethyl acetate. Advanced extraction techniques using supercritical ethyl acetate are being developed for the isolation of natural compounds from plant materials. This method offers advantages over traditional extraction methods, including higher selectivity and purity of extracted compounds, with potential applications in the production of nutraceuticals and functional foods.
Lastly, in the field of energy storage, ethyl acetate is being investigated as a component in next-generation electrolytes for lithium-ion batteries. Its unique combination of properties, including high dielectric constant and low viscosity, makes it a promising candidate for improving battery performance and safety. Research in this area could lead to the development of more efficient and longer-lasting energy storage solutions for electric vehicles and renewable energy systems.
Regulatory Framework
The regulatory framework surrounding ethyl acetate is complex and multifaceted, reflecting the compound's widespread use and potential hazards. In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA), which requires manufacturers and importers to report production volumes, uses, and potential exposures. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in workplace environments, setting the standard at 400 parts per million (ppm) as an 8-hour time-weighted average.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the use and import of ethyl acetate within EU member states. Under REACH, manufacturers and importers must register ethyl acetate with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The EU has also classified ethyl acetate as a volatile organic compound (VOC), subject to emissions controls under the Industrial Emissions Directive.
In the realm of transportation, ethyl acetate is classified as a flammable liquid under various international agreements, including the United Nations Recommendations on the Transport of Dangerous Goods. This classification imposes specific packaging, labeling, and handling requirements for shipment by road, rail, sea, and air. The International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations provide detailed guidelines for the safe transport of ethyl acetate across borders.
Food safety regulations also play a crucial role in the regulatory landscape of ethyl acetate. In the United States, the Food and Drug Administration (FDA) has approved ethyl acetate as a food additive and solvent, listing it as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and deemed it safe for use as a food flavoring substance and extraction solvent.
Environmental regulations addressing air quality and waste management also impact the use and disposal of ethyl acetate. Many countries have implemented strict controls on VOC emissions from industrial processes involving ethyl acetate, requiring the use of abatement technologies or process modifications to minimize environmental impact. Additionally, waste streams containing ethyl acetate are often subject to special handling and disposal requirements to prevent soil and groundwater contamination.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the use and import of ethyl acetate within EU member states. Under REACH, manufacturers and importers must register ethyl acetate with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The EU has also classified ethyl acetate as a volatile organic compound (VOC), subject to emissions controls under the Industrial Emissions Directive.
In the realm of transportation, ethyl acetate is classified as a flammable liquid under various international agreements, including the United Nations Recommendations on the Transport of Dangerous Goods. This classification imposes specific packaging, labeling, and handling requirements for shipment by road, rail, sea, and air. The International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations provide detailed guidelines for the safe transport of ethyl acetate across borders.
Food safety regulations also play a crucial role in the regulatory landscape of ethyl acetate. In the United States, the Food and Drug Administration (FDA) has approved ethyl acetate as a food additive and solvent, listing it as Generally Recognized as Safe (GRAS) when used in accordance with good manufacturing practices. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and deemed it safe for use as a food flavoring substance and extraction solvent.
Environmental regulations addressing air quality and waste management also impact the use and disposal of ethyl acetate. Many countries have implemented strict controls on VOC emissions from industrial processes involving ethyl acetate, requiring the use of abatement technologies or process modifications to minimize environmental impact. Additionally, waste streams containing ethyl acetate are often subject to special handling and disposal requirements to prevent soil and groundwater contamination.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!