Ethyl Acetate Production: Enhancing Yield and Purity
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Synthesis Background and Objectives
Ethyl acetate, a versatile organic compound, has been a cornerstone in various industries for decades. Its production process has undergone significant evolution since its initial synthesis in the early 19th century. The journey of ethyl acetate synthesis reflects the broader trends in chemical engineering and industrial processes, showcasing the industry's continuous pursuit of efficiency and sustainability.
The primary objective in ethyl acetate production has consistently been to enhance yield and purity while minimizing production costs and environmental impact. This goal has driven researchers and engineers to explore various synthesis routes and optimization techniques over the years. The traditional Fischer esterification method, involving the reaction of ethanol and acetic acid, has been the foundation of ethyl acetate production for many years.
As the demand for ethyl acetate grew across diverse sectors such as pharmaceuticals, cosmetics, and electronics, the need for more efficient production methods became apparent. This led to the development of alternative synthesis routes, including the Tishchenko reaction and the dehydrogenation of ethanol. Each of these methods brought its own set of advantages and challenges, contributing to the rich tapestry of ethyl acetate production technology.
The technological evolution in this field has been marked by several key milestones. The introduction of catalysts to enhance reaction rates and selectivity was a significant breakthrough. Heterogeneous catalysts, in particular, have played a crucial role in improving the efficiency of ethyl acetate synthesis. Another important development was the implementation of continuous flow processes, which allowed for increased production capacity and better control over reaction parameters.
In recent years, the focus has shifted towards more sustainable production methods. This trend aligns with the global push for greener chemical processes and reduced carbon footprints. Researchers are now exploring bio-based routes for ethyl acetate production, utilizing renewable resources as feedstocks. Additionally, there is growing interest in developing novel reactor designs and process intensification techniques to further optimize the production process.
The current technological landscape of ethyl acetate production is characterized by a blend of traditional methods and innovative approaches. While the Fischer esterification remains relevant, especially in smaller-scale productions, newer technologies are gaining traction in larger industrial settings. The industry is witnessing a gradual shift towards more integrated and efficient production systems, often combining multiple unit operations to achieve higher yields and purities.
Looking ahead, the objectives for ethyl acetate production are multifaceted. There is a strong emphasis on developing more energy-efficient processes, reducing waste generation, and exploring alternative raw materials. The integration of advanced process control systems and the application of artificial intelligence in process optimization are emerging trends that promise to further enhance the production of this important chemical compound.
The primary objective in ethyl acetate production has consistently been to enhance yield and purity while minimizing production costs and environmental impact. This goal has driven researchers and engineers to explore various synthesis routes and optimization techniques over the years. The traditional Fischer esterification method, involving the reaction of ethanol and acetic acid, has been the foundation of ethyl acetate production for many years.
As the demand for ethyl acetate grew across diverse sectors such as pharmaceuticals, cosmetics, and electronics, the need for more efficient production methods became apparent. This led to the development of alternative synthesis routes, including the Tishchenko reaction and the dehydrogenation of ethanol. Each of these methods brought its own set of advantages and challenges, contributing to the rich tapestry of ethyl acetate production technology.
The technological evolution in this field has been marked by several key milestones. The introduction of catalysts to enhance reaction rates and selectivity was a significant breakthrough. Heterogeneous catalysts, in particular, have played a crucial role in improving the efficiency of ethyl acetate synthesis. Another important development was the implementation of continuous flow processes, which allowed for increased production capacity and better control over reaction parameters.
In recent years, the focus has shifted towards more sustainable production methods. This trend aligns with the global push for greener chemical processes and reduced carbon footprints. Researchers are now exploring bio-based routes for ethyl acetate production, utilizing renewable resources as feedstocks. Additionally, there is growing interest in developing novel reactor designs and process intensification techniques to further optimize the production process.
The current technological landscape of ethyl acetate production is characterized by a blend of traditional methods and innovative approaches. While the Fischer esterification remains relevant, especially in smaller-scale productions, newer technologies are gaining traction in larger industrial settings. The industry is witnessing a gradual shift towards more integrated and efficient production systems, often combining multiple unit operations to achieve higher yields and purities.
Looking ahead, the objectives for ethyl acetate production are multifaceted. There is a strong emphasis on developing more energy-efficient processes, reducing waste generation, and exploring alternative raw materials. The integration of advanced process control systems and the application of artificial intelligence in process optimization are emerging trends that promise to further enhance the production of this important chemical compound.
Market Analysis for High-Purity Ethyl Acetate
The global market for high-purity ethyl acetate has been experiencing steady growth, driven by increasing demand from various end-use industries such as pharmaceuticals, electronics, and specialty chemicals. This solvent's unique properties, including low toxicity, high solvency, and rapid evaporation rate, make it an essential component in numerous applications.
In the pharmaceutical sector, high-purity ethyl acetate is crucial for drug formulation and as a reaction medium in the synthesis of active pharmaceutical ingredients (APIs). The growing pharmaceutical industry, particularly in emerging markets, is expected to fuel the demand for high-purity ethyl acetate in the coming years.
The electronics industry represents another significant market for high-purity ethyl acetate. Its use in the production of printed circuit boards, semiconductors, and other electronic components has been increasing due to the rapid growth of the consumer electronics market and the expansion of 5G technology.
The paints and coatings industry also contributes substantially to the demand for high-purity ethyl acetate. As a fast-evaporating solvent, it is widely used in formulating industrial coatings, automotive paints, and printing inks. The recovery of the construction and automotive sectors post-pandemic is likely to boost the consumption of high-purity ethyl acetate in this segment.
Geographically, Asia-Pacific dominates the high-purity ethyl acetate market, with China and India being the major consumers and producers. The region's robust industrial growth, coupled with increasing investments in manufacturing sectors, is expected to maintain its market leadership position.
North America and Europe follow as significant markets, primarily driven by the pharmaceutical and specialty chemicals industries. These regions are also witnessing a trend towards bio-based ethyl acetate production, aligning with sustainability goals and regulatory pressures.
The market is characterized by intense competition among key players, leading to continuous efforts in product innovation and capacity expansion. Major manufacturers are focusing on developing more efficient production processes to meet the growing demand for high-purity grades while maintaining cost-effectiveness.
Despite the positive outlook, the high-purity ethyl acetate market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of ethanol and acetic acid, the primary raw materials, can significantly impact production costs and market dynamics.
In the pharmaceutical sector, high-purity ethyl acetate is crucial for drug formulation and as a reaction medium in the synthesis of active pharmaceutical ingredients (APIs). The growing pharmaceutical industry, particularly in emerging markets, is expected to fuel the demand for high-purity ethyl acetate in the coming years.
The electronics industry represents another significant market for high-purity ethyl acetate. Its use in the production of printed circuit boards, semiconductors, and other electronic components has been increasing due to the rapid growth of the consumer electronics market and the expansion of 5G technology.
The paints and coatings industry also contributes substantially to the demand for high-purity ethyl acetate. As a fast-evaporating solvent, it is widely used in formulating industrial coatings, automotive paints, and printing inks. The recovery of the construction and automotive sectors post-pandemic is likely to boost the consumption of high-purity ethyl acetate in this segment.
Geographically, Asia-Pacific dominates the high-purity ethyl acetate market, with China and India being the major consumers and producers. The region's robust industrial growth, coupled with increasing investments in manufacturing sectors, is expected to maintain its market leadership position.
North America and Europe follow as significant markets, primarily driven by the pharmaceutical and specialty chemicals industries. These regions are also witnessing a trend towards bio-based ethyl acetate production, aligning with sustainability goals and regulatory pressures.
The market is characterized by intense competition among key players, leading to continuous efforts in product innovation and capacity expansion. Major manufacturers are focusing on developing more efficient production processes to meet the growing demand for high-purity grades while maintaining cost-effectiveness.
Despite the positive outlook, the high-purity ethyl acetate market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of ethanol and acetic acid, the primary raw materials, can significantly impact production costs and market dynamics.
Current Challenges in Ethyl Acetate Production
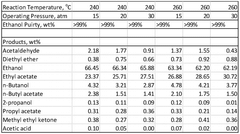
The production of ethyl acetate faces several significant challenges that hinder the optimization of yield and purity. One of the primary issues is the reversible nature of the esterification reaction between ethanol and acetic acid. This equilibrium limitation restricts the maximum theoretical yield, making it difficult to achieve high conversion rates without employing advanced techniques or process modifications.
Another major challenge is the formation of azeotropes during the production process. Ethyl acetate forms azeotropes with both water and ethanol, which complicates the separation and purification steps. These azeotropes make conventional distillation ineffective for achieving high purity levels, necessitating more complex and energy-intensive separation methods.
The presence of side reactions also poses a significant hurdle in ethyl acetate production. Unwanted reactions, such as the formation of diethyl ether or ethyl acetate hydrolysis, can occur under certain conditions, reducing the overall yield and introducing impurities that are challenging to remove.
Catalyst deactivation is another critical issue affecting the efficiency and longevity of the production process. Traditional acid catalysts used in esterification reactions, such as sulfuric acid, can lead to equipment corrosion and require frequent replacement or regeneration, impacting both the economics and sustainability of the process.
Energy consumption remains a persistent challenge in ethyl acetate production. The need for high temperatures in the reaction and separation stages, coupled with the energy-intensive nature of azeotropic distillation, contributes significantly to the overall production costs and environmental footprint.
Water management is also a crucial aspect that requires careful consideration. The presence of water as a byproduct of the esterification reaction can shift the equilibrium unfavorably, necessitating its continuous removal to drive the reaction towards completion. However, efficient water removal without compromising product quality or increasing energy consumption remains a challenge.
Lastly, the industry faces increasing pressure to adopt more sustainable and environmentally friendly production methods. This includes reducing the use of hazardous chemicals, minimizing waste generation, and improving overall energy efficiency. Balancing these sustainability goals with the need for high yield and purity presents a complex challenge for ethyl acetate manufacturers.
Another major challenge is the formation of azeotropes during the production process. Ethyl acetate forms azeotropes with both water and ethanol, which complicates the separation and purification steps. These azeotropes make conventional distillation ineffective for achieving high purity levels, necessitating more complex and energy-intensive separation methods.
The presence of side reactions also poses a significant hurdle in ethyl acetate production. Unwanted reactions, such as the formation of diethyl ether or ethyl acetate hydrolysis, can occur under certain conditions, reducing the overall yield and introducing impurities that are challenging to remove.
Catalyst deactivation is another critical issue affecting the efficiency and longevity of the production process. Traditional acid catalysts used in esterification reactions, such as sulfuric acid, can lead to equipment corrosion and require frequent replacement or regeneration, impacting both the economics and sustainability of the process.
Energy consumption remains a persistent challenge in ethyl acetate production. The need for high temperatures in the reaction and separation stages, coupled with the energy-intensive nature of azeotropic distillation, contributes significantly to the overall production costs and environmental footprint.
Water management is also a crucial aspect that requires careful consideration. The presence of water as a byproduct of the esterification reaction can shift the equilibrium unfavorably, necessitating its continuous removal to drive the reaction towards completion. However, efficient water removal without compromising product quality or increasing energy consumption remains a challenge.
Lastly, the industry faces increasing pressure to adopt more sustainable and environmentally friendly production methods. This includes reducing the use of hazardous chemicals, minimizing waste generation, and improving overall energy efficiency. Balancing these sustainability goals with the need for high yield and purity presents a complex challenge for ethyl acetate manufacturers.
State-of-the-Art Ethyl Acetate Production Methods
01 Optimization of reaction conditions
Improving ethyl acetate yield and purity can be achieved by optimizing reaction conditions such as temperature, pressure, catalyst type and concentration, and reactant ratios. Fine-tuning these parameters can lead to higher conversion rates and reduced formation of byproducts, resulting in increased yield and purity of the final product.- Improved production methods for ethyl acetate: Various methods have been developed to enhance the yield and purity of ethyl acetate production. These include optimized reaction conditions, novel catalysts, and improved separation techniques. Some processes focus on continuous production methods, while others utilize specific feedstocks or employ unique reactor designs to increase efficiency and product quality.
- Purification techniques for ethyl acetate: Several purification methods have been developed to increase the purity of ethyl acetate. These include advanced distillation techniques, membrane separation processes, and the use of specific adsorbents. Some approaches focus on removing impurities like water, acetic acid, or other byproducts, while others aim to achieve high-purity ethyl acetate for specialized applications.
- Catalytic processes for ethyl acetate synthesis: Various catalytic processes have been developed to improve the yield and selectivity of ethyl acetate production. These include heterogeneous catalysts, enzyme-catalyzed reactions, and novel catalyst support materials. Some processes focus on reducing side reactions, while others aim to enhance the conversion of reactants or improve catalyst stability and reusability.
- Green chemistry approaches for ethyl acetate production: Environmentally friendly methods for producing ethyl acetate with high yield and purity have been developed. These include the use of renewable feedstocks, bio-based catalysts, and low-energy processes. Some approaches focus on reducing waste generation, while others aim to minimize the use of harmful solvents or reagents in the production and purification stages.
- Process intensification for ethyl acetate production: Various process intensification techniques have been applied to ethyl acetate production to improve yield and purity. These include reactive distillation, microreactor technology, and integrated reaction-separation processes. Some approaches focus on reducing equipment size and energy consumption, while others aim to enhance mass transfer and reaction kinetics for improved product quality and efficiency.
02 Purification techniques
Various purification techniques can be employed to enhance the purity of ethyl acetate. These may include distillation, extraction, adsorption, and membrane separation processes. Advanced purification methods can effectively remove impurities and byproducts, leading to high-purity ethyl acetate.Expand Specific Solutions03 Catalyst development
Development of novel catalysts or improvement of existing catalysts can significantly impact ethyl acetate yield and purity. Catalysts with higher selectivity, stability, and activity can promote the desired reaction while minimizing side reactions, resulting in improved product quality and quantity.Expand Specific Solutions04 Continuous production processes
Implementation of continuous production processes, such as reactive distillation or membrane reactors, can enhance ethyl acetate yield and purity. These processes often allow for better control of reaction conditions, improved mass transfer, and in-situ product separation, leading to higher efficiency and product quality.Expand Specific Solutions05 Raw material selection and pretreatment
Careful selection and pretreatment of raw materials can contribute to improved ethyl acetate yield and purity. Using high-quality starting materials and implementing effective pretreatment methods can reduce impurities, enhance reaction efficiency, and ultimately lead to better product characteristics.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The ethyl acetate production market is in a mature stage, with established players like Celanese, BASF, and Solvay dominating the industry. The global market size is estimated to be around $3-4 billion, growing steadily due to increasing demand in various applications such as coatings, adhesives, and pharmaceuticals. Technologically, the process is well-established, with most major players using traditional esterification methods. However, emerging companies like Viridis Chemical are introducing innovative bio-based production techniques, indicating a shift towards more sustainable practices. Companies such as Resonac and SABIC are also investing in research and development to enhance yield and purity, suggesting ongoing technological advancements in this mature market.
Celanese International Corp.
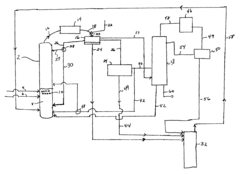
Technical Solution: Celanese has developed an advanced esterification process for ethyl acetate production, utilizing a reactive distillation technology. This method combines reaction and separation in a single unit operation, significantly enhancing yield and purity. The process employs a heterogeneous acid catalyst, typically a strong cation exchange resin, which promotes the reaction between ethanol and acetic acid[1]. The reactive distillation column is designed with specific internals to maximize contact between reactants and catalyst while facilitating the separation of the product. This approach allows for continuous removal of the ethyl acetate product, driving the equilibrium towards higher conversion rates. The company has also implemented a heat integration system that recovers energy from the distillation process, improving overall energy efficiency[3].
Strengths: Higher conversion rates, improved energy efficiency, and reduced equipment footprint. Weaknesses: Potential catalyst deactivation over time, complexity in column design and control.
BASF Corp.
Technical Solution: BASF has innovated a novel approach to ethyl acetate production using a gas-phase process. This method involves the direct addition of ethylene to acetic acid over a heterogeneous catalyst, typically a supported noble metal such as palladium. The reaction occurs in a fixed-bed reactor under carefully controlled temperature and pressure conditions. BASF's process achieves high selectivity towards ethyl acetate, minimizing unwanted side reactions[2]. To enhance yield and purity, the company has implemented an advanced separation train, including a series of distillation columns with inter-stage cooling. This setup allows for efficient removal of unreacted raw materials and byproducts. Additionally, BASF has developed a proprietary catalyst regeneration technique that extends catalyst life and maintains high activity levels over extended production runs[4].
Strengths: High selectivity, efficient use of raw materials, and extended catalyst life. Weaknesses: Higher initial capital investment and potential sensitivity to feedstock impurities.
Innovative Technologies for Yield and Purity Enhancement
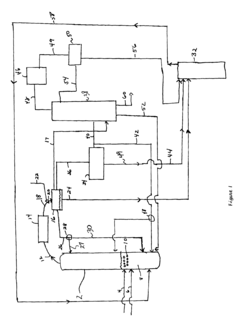
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
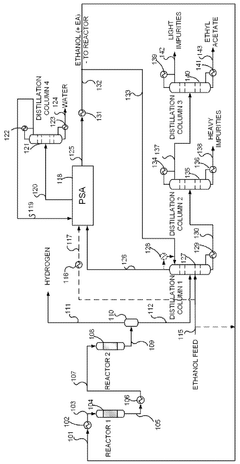
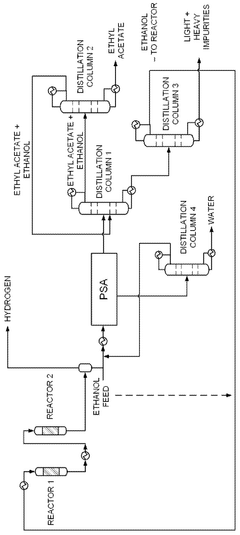
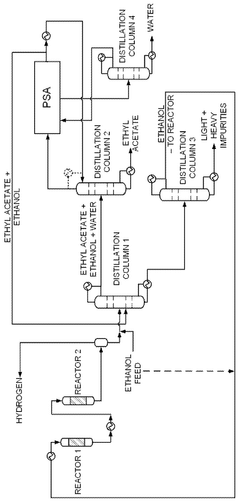
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Environmental Impact and Sustainability Considerations
The production of ethyl acetate, while an essential process in various industries, carries significant environmental implications that demand careful consideration. The traditional manufacturing methods often involve the use of fossil fuel-based feedstocks and energy-intensive processes, contributing to greenhouse gas emissions and climate change. To address these concerns, the industry is increasingly focusing on developing more sustainable production techniques.
One of the primary environmental challenges in ethyl acetate production is the generation of waste and by-products. Conventional processes may result in the formation of unwanted side products, which not only reduce yield but also require additional treatment and disposal. This waste management aspect adds to the overall environmental footprint of the production process. Implementing more selective catalysts and optimizing reaction conditions can help minimize waste generation and improve atom economy.
Water consumption and wastewater management are also critical factors in the environmental impact of ethyl acetate production. The purification and separation stages often require substantial amounts of water, and the resulting wastewater may contain organic contaminants. Developing water-efficient processes and implementing effective wastewater treatment systems are essential steps towards reducing the water footprint of ethyl acetate manufacturing.
Energy efficiency is another key consideration in enhancing the sustainability of ethyl acetate production. The distillation and purification steps are typically energy-intensive, contributing significantly to the overall carbon footprint of the process. Exploring alternative separation technologies, such as membrane-based systems or reactive distillation, could potentially reduce energy consumption and associated emissions.
The choice of raw materials plays a crucial role in the sustainability of ethyl acetate production. Shifting towards bio-based feedstocks, such as ethanol derived from renewable sources, can significantly reduce the reliance on fossil fuels and decrease the overall carbon footprint. However, this transition must be carefully evaluated to ensure that it does not lead to unintended consequences, such as competition with food crops or increased land use.
Life cycle assessment (LCA) is an invaluable tool in comprehensively evaluating the environmental impact of ethyl acetate production. By considering all stages of the product's life cycle, from raw material extraction to end-of-life disposal, LCA can identify hotspots for environmental improvement and guide decision-making towards more sustainable production methods.
As regulatory pressures and consumer demand for environmentally friendly products continue to grow, the ethyl acetate industry must prioritize sustainability in its production processes. This includes not only optimizing current technologies but also investing in research and development of novel, green chemistry approaches that can revolutionize the manufacturing landscape.
One of the primary environmental challenges in ethyl acetate production is the generation of waste and by-products. Conventional processes may result in the formation of unwanted side products, which not only reduce yield but also require additional treatment and disposal. This waste management aspect adds to the overall environmental footprint of the production process. Implementing more selective catalysts and optimizing reaction conditions can help minimize waste generation and improve atom economy.
Water consumption and wastewater management are also critical factors in the environmental impact of ethyl acetate production. The purification and separation stages often require substantial amounts of water, and the resulting wastewater may contain organic contaminants. Developing water-efficient processes and implementing effective wastewater treatment systems are essential steps towards reducing the water footprint of ethyl acetate manufacturing.
Energy efficiency is another key consideration in enhancing the sustainability of ethyl acetate production. The distillation and purification steps are typically energy-intensive, contributing significantly to the overall carbon footprint of the process. Exploring alternative separation technologies, such as membrane-based systems or reactive distillation, could potentially reduce energy consumption and associated emissions.
The choice of raw materials plays a crucial role in the sustainability of ethyl acetate production. Shifting towards bio-based feedstocks, such as ethanol derived from renewable sources, can significantly reduce the reliance on fossil fuels and decrease the overall carbon footprint. However, this transition must be carefully evaluated to ensure that it does not lead to unintended consequences, such as competition with food crops or increased land use.
Life cycle assessment (LCA) is an invaluable tool in comprehensively evaluating the environmental impact of ethyl acetate production. By considering all stages of the product's life cycle, from raw material extraction to end-of-life disposal, LCA can identify hotspots for environmental improvement and guide decision-making towards more sustainable production methods.
As regulatory pressures and consumer demand for environmentally friendly products continue to grow, the ethyl acetate industry must prioritize sustainability in its production processes. This includes not only optimizing current technologies but also investing in research and development of novel, green chemistry approaches that can revolutionize the manufacturing landscape.
Economic Feasibility of Advanced Production Techniques
The economic feasibility of advanced production techniques for ethyl acetate is a critical consideration for manufacturers seeking to enhance yield and purity. These techniques often require significant capital investment but can lead to substantial long-term benefits in terms of production efficiency and product quality.
One of the most promising advanced techniques is the use of reactive distillation, which combines reaction and separation processes in a single unit operation. This method can significantly reduce equipment and energy costs compared to conventional processes. Initial capital expenditure for reactive distillation columns may be higher, but the operational cost savings can result in a shorter payback period, typically within 2-3 years for large-scale production facilities.
Membrane-based separation technologies represent another economically viable option for improving ethyl acetate production. These systems offer lower energy consumption and reduced operational costs compared to traditional distillation methods. The initial investment in membrane technology can be offset by savings in utility costs and increased product recovery rates, with some studies reporting energy savings of up to 30% in ethyl acetate purification processes.
Catalytic distillation is an emerging technique that shows promise for enhancing both yield and purity in ethyl acetate production. By integrating catalysts directly into distillation columns, this method can achieve higher conversion rates and improved selectivity. While the upfront costs for catalytic distillation systems are considerable, the increased product quality and reduced waste generation can lead to significant economic benefits over time.
Advanced process control and optimization systems, such as model predictive control (MPC), can also contribute to the economic feasibility of ethyl acetate production. These systems can fine-tune production parameters in real-time, leading to improved yield, reduced energy consumption, and more consistent product quality. The implementation of MPC typically requires a moderate investment in software and training but can result in operational cost reductions of 3-5% annually.
When evaluating the economic feasibility of these advanced techniques, it is essential to consider not only the direct production costs but also the potential for value-added products and market differentiation. Higher purity ethyl acetate can command premium prices in certain industries, such as electronics and pharmaceuticals, potentially offsetting the higher production costs associated with advanced techniques.
Furthermore, the adoption of advanced production methods can lead to improved sustainability profiles, which may provide additional economic benefits through regulatory compliance, reduced waste management costs, and enhanced corporate reputation. As environmental regulations become more stringent, the economic advantages of cleaner production processes are likely to become increasingly significant.
One of the most promising advanced techniques is the use of reactive distillation, which combines reaction and separation processes in a single unit operation. This method can significantly reduce equipment and energy costs compared to conventional processes. Initial capital expenditure for reactive distillation columns may be higher, but the operational cost savings can result in a shorter payback period, typically within 2-3 years for large-scale production facilities.
Membrane-based separation technologies represent another economically viable option for improving ethyl acetate production. These systems offer lower energy consumption and reduced operational costs compared to traditional distillation methods. The initial investment in membrane technology can be offset by savings in utility costs and increased product recovery rates, with some studies reporting energy savings of up to 30% in ethyl acetate purification processes.
Catalytic distillation is an emerging technique that shows promise for enhancing both yield and purity in ethyl acetate production. By integrating catalysts directly into distillation columns, this method can achieve higher conversion rates and improved selectivity. While the upfront costs for catalytic distillation systems are considerable, the increased product quality and reduced waste generation can lead to significant economic benefits over time.
Advanced process control and optimization systems, such as model predictive control (MPC), can also contribute to the economic feasibility of ethyl acetate production. These systems can fine-tune production parameters in real-time, leading to improved yield, reduced energy consumption, and more consistent product quality. The implementation of MPC typically requires a moderate investment in software and training but can result in operational cost reductions of 3-5% annually.
When evaluating the economic feasibility of these advanced techniques, it is essential to consider not only the direct production costs but also the potential for value-added products and market differentiation. Higher purity ethyl acetate can command premium prices in certain industries, such as electronics and pharmaceuticals, potentially offsetting the higher production costs associated with advanced techniques.
Furthermore, the adoption of advanced production methods can lead to improved sustainability profiles, which may provide additional economic benefits through regulatory compliance, reduced waste management costs, and enhanced corporate reputation. As environmental regulations become more stringent, the economic advantages of cleaner production processes are likely to become increasingly significant.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!