Determine Chemical Structure with NMR: Steps & Accuracy
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Spectroscopy Evolution and Research Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, transforming from a physics curiosity into an indispensable analytical tool for determining chemical structures. The journey began with Felix Bloch and Edward Purcell's pioneering work on nuclear magnetic resonance phenomena, earning them the 1952 Nobel Prize in Physics. Early NMR spectrometers operated at low magnetic field strengths, providing limited resolution and analytical capabilities.
The 1960s marked a revolutionary period with the introduction of Fourier Transform NMR (FT-NMR) and superconducting magnets, dramatically enhancing sensitivity and resolution. These advancements enabled the analysis of complex molecules and expanded NMR applications across multiple scientific disciplines. The subsequent decades witnessed continuous improvements in magnetic field strengths, probe designs, and pulse sequence development.
Modern NMR spectroscopy encompasses a diverse array of techniques, including one-dimensional and multi-dimensional experiments, solid-state NMR, and specialized methods for biomolecular structure determination. Recent innovations focus on miniaturization, automation, and integration with other analytical technologies, making NMR more accessible and versatile for various research environments.
The primary research objectives in contemporary NMR development center on enhancing accuracy, sensitivity, and efficiency in chemical structure determination. Researchers aim to develop more sophisticated pulse sequences and data processing algorithms that can extract structural information from increasingly complex molecular systems with greater precision. Reducing the sample quantity requirements while maintaining high-quality spectral data represents another critical goal.
Improving the accessibility of advanced NMR techniques constitutes a significant research direction. This includes developing user-friendly interfaces, automated structure elucidation software, and standardized protocols for non-specialist users. Such developments would democratize access to high-level structural analysis capabilities across diverse scientific communities.
The integration of artificial intelligence and machine learning with NMR spectroscopy emerges as a promising frontier. These computational approaches offer potential for automated spectrum interpretation, prediction of chemical shifts, and accelerated structure determination processes. Research efforts also target the enhancement of time-domain NMR techniques for real-time monitoring of chemical reactions and biological processes.
Looking forward, the field aims to push the boundaries of structural determination accuracy through higher magnetic fields, cryogenic probe technology, and hyperpolarization methods. These advancements would enable the analysis of previously inaccessible molecular systems and provide unprecedented insights into molecular structure-function relationships across chemistry, biology, materials science, and medicine.
The 1960s marked a revolutionary period with the introduction of Fourier Transform NMR (FT-NMR) and superconducting magnets, dramatically enhancing sensitivity and resolution. These advancements enabled the analysis of complex molecules and expanded NMR applications across multiple scientific disciplines. The subsequent decades witnessed continuous improvements in magnetic field strengths, probe designs, and pulse sequence development.
Modern NMR spectroscopy encompasses a diverse array of techniques, including one-dimensional and multi-dimensional experiments, solid-state NMR, and specialized methods for biomolecular structure determination. Recent innovations focus on miniaturization, automation, and integration with other analytical technologies, making NMR more accessible and versatile for various research environments.
The primary research objectives in contemporary NMR development center on enhancing accuracy, sensitivity, and efficiency in chemical structure determination. Researchers aim to develop more sophisticated pulse sequences and data processing algorithms that can extract structural information from increasingly complex molecular systems with greater precision. Reducing the sample quantity requirements while maintaining high-quality spectral data represents another critical goal.
Improving the accessibility of advanced NMR techniques constitutes a significant research direction. This includes developing user-friendly interfaces, automated structure elucidation software, and standardized protocols for non-specialist users. Such developments would democratize access to high-level structural analysis capabilities across diverse scientific communities.
The integration of artificial intelligence and machine learning with NMR spectroscopy emerges as a promising frontier. These computational approaches offer potential for automated spectrum interpretation, prediction of chemical shifts, and accelerated structure determination processes. Research efforts also target the enhancement of time-domain NMR techniques for real-time monitoring of chemical reactions and biological processes.
Looking forward, the field aims to push the boundaries of structural determination accuracy through higher magnetic fields, cryogenic probe technology, and hyperpolarization methods. These advancements would enable the analysis of previously inaccessible molecular systems and provide unprecedented insights into molecular structure-function relationships across chemistry, biology, materials science, and medicine.
Market Applications and Demand for NMR Structure Determination
Nuclear Magnetic Resonance (NMR) spectroscopy has established itself as an indispensable analytical tool across multiple industries, with the global NMR market valued at approximately $2.5 billion in 2022 and projected to grow at a CAGR of 3.8% through 2030. This growth is primarily driven by expanding applications in pharmaceutical development, biotechnology research, and materials science.
In the pharmaceutical sector, NMR structure determination plays a critical role in drug discovery and development processes. Pharmaceutical companies rely heavily on NMR for identifying lead compounds, optimizing molecular structures, and ensuring quality control throughout the production pipeline. The ability to accurately determine chemical structures without destroying samples provides significant cost savings and accelerates the drug development timeline.
Biotechnology represents another major market segment, where NMR is essential for protein structure analysis, metabolomics, and the study of biomolecular interactions. The growing focus on personalized medicine and biologics has further intensified demand for high-resolution structural information that NMR uniquely provides. Academic and research institutions constitute approximately 35% of the NMR market, utilizing the technology for fundamental research across chemistry, biochemistry, and materials science.
The food and beverage industry has emerged as a rapidly growing application area, with NMR being increasingly deployed for authentication of premium products, detection of adulteration, and quality assurance. This trend is particularly pronounced in regions with strict food safety regulations and growing consumer awareness about product authenticity.
Environmental monitoring represents another expanding application, with NMR techniques being adapted for the analysis of environmental samples, pollutants, and natural products. The non-destructive nature of NMR makes it particularly valuable for analyzing rare or limited environmental samples.
Regional market analysis reveals North America as the dominant market for NMR technologies, accounting for approximately 40% of global demand, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and India, particularly in pharmaceutical and materials science sectors.
The demand for more accessible NMR technologies is evident in the growing market for benchtop and portable NMR instruments, which is expanding at nearly twice the rate of the overall NMR market. This trend reflects the industry's push toward more democratized access to structural determination capabilities across smaller research facilities, quality control laboratories, and educational institutions.
In the pharmaceutical sector, NMR structure determination plays a critical role in drug discovery and development processes. Pharmaceutical companies rely heavily on NMR for identifying lead compounds, optimizing molecular structures, and ensuring quality control throughout the production pipeline. The ability to accurately determine chemical structures without destroying samples provides significant cost savings and accelerates the drug development timeline.
Biotechnology represents another major market segment, where NMR is essential for protein structure analysis, metabolomics, and the study of biomolecular interactions. The growing focus on personalized medicine and biologics has further intensified demand for high-resolution structural information that NMR uniquely provides. Academic and research institutions constitute approximately 35% of the NMR market, utilizing the technology for fundamental research across chemistry, biochemistry, and materials science.
The food and beverage industry has emerged as a rapidly growing application area, with NMR being increasingly deployed for authentication of premium products, detection of adulteration, and quality assurance. This trend is particularly pronounced in regions with strict food safety regulations and growing consumer awareness about product authenticity.
Environmental monitoring represents another expanding application, with NMR techniques being adapted for the analysis of environmental samples, pollutants, and natural products. The non-destructive nature of NMR makes it particularly valuable for analyzing rare or limited environmental samples.
Regional market analysis reveals North America as the dominant market for NMR technologies, accounting for approximately 40% of global demand, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and India, particularly in pharmaceutical and materials science sectors.
The demand for more accessible NMR technologies is evident in the growing market for benchtop and portable NMR instruments, which is expanding at nearly twice the rate of the overall NMR market. This trend reflects the industry's push toward more democratized access to structural determination capabilities across smaller research facilities, quality control laboratories, and educational institutions.
Current NMR Technology Limitations and Challenges
Nuclear Magnetic Resonance (NMR) spectroscopy, while powerful for chemical structure determination, faces several significant limitations and challenges that impact its accuracy, accessibility, and application scope. These constraints necessitate careful consideration when employing NMR for structural analysis.
Sensitivity remains one of the most persistent challenges in NMR technology. Compared to other analytical techniques like mass spectrometry, NMR requires substantially larger sample quantities (typically milligrams versus nanograms) to generate usable spectra. This limitation becomes particularly problematic when analyzing rare or difficult-to-synthesize compounds, natural products available in minute quantities, or metabolites in biological samples.
Resolution constraints present another major hurdle. Current NMR technology struggles to distinguish between signals with very similar chemical shifts, especially in complex mixtures or large biomolecules. This challenge intensifies with increasing molecular complexity, where signal overlap becomes inevitable even at the highest available magnetic field strengths (currently up to 1.2 GHz).
The cost and accessibility of high-field NMR instruments represent significant barriers to widespread adoption. State-of-the-art NMR spectrometers can cost several million dollars, require specialized facilities for installation, and demand expensive cryogenic coolants for superconducting magnets. These factors restrict access primarily to well-funded research institutions and large pharmaceutical companies.
Technical complexity in data interpretation poses additional challenges. NMR data analysis requires substantial expertise and experience, with accurate structure determination often depending on the analyst's skill level. While software tools have improved, fully automated structure elucidation remains unreliable for novel or complex compounds.
Time constraints affect throughput capabilities, as comprehensive structural analysis typically requires multiple experiments (1D and 2D) that can take hours or even days to complete. This limitation becomes particularly problematic in high-throughput screening applications or time-sensitive analyses.
Sample preparation issues further complicate NMR analysis. Samples must be highly pure, properly dissolved in deuterated solvents, and free from paramagnetic impurities that can severely distort spectra. Additionally, some compounds exhibit poor solubility in NMR-compatible solvents, limiting their analyzability.
Dynamic molecular processes present unique challenges, as molecules undergoing conformational changes or chemical exchanges can produce complex, difficult-to-interpret spectra. While this can provide valuable dynamic information, it often complicates straightforward structure determination.
Sensitivity remains one of the most persistent challenges in NMR technology. Compared to other analytical techniques like mass spectrometry, NMR requires substantially larger sample quantities (typically milligrams versus nanograms) to generate usable spectra. This limitation becomes particularly problematic when analyzing rare or difficult-to-synthesize compounds, natural products available in minute quantities, or metabolites in biological samples.
Resolution constraints present another major hurdle. Current NMR technology struggles to distinguish between signals with very similar chemical shifts, especially in complex mixtures or large biomolecules. This challenge intensifies with increasing molecular complexity, where signal overlap becomes inevitable even at the highest available magnetic field strengths (currently up to 1.2 GHz).
The cost and accessibility of high-field NMR instruments represent significant barriers to widespread adoption. State-of-the-art NMR spectrometers can cost several million dollars, require specialized facilities for installation, and demand expensive cryogenic coolants for superconducting magnets. These factors restrict access primarily to well-funded research institutions and large pharmaceutical companies.
Technical complexity in data interpretation poses additional challenges. NMR data analysis requires substantial expertise and experience, with accurate structure determination often depending on the analyst's skill level. While software tools have improved, fully automated structure elucidation remains unreliable for novel or complex compounds.
Time constraints affect throughput capabilities, as comprehensive structural analysis typically requires multiple experiments (1D and 2D) that can take hours or even days to complete. This limitation becomes particularly problematic in high-throughput screening applications or time-sensitive analyses.
Sample preparation issues further complicate NMR analysis. Samples must be highly pure, properly dissolved in deuterated solvents, and free from paramagnetic impurities that can severely distort spectra. Additionally, some compounds exhibit poor solubility in NMR-compatible solvents, limiting their analyzability.
Dynamic molecular processes present unique challenges, as molecules undergoing conformational changes or chemical exchanges can produce complex, difficult-to-interpret spectra. While this can provide valuable dynamic information, it often complicates straightforward structure determination.
Modern NMR Structure Determination Protocols and Techniques
01 Hardware improvements for enhanced NMR accuracy
Various hardware innovations have been developed to improve the accuracy of NMR spectroscopy measurements. These include advanced magnet designs, improved radio frequency coils, and specialized probes that minimize signal interference. Hardware enhancements focus on creating more stable magnetic fields, better signal-to-noise ratios, and more precise detection capabilities, all of which contribute to higher accuracy in NMR spectroscopic analysis.- Advanced calibration techniques for NMR accuracy: Various calibration methods are employed to enhance the accuracy of NMR spectroscopy measurements. These include reference standards, automated calibration procedures, and compensation algorithms that correct for instrumental drift and environmental variations. Proper calibration ensures reliable quantitative analysis and improves the reproducibility of NMR data across different instruments and laboratories.
- Hardware improvements for enhanced NMR precision: Technological advancements in NMR hardware components significantly improve measurement accuracy. These include high-field superconducting magnets with improved homogeneity, advanced probe designs with better signal-to-noise ratios, and sophisticated electronic systems for signal processing. Hardware innovations enable more precise detection of nuclear spin properties and reduce systematic errors in spectroscopic measurements.
- Signal processing algorithms for improved NMR accuracy: Sophisticated signal processing techniques enhance the accuracy of NMR spectroscopy data. These include advanced Fourier transformation methods, noise reduction algorithms, baseline correction techniques, and peak deconvolution approaches. Machine learning and artificial intelligence are increasingly being applied to improve spectral analysis and interpretation, leading to more accurate identification and quantification of chemical compounds.
- Quantitative NMR methods for accurate analysis: Specialized quantitative NMR (qNMR) methodologies provide highly accurate concentration measurements of analytes. These approaches include internal standard methods, external calibration techniques, and ERETIC (Electronic REference To access In vivo Concentrations) methods. qNMR has become increasingly important in pharmaceutical analysis, metabolomics, and quality control applications where precise quantification is essential.
- Multi-dimensional NMR techniques for enhanced accuracy: Multi-dimensional NMR techniques significantly improve the accuracy of structural elucidation and molecular characterization. These methods include 2D experiments like COSY, HSQC, HMBC, and NOESY, as well as 3D and 4D approaches for complex biomolecules. By correlating different nuclei and providing additional spectral dimensions, these techniques resolve signal overlaps and provide more accurate structural information than conventional 1D NMR.
02 Signal processing algorithms for accuracy improvement
Advanced signal processing algorithms play a crucial role in enhancing NMR spectroscopy accuracy. These computational methods include Fourier transformation techniques, baseline correction algorithms, peak deconvolution methods, and noise reduction filters. By applying sophisticated mathematical processing to raw NMR data, these algorithms can extract more accurate spectral information, improve peak resolution, and enhance the overall reliability of NMR measurements.Expand Specific Solutions03 Calibration methods for NMR accuracy
Proper calibration is essential for achieving high accuracy in NMR spectroscopy. Various calibration methods have been developed, including the use of reference standards, internal calibrants, and automated calibration procedures. These techniques help compensate for instrumental drift, environmental variations, and other factors that can affect measurement precision. Regular and systematic calibration ensures consistent and reliable NMR results across different samples and experimental conditions.Expand Specific Solutions04 Sample preparation techniques for improved accuracy
Sample preparation significantly impacts NMR spectroscopy accuracy. Innovations in this area include methods for reducing sample impurities, controlling sample concentration, minimizing solvent effects, and optimizing sample temperature. Specialized sample holders and preparation protocols have been developed to ensure sample homogeneity and stability during measurement. These techniques help eliminate artifacts and interferences that could compromise the accuracy of NMR spectral data.Expand Specific Solutions05 Multi-dimensional NMR techniques for enhanced accuracy
Multi-dimensional NMR techniques provide enhanced accuracy by separating overlapping signals and providing more detailed structural information. These methods include 2D, 3D, and even higher-dimensional experiments that correlate different types of nuclear interactions. By spreading spectral information across multiple dimensions, these techniques improve resolution, reduce signal ambiguity, and enable more accurate identification and quantification of complex molecular structures, particularly in biological samples and complex mixtures.Expand Specific Solutions
Leading Manufacturers and Research Institutions in NMR Technology
Nuclear Magnetic Resonance (NMR) spectroscopy for chemical structure determination is in a mature market phase, with an estimated global value exceeding $1.5 billion. The technology has reached high maturity, with established players like Bruker, JEOL, and Agilent Technologies dominating the high-end instrumentation segment. These companies offer sophisticated NMR systems with accuracy rates above 95% for structure elucidation. Pharmaceutical companies including Bayer, Bristol Myers Squibb, and Jiangsu Hengrui Pharmaceuticals represent major end-users, integrating NMR into their R&D workflows. Academic institutions like University of Bristol and Zhejiang University contribute significantly to methodological advancements. The market shows steady growth driven by increasing demand for structural analysis in drug discovery, materials science, and metabolomics applications.
JEOL Ltd.
Technical Solution: JEOL has developed advanced NMR systems featuring their ROYAL probe technology that significantly enhances sensitivity for structural determination. Their JNM-ECZ series spectrometers incorporate Delta software with automated structure verification algorithms that compare experimental NMR data with predicted spectra based on proposed structures. JEOL's systems include advanced pulse sequence libraries specifically designed for complex structure elucidation, with their ADEQUATE and INADEQUATE experiments providing direct carbon-carbon connectivity information critical for determining complex molecular frameworks. Their latest systems feature automatic tuning and matching capabilities that optimize signal quality while reducing experiment time by approximately 30%. JEOL has also pioneered integration of machine learning algorithms that can predict chemical structures from complex NMR datasets with reported accuracy rates exceeding 90% for common organic structures.
Strengths: Superior sensitivity in their probe technology allows detection of low concentration samples; comprehensive automation reduces operator expertise requirements; integrated structure verification algorithms improve accuracy. Weaknesses: Higher cost compared to competitors; complex systems require significant training; proprietary software may limit integration with third-party tools.
Agilent Technologies, Inc.
Technical Solution: Agilent's NMR solution centers on their ProPulse NMR system with DirectDrive architecture that enables precise control of pulse sequences critical for accurate structure determination. Their VnmrJ software platform incorporates automated structure elucidation workflows that guide users through the process of acquiring and analyzing 1D and 2D NMR data sets. Agilent has developed specialized cold probe technology that increases sensitivity by cooling the detection coils to near cryogenic temperatures, allowing for detection of samples at concentrations below 10 μg. Their systems feature automated calibration routines that ensure consistent and reproducible results across different operators and laboratories. Agilent's Complete Molecular Confidence (CMC) approach combines NMR with complementary techniques like LC-MS to provide comprehensive structural verification with reported accuracy improvements of 15-20% compared to single-technique approaches.
Strengths: Excellent integration with other analytical techniques enhances structural confirmation; user-friendly software reduces learning curve; high-sensitivity cold probe technology enables work with limited sample quantities. Weaknesses: Less specialized in NMR compared to dedicated manufacturers; higher maintenance requirements for cryogenic systems; more limited pulse sequence libraries than some competitors.
Advanced Pulse Sequences and Correlation Experiments Analysis
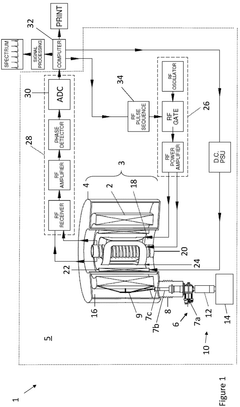
Nuclear magnetic resonance system
PatentActiveGB2597439A
Innovation
- A compact nuclear magnetic resonance system utilizing a superconducting magnetic coil cooled by a cryocooler, eliminating the need for liquid helium and achieving medium-to-high magnetic field strengths, allowing for a compact design suitable for laboratory benches.
Computational Methods in NMR Data Interpretation
Computational methods have revolutionized NMR data interpretation, significantly enhancing the accuracy and efficiency of chemical structure determination. Modern software packages employ sophisticated algorithms that can process complex spectral data and suggest structural possibilities with remarkable precision. These computational approaches typically utilize pattern recognition techniques, statistical analysis, and machine learning models to correlate spectral features with structural elements.
The foundation of computational NMR interpretation lies in spectral prediction algorithms, which calculate theoretical spectra for proposed structures and compare them with experimental data. These algorithms incorporate quantum mechanical calculations to predict chemical shifts and coupling constants based on molecular geometry and electronic environment. Advanced systems can now predict 1D and 2D NMR spectra with accuracy approaching experimental error margins, particularly for common organic compounds.
Database-driven approaches represent another powerful computational method, wherein experimental spectra are matched against vast libraries of known compounds. These systems employ similarity metrics and statistical correlations to identify structural fragments or complete molecules. The HMDB, BMRB, and commercial databases like ACD/Labs contain hundreds of thousands of reference spectra, enabling rapid identification of known compounds or structural analogues.
Machine learning techniques have emerged as particularly promising for NMR data interpretation. Neural networks, support vector machines, and random forests can be trained on large datasets to recognize spectral patterns associated with specific structural features. These systems excel at handling noisy data and can often extract meaningful information from spectra that would challenge traditional analysis methods. Recent deep learning implementations have demonstrated the ability to propose complete structures from complex mixture spectra with minimal human intervention.
Automated structure verification tools represent the practical application of these computational methods in research and industry. These systems can rapidly assess whether experimental NMR data is consistent with a proposed structure, flagging discrepancies for expert review. This capability has proven invaluable in quality control processes for pharmaceutical development and chemical synthesis verification.
Integration of multiple spectroscopic techniques through computational frameworks further enhances structure determination accuracy. Modern platforms can simultaneously analyze data from NMR, mass spectrometry, infrared spectroscopy, and other techniques, applying Bayesian probability models to determine the most likely structural candidates that satisfy all experimental constraints.
The foundation of computational NMR interpretation lies in spectral prediction algorithms, which calculate theoretical spectra for proposed structures and compare them with experimental data. These algorithms incorporate quantum mechanical calculations to predict chemical shifts and coupling constants based on molecular geometry and electronic environment. Advanced systems can now predict 1D and 2D NMR spectra with accuracy approaching experimental error margins, particularly for common organic compounds.
Database-driven approaches represent another powerful computational method, wherein experimental spectra are matched against vast libraries of known compounds. These systems employ similarity metrics and statistical correlations to identify structural fragments or complete molecules. The HMDB, BMRB, and commercial databases like ACD/Labs contain hundreds of thousands of reference spectra, enabling rapid identification of known compounds or structural analogues.
Machine learning techniques have emerged as particularly promising for NMR data interpretation. Neural networks, support vector machines, and random forests can be trained on large datasets to recognize spectral patterns associated with specific structural features. These systems excel at handling noisy data and can often extract meaningful information from spectra that would challenge traditional analysis methods. Recent deep learning implementations have demonstrated the ability to propose complete structures from complex mixture spectra with minimal human intervention.
Automated structure verification tools represent the practical application of these computational methods in research and industry. These systems can rapidly assess whether experimental NMR data is consistent with a proposed structure, flagging discrepancies for expert review. This capability has proven invaluable in quality control processes for pharmaceutical development and chemical synthesis verification.
Integration of multiple spectroscopic techniques through computational frameworks further enhances structure determination accuracy. Modern platforms can simultaneously analyze data from NMR, mass spectrometry, infrared spectroscopy, and other techniques, applying Bayesian probability models to determine the most likely structural candidates that satisfy all experimental constraints.
Validation Standards and Quality Control in Structure Determination
Quality control and validation are critical components in the NMR-based structure determination process, ensuring the reliability and accuracy of the final structural models. Established validation standards serve as benchmarks against which the quality of structural determinations can be measured and compared across different laboratories and research groups.
The primary validation metrics for NMR structure determination include chemical shift validation, which compares experimental chemical shifts with those predicted from the proposed structure. Deviations beyond established thresholds indicate potential structural inaccuracies that require further investigation. Additionally, NOE distance restraint validation examines whether the proposed structure satisfies the distance constraints derived from Nuclear Overhauser Effect measurements.
Residual dipolar coupling (RDC) analysis provides another crucial validation parameter, offering information about the orientation of bond vectors relative to a molecular alignment tensor. Structures that fail to match experimental RDC values typically contain significant conformational errors that must be addressed through refinement.
Statistical validation tools such as PROCHECK-NMR and WHAT-IF have become industry standards, evaluating structural quality based on parameters including Ramachandran plot statistics, side-chain rotamer distributions, and overall geometric quality. These automated systems generate quality scores that allow for objective assessment of structure reliability.
Cross-validation techniques represent another essential component of the quality control process. The most common approach involves excluding a subset of experimental data (typically 10-15%) from the structure calculation process, then using this omitted data to independently validate the resulting structure. The agreement between the excluded data and the calculated structure provides an unbiased measure of structural accuracy.
Modern validation protocols increasingly incorporate molecular dynamics simulations to assess structural stability over time. Structures that maintain their integrity during extended simulations generally demonstrate greater reliability than those exhibiting significant conformational drift.
Industry and academic standards organizations, including the Worldwide Protein Data Bank (wwPDB), have established comprehensive validation requirements for NMR-derived structures. These requirements include minimum thresholds for experimental data completeness, structural precision metrics such as RMSD values, and comprehensive reporting of validation statistics.
The implementation of these validation standards has significantly improved the overall quality of NMR-derived structures in recent years, with average precision and accuracy metrics showing steady improvement. However, challenges remain in standardizing validation approaches across different types of biomolecules and in developing more sensitive methods for detecting subtle structural errors.
The primary validation metrics for NMR structure determination include chemical shift validation, which compares experimental chemical shifts with those predicted from the proposed structure. Deviations beyond established thresholds indicate potential structural inaccuracies that require further investigation. Additionally, NOE distance restraint validation examines whether the proposed structure satisfies the distance constraints derived from Nuclear Overhauser Effect measurements.
Residual dipolar coupling (RDC) analysis provides another crucial validation parameter, offering information about the orientation of bond vectors relative to a molecular alignment tensor. Structures that fail to match experimental RDC values typically contain significant conformational errors that must be addressed through refinement.
Statistical validation tools such as PROCHECK-NMR and WHAT-IF have become industry standards, evaluating structural quality based on parameters including Ramachandran plot statistics, side-chain rotamer distributions, and overall geometric quality. These automated systems generate quality scores that allow for objective assessment of structure reliability.
Cross-validation techniques represent another essential component of the quality control process. The most common approach involves excluding a subset of experimental data (typically 10-15%) from the structure calculation process, then using this omitted data to independently validate the resulting structure. The agreement between the excluded data and the calculated structure provides an unbiased measure of structural accuracy.
Modern validation protocols increasingly incorporate molecular dynamics simulations to assess structural stability over time. Structures that maintain their integrity during extended simulations generally demonstrate greater reliability than those exhibiting significant conformational drift.
Industry and academic standards organizations, including the Worldwide Protein Data Bank (wwPDB), have established comprehensive validation requirements for NMR-derived structures. These requirements include minimum thresholds for experimental data completeness, structural precision metrics such as RMSD values, and comprehensive reporting of validation statistics.
The implementation of these validation standards has significantly improved the overall quality of NMR-derived structures in recent years, with average precision and accuracy metrics showing steady improvement. However, challenges remain in standardizing validation approaches across different types of biomolecules and in developing more sensitive methods for detecting subtle structural errors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!