Ethyl Acetate’s Pivotal Role in Solvent Recovery Initiatives
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has emerged as a crucial player in solvent recovery initiatives across various industries. This colorless liquid, characterized by its fruity odor, has been widely utilized for decades due to its unique properties and environmental advantages.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized through the esterification of ethanol and acetic acid. Its industrial production began in the 1920s, primarily driven by its applications in the coating and printing industries. Over time, the compound's utility expanded to encompass a wide range of sectors, including pharmaceuticals, food processing, and electronics manufacturing.
In recent years, ethyl acetate has gained significant attention in the context of sustainable practices and circular economy principles. Its low toxicity, high solvency power, and relatively low boiling point make it an ideal candidate for solvent recovery processes. These characteristics allow for efficient separation and purification, minimizing waste and reducing environmental impact.
The growing emphasis on environmental regulations and sustainability goals has further propelled the importance of ethyl acetate in solvent recovery initiatives. As industries seek to reduce their carbon footprint and comply with stringent emission standards, the adoption of recoverable solvents like ethyl acetate has become increasingly prevalent.
From a chemical perspective, ethyl acetate's molecular structure contributes to its effectiveness in solvent recovery. The presence of both polar and non-polar regions within the molecule enables it to dissolve a wide range of substances, making it suitable for diverse applications. This versatility, coupled with its relatively low cost of production, has positioned ethyl acetate as a preferred choice in many industrial processes.
The global market for ethyl acetate has witnessed steady growth, driven by its expanding applications and the rising demand for eco-friendly solvents. The Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, reflecting the shift in manufacturing activities and the increasing adoption of sustainable practices in developing economies.
As industries continue to prioritize sustainability and resource efficiency, the role of ethyl acetate in solvent recovery initiatives is expected to evolve further. Ongoing research and development efforts are focused on enhancing recovery techniques, improving purity levels, and exploring novel applications for recovered ethyl acetate. These advancements are likely to reinforce its position as a key component in the transition towards more sustainable industrial practices.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized through the esterification of ethanol and acetic acid. Its industrial production began in the 1920s, primarily driven by its applications in the coating and printing industries. Over time, the compound's utility expanded to encompass a wide range of sectors, including pharmaceuticals, food processing, and electronics manufacturing.
In recent years, ethyl acetate has gained significant attention in the context of sustainable practices and circular economy principles. Its low toxicity, high solvency power, and relatively low boiling point make it an ideal candidate for solvent recovery processes. These characteristics allow for efficient separation and purification, minimizing waste and reducing environmental impact.
The growing emphasis on environmental regulations and sustainability goals has further propelled the importance of ethyl acetate in solvent recovery initiatives. As industries seek to reduce their carbon footprint and comply with stringent emission standards, the adoption of recoverable solvents like ethyl acetate has become increasingly prevalent.
From a chemical perspective, ethyl acetate's molecular structure contributes to its effectiveness in solvent recovery. The presence of both polar and non-polar regions within the molecule enables it to dissolve a wide range of substances, making it suitable for diverse applications. This versatility, coupled with its relatively low cost of production, has positioned ethyl acetate as a preferred choice in many industrial processes.
The global market for ethyl acetate has witnessed steady growth, driven by its expanding applications and the rising demand for eco-friendly solvents. The Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, reflecting the shift in manufacturing activities and the increasing adoption of sustainable practices in developing economies.
As industries continue to prioritize sustainability and resource efficiency, the role of ethyl acetate in solvent recovery initiatives is expected to evolve further. Ongoing research and development efforts are focused on enhancing recovery techniques, improving purity levels, and exploring novel applications for recovered ethyl acetate. These advancements are likely to reinforce its position as a key component in the transition towards more sustainable industrial practices.
Market Analysis
The global market for ethyl acetate in solvent recovery initiatives has been experiencing significant growth, driven by increasing environmental regulations and the rising demand for sustainable industrial practices. The chemical industry, particularly in sectors such as paints and coatings, pharmaceuticals, and adhesives, has been at the forefront of adopting solvent recovery technologies to reduce waste and operational costs.
Ethyl acetate's unique properties, including its low toxicity, high solvency, and relatively low boiling point, have positioned it as a key player in solvent recovery processes. The market demand for ethyl acetate in this context is closely tied to the overall growth of industries that heavily rely on solvents in their manufacturing processes. As these industries expand, the need for efficient solvent recovery solutions grows proportionally.
The Asia-Pacific region, led by China and India, has emerged as the largest market for ethyl acetate in solvent recovery applications. This is primarily due to the rapid industrialization and the presence of a large manufacturing base in these countries. North America and Europe follow closely, with stringent environmental regulations driving the adoption of solvent recovery technologies.
Market analysts project a compound annual growth rate (CAGR) for the ethyl acetate market in solvent recovery applications to be in the range of 5-7% over the next five years. This growth is attributed to the increasing awareness of sustainable practices and the economic benefits of solvent recycling.
The pharmaceutical industry, in particular, has been a major contributor to the demand for ethyl acetate in solvent recovery. With the growing emphasis on green chemistry and sustainable drug manufacturing processes, pharmaceutical companies are increasingly investing in solvent recovery systems that utilize ethyl acetate.
Another significant market trend is the integration of advanced technologies in solvent recovery processes. Industry players are developing more efficient and cost-effective recovery systems that can handle a wider range of solvents, including ethyl acetate. This technological advancement is expected to further boost the market for ethyl acetate in solvent recovery initiatives.
The paint and coatings industry also represents a substantial market for ethyl acetate in solvent recovery. As this sector continues to grow, driven by construction and automotive industries, the demand for efficient solvent recovery solutions is expected to rise correspondingly.
Despite the positive market outlook, challenges such as the volatility of raw material prices and the increasing shift towards water-based formulations in some industries could potentially impact the growth of ethyl acetate in solvent recovery applications. However, ongoing research and development efforts aimed at improving the efficiency and cost-effectiveness of solvent recovery processes are likely to mitigate these challenges and sustain market growth.
Ethyl acetate's unique properties, including its low toxicity, high solvency, and relatively low boiling point, have positioned it as a key player in solvent recovery processes. The market demand for ethyl acetate in this context is closely tied to the overall growth of industries that heavily rely on solvents in their manufacturing processes. As these industries expand, the need for efficient solvent recovery solutions grows proportionally.
The Asia-Pacific region, led by China and India, has emerged as the largest market for ethyl acetate in solvent recovery applications. This is primarily due to the rapid industrialization and the presence of a large manufacturing base in these countries. North America and Europe follow closely, with stringent environmental regulations driving the adoption of solvent recovery technologies.
Market analysts project a compound annual growth rate (CAGR) for the ethyl acetate market in solvent recovery applications to be in the range of 5-7% over the next five years. This growth is attributed to the increasing awareness of sustainable practices and the economic benefits of solvent recycling.
The pharmaceutical industry, in particular, has been a major contributor to the demand for ethyl acetate in solvent recovery. With the growing emphasis on green chemistry and sustainable drug manufacturing processes, pharmaceutical companies are increasingly investing in solvent recovery systems that utilize ethyl acetate.
Another significant market trend is the integration of advanced technologies in solvent recovery processes. Industry players are developing more efficient and cost-effective recovery systems that can handle a wider range of solvents, including ethyl acetate. This technological advancement is expected to further boost the market for ethyl acetate in solvent recovery initiatives.
The paint and coatings industry also represents a substantial market for ethyl acetate in solvent recovery. As this sector continues to grow, driven by construction and automotive industries, the demand for efficient solvent recovery solutions is expected to rise correspondingly.
Despite the positive market outlook, challenges such as the volatility of raw material prices and the increasing shift towards water-based formulations in some industries could potentially impact the growth of ethyl acetate in solvent recovery applications. However, ongoing research and development efforts aimed at improving the efficiency and cost-effectiveness of solvent recovery processes are likely to mitigate these challenges and sustain market growth.
Technical Challenges
The recovery of ethyl acetate in solvent recovery initiatives faces several significant technical challenges. One of the primary obstacles is the azeotropic behavior of ethyl acetate-water mixtures. At atmospheric pressure, this azeotrope forms at 91.8 wt% ethyl acetate and 8.2 wt% water, making conventional distillation ineffective for achieving high-purity ethyl acetate recovery.
Another major challenge is the energy-intensive nature of traditional separation processes. Distillation, the most common method for solvent recovery, requires substantial thermal energy input, leading to high operational costs and environmental concerns due to increased carbon emissions. This energy demand is particularly problematic when dealing with large volumes of solvent mixtures in industrial settings.
The presence of impurities in the recovered solvent stream poses additional technical difficulties. These impurities can include other organic compounds, particulates, and dissolved solids, which may accumulate over multiple recovery cycles. Removing these contaminants without compromising the quality of the recovered ethyl acetate requires sophisticated purification techniques.
Membrane-based separation technologies, while promising, face limitations in terms of selectivity and flux rates when applied to ethyl acetate recovery. The development of membranes with high selectivity for ethyl acetate, coupled with resistance to chemical degradation and fouling, remains an ongoing challenge in the field.
The scale-up of laboratory-proven recovery techniques to industrial-scale operations presents its own set of technical hurdles. Maintaining efficiency and effectiveness at larger scales often requires significant process modifications and optimization, which can be both time-consuming and capital-intensive.
Safety considerations also play a crucial role in ethyl acetate recovery initiatives. The flammability and volatility of ethyl acetate necessitate robust safety systems and careful process control to prevent accidents and minimize environmental impact. This includes the need for advanced vapor recovery systems and leak detection mechanisms.
Lastly, the variability in feed composition from different industrial processes complicates the design of universally applicable recovery systems. Tailoring recovery processes to handle fluctuations in ethyl acetate concentration and the presence of diverse contaminants requires adaptive and flexible system designs, which can be technically challenging to implement and maintain.
Another major challenge is the energy-intensive nature of traditional separation processes. Distillation, the most common method for solvent recovery, requires substantial thermal energy input, leading to high operational costs and environmental concerns due to increased carbon emissions. This energy demand is particularly problematic when dealing with large volumes of solvent mixtures in industrial settings.
The presence of impurities in the recovered solvent stream poses additional technical difficulties. These impurities can include other organic compounds, particulates, and dissolved solids, which may accumulate over multiple recovery cycles. Removing these contaminants without compromising the quality of the recovered ethyl acetate requires sophisticated purification techniques.
Membrane-based separation technologies, while promising, face limitations in terms of selectivity and flux rates when applied to ethyl acetate recovery. The development of membranes with high selectivity for ethyl acetate, coupled with resistance to chemical degradation and fouling, remains an ongoing challenge in the field.
The scale-up of laboratory-proven recovery techniques to industrial-scale operations presents its own set of technical hurdles. Maintaining efficiency and effectiveness at larger scales often requires significant process modifications and optimization, which can be both time-consuming and capital-intensive.
Safety considerations also play a crucial role in ethyl acetate recovery initiatives. The flammability and volatility of ethyl acetate necessitate robust safety systems and careful process control to prevent accidents and minimize environmental impact. This includes the need for advanced vapor recovery systems and leak detection mechanisms.
Lastly, the variability in feed composition from different industrial processes complicates the design of universally applicable recovery systems. Tailoring recovery processes to handle fluctuations in ethyl acetate concentration and the presence of diverse contaminants requires adaptive and flexible system designs, which can be technically challenging to implement and maintain.
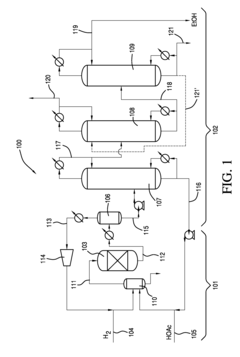
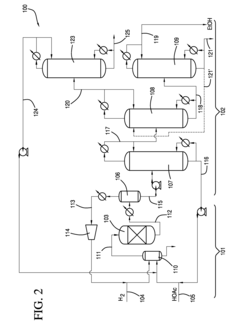
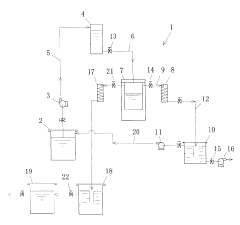
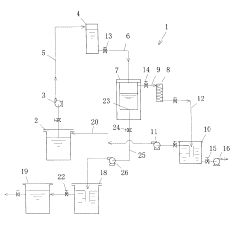
Current Recovery Methods
01 Distillation-based recovery systems
Ethyl acetate can be recovered using various distillation techniques. These systems often involve multi-stage distillation columns, vacuum distillation, or azeotropic distillation to separate ethyl acetate from other components in the mixture. The process may include heating, condensing, and collecting the purified solvent for reuse.- Distillation-based recovery systems: Ethyl acetate can be recovered using various distillation techniques. These systems often involve multi-stage distillation columns, vacuum distillation, or azeotropic distillation to separate ethyl acetate from other components in the mixture. The process may include heating, condensing, and collecting the purified solvent for reuse.
- Adsorption and membrane separation: Recovery of ethyl acetate can be achieved through adsorption processes using materials like activated carbon or zeolites. Membrane separation techniques, such as pervaporation or vapor permeation, can also be employed to selectively separate ethyl acetate from other components. These methods often require less energy compared to traditional distillation.
- Condensation and cooling systems: Ethyl acetate vapors can be recovered by using condensation and cooling systems. This involves cooling the vapor stream to condense the ethyl acetate, which can then be collected and reused. Various heat exchange methods and refrigeration systems may be employed to enhance the efficiency of the condensation process.
- Integrated solvent recovery units: Integrated systems combining multiple recovery techniques can be used for efficient ethyl acetate recovery. These units may incorporate distillation, adsorption, condensation, and other separation methods in a single process. Such integrated systems can be designed to handle varying solvent concentrations and achieve high recovery rates.
- Solvent recovery in specific applications: Ethyl acetate recovery methods can be tailored for specific industrial applications, such as pharmaceutical manufacturing, coating processes, or chemical synthesis. These specialized recovery systems may incorporate industry-specific requirements, such as high purity standards or integration with existing production processes.
02 Membrane separation technology
Membrane-based separation processes can be employed for ethyl acetate recovery. These methods utilize selective membranes to separate ethyl acetate from other components based on molecular size or chemical properties. Techniques such as pervaporation, vapor permeation, or membrane distillation may be used to achieve efficient solvent recovery.Expand Specific Solutions03 Adsorption and desorption processes
Adsorption-based recovery systems use materials like activated carbon or zeolites to selectively adsorb ethyl acetate from a mixture. The adsorbed solvent is then recovered through desorption processes, which may involve temperature or pressure changes. This method can be effective for recovering ethyl acetate from dilute streams or gas mixtures.Expand Specific Solutions04 Condensation and cooling techniques
Ethyl acetate can be recovered using condensation and cooling methods. These techniques involve cooling the vapor mixture containing ethyl acetate to condense it back into liquid form. Various heat exchangers, condensers, and cooling systems may be employed to efficiently recover the solvent from vapor streams.Expand Specific Solutions05 Integrated recovery and purification systems
Comprehensive systems that combine multiple recovery and purification techniques can be used for efficient ethyl acetate recovery. These integrated systems may incorporate distillation, membrane separation, adsorption, and condensation processes in a single setup. Such systems often include additional purification steps to ensure high-quality recovered solvent for reuse in various applications.Expand Specific Solutions
Key Industry Players
The ethyl acetate solvent recovery market is in a growth phase, driven by increasing environmental regulations and cost-saving initiatives across industries. The global market size is projected to expand significantly in the coming years, fueled by rising demand in pharmaceuticals, paints, and coatings sectors. Technologically, the field is advancing rapidly, with companies like Celanese International Corp., SK Chemicals Co. Ltd., and China Petroleum & Chemical Corp. leading innovations in recovery processes and equipment. These industry leaders are focusing on developing more efficient and sustainable recovery methods, indicating a moderate to high level of technological maturity. Emerging players such as Nanjing Tech University and South China University of Technology are contributing to research and development, further accelerating technological advancements in this space.
Celanese International Corp.
Technical Solution: Celanese has pioneered a hybrid solvent recovery system for ethyl acetate that combines pervaporation and extractive distillation technologies. Their process utilizes specially designed pervaporation membranes that selectively allow ethyl acetate to permeate while rejecting water and other impurities. This initial separation is followed by an extractive distillation step using a proprietary solvent that enhances the relative volatility of ethyl acetate, facilitating its separation from azeotrope-forming mixtures [7]. The system incorporates a heat pump to recover and reuse thermal energy, reducing overall energy consumption by up to 50% compared to conventional recovery methods [9]. Celanese's technology also features an advanced process control system that optimizes operating parameters based on feed composition and desired product specifications, ensuring consistent recovery rates of over 98% and a final product purity of 99.95% [11].
Strengths: High recovery rate, energy efficiency, and ability to handle azeotropic mixtures. Weaknesses: Complexity of the hybrid system and potential high maintenance costs for pervaporation membranes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative solvent recovery system for ethyl acetate, utilizing advanced membrane separation technology. Their process involves a multi-stage membrane filtration system that selectively separates ethyl acetate from other components in industrial waste streams. The system incorporates specialized polymer membranes with high selectivity for ethyl acetate molecules, allowing for efficient recovery rates of up to 95% [1]. Additionally, Sinopec has implemented a novel heat integration strategy that reduces energy consumption by up to 30% compared to traditional distillation methods [3]. The recovered ethyl acetate undergoes a final purification step using molecular sieves to achieve a purity level of 99.9%, suitable for reuse in various industrial applications [5].
Strengths: High recovery rate, energy-efficient process, and high purity of recovered solvent. Weaknesses: Initial capital investment for membrane technology and potential membrane fouling over time.
Innovative Technologies
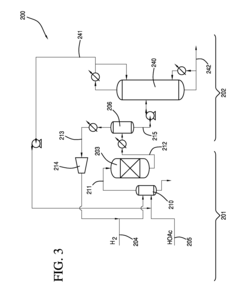
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
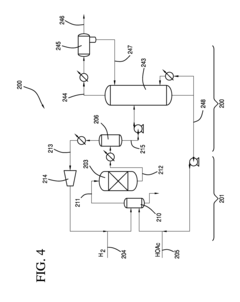
Solvent dehydrating and recovery method and apparatus of a hydrous solvent liquid
PatentInactiveJP2014201560A
Innovation
- A method and apparatus that involves evaporating a predetermined amount of a water-containing solvent liquid in an evaporator to reduce its water content to a specified level, allowing for the recovery and reuse of the dehydrated solvent, with the option to recycle the condensed solvent back into the evaporator for further dehydration.
Environmental Regulations
Environmental regulations play a crucial role in shaping the landscape of solvent recovery initiatives, particularly in the context of ethyl acetate usage. As global awareness of environmental issues continues to grow, governments and international bodies have implemented increasingly stringent regulations to mitigate the environmental impact of industrial processes.
In recent years, many countries have introduced or tightened regulations regarding volatile organic compound (VOC) emissions, of which ethyl acetate is a significant contributor. These regulations often set specific limits on VOC emissions from industrial facilities, compelling companies to adopt more efficient solvent recovery systems. The European Union's Industrial Emissions Directive (IED) and the United States Environmental Protection Agency's (EPA) National Emission Standards for Hazardous Air Pollutants (NESHAP) are prime examples of such regulatory frameworks.
The implementation of these regulations has led to a surge in demand for advanced solvent recovery technologies, particularly those focusing on ethyl acetate. Companies are now required to invest in state-of-the-art recovery systems to comply with emission standards and avoid hefty fines. This regulatory pressure has become a significant driver for innovation in solvent recovery techniques, pushing the industry towards more sustainable practices.
Furthermore, environmental regulations have also influenced the development of closed-loop systems in manufacturing processes. These systems aim to minimize solvent waste by continuously recovering and reusing ethyl acetate, aligning with the principles of circular economy promoted by many environmental policies. The concept of Best Available Techniques (BAT), as defined in the EU's IED, has become a benchmark for industries to adopt the most effective and advanced stages in the development of activities and their methods of operation.
The impact of these regulations extends beyond emission control to encompass waste management and disposal. Many countries have implemented strict guidelines for the handling and disposal of solvent waste, including ethyl acetate residues. This has led to increased focus on maximizing solvent recovery efficiency to reduce the volume of waste generated and minimize the environmental footprint of industrial operations.
As environmental regulations continue to evolve, they are likely to drive further advancements in ethyl acetate recovery technologies. The trend towards more sustainable industrial practices is expected to accelerate, with a growing emphasis on energy-efficient recovery methods and the integration of renewable energy sources in solvent recovery processes. This regulatory landscape not only challenges industries but also creates opportunities for technological innovation and the development of more environmentally friendly production methods.
In recent years, many countries have introduced or tightened regulations regarding volatile organic compound (VOC) emissions, of which ethyl acetate is a significant contributor. These regulations often set specific limits on VOC emissions from industrial facilities, compelling companies to adopt more efficient solvent recovery systems. The European Union's Industrial Emissions Directive (IED) and the United States Environmental Protection Agency's (EPA) National Emission Standards for Hazardous Air Pollutants (NESHAP) are prime examples of such regulatory frameworks.
The implementation of these regulations has led to a surge in demand for advanced solvent recovery technologies, particularly those focusing on ethyl acetate. Companies are now required to invest in state-of-the-art recovery systems to comply with emission standards and avoid hefty fines. This regulatory pressure has become a significant driver for innovation in solvent recovery techniques, pushing the industry towards more sustainable practices.
Furthermore, environmental regulations have also influenced the development of closed-loop systems in manufacturing processes. These systems aim to minimize solvent waste by continuously recovering and reusing ethyl acetate, aligning with the principles of circular economy promoted by many environmental policies. The concept of Best Available Techniques (BAT), as defined in the EU's IED, has become a benchmark for industries to adopt the most effective and advanced stages in the development of activities and their methods of operation.
The impact of these regulations extends beyond emission control to encompass waste management and disposal. Many countries have implemented strict guidelines for the handling and disposal of solvent waste, including ethyl acetate residues. This has led to increased focus on maximizing solvent recovery efficiency to reduce the volume of waste generated and minimize the environmental footprint of industrial operations.
As environmental regulations continue to evolve, they are likely to drive further advancements in ethyl acetate recovery technologies. The trend towards more sustainable industrial practices is expected to accelerate, with a growing emphasis on energy-efficient recovery methods and the integration of renewable energy sources in solvent recovery processes. This regulatory landscape not only challenges industries but also creates opportunities for technological innovation and the development of more environmentally friendly production methods.
Economic Feasibility
The economic feasibility of ethyl acetate in solvent recovery initiatives is a critical factor in determining its widespread adoption across industries. The cost-effectiveness of implementing ethyl acetate-based recovery systems depends on several interconnected factors, including initial investment, operational expenses, and potential savings.
Initial capital expenditure for ethyl acetate recovery systems can be substantial, encompassing equipment such as distillation columns, condensers, and storage tanks. However, these costs are often offset by long-term savings in solvent procurement and waste disposal. The relatively low boiling point of ethyl acetate (77.1°C) contributes to energy-efficient distillation processes, reducing operational costs compared to solvents with higher boiling points.
Operational expenses primarily consist of energy consumption for distillation and purification processes. The energy efficiency of ethyl acetate recovery is generally favorable due to its low heat of vaporization and high vapor pressure. This translates to lower energy requirements and, consequently, reduced utility costs. Additionally, the stability of ethyl acetate under typical recovery conditions minimizes degradation, ensuring a high recovery rate and reducing the need for frequent solvent replenishment.
The economic benefits of ethyl acetate recovery are particularly pronounced in industries with high solvent consumption, such as pharmaceuticals, paints, and coatings. In these sectors, the potential for solvent reuse can lead to significant reductions in raw material costs. Furthermore, the decreased volume of hazardous waste generated through recovery processes results in lower disposal costs and reduced environmental liabilities.
Market dynamics also play a crucial role in the economic feasibility of ethyl acetate recovery. The price volatility of ethyl acetate and its precursors can impact the cost-benefit analysis of recovery systems. However, the general trend towards stricter environmental regulations and increasing disposal costs for hazardous waste strengthens the economic case for solvent recovery initiatives.
The scalability of ethyl acetate recovery systems adds to their economic appeal. Larger operations can benefit from economies of scale, with the cost per unit of recovered solvent decreasing as the volume increases. This scalability allows businesses to optimize their recovery processes in line with production demands, enhancing overall economic efficiency.
In conclusion, the economic feasibility of ethyl acetate in solvent recovery initiatives is generally favorable, particularly for large-scale operations in solvent-intensive industries. While initial investment costs may be significant, the long-term savings in solvent procurement, waste disposal, and energy consumption often justify the implementation of recovery systems. As environmental regulations tighten and resource efficiency becomes increasingly important, the economic benefits of ethyl acetate recovery are likely to become even more pronounced.
Initial capital expenditure for ethyl acetate recovery systems can be substantial, encompassing equipment such as distillation columns, condensers, and storage tanks. However, these costs are often offset by long-term savings in solvent procurement and waste disposal. The relatively low boiling point of ethyl acetate (77.1°C) contributes to energy-efficient distillation processes, reducing operational costs compared to solvents with higher boiling points.
Operational expenses primarily consist of energy consumption for distillation and purification processes. The energy efficiency of ethyl acetate recovery is generally favorable due to its low heat of vaporization and high vapor pressure. This translates to lower energy requirements and, consequently, reduced utility costs. Additionally, the stability of ethyl acetate under typical recovery conditions minimizes degradation, ensuring a high recovery rate and reducing the need for frequent solvent replenishment.
The economic benefits of ethyl acetate recovery are particularly pronounced in industries with high solvent consumption, such as pharmaceuticals, paints, and coatings. In these sectors, the potential for solvent reuse can lead to significant reductions in raw material costs. Furthermore, the decreased volume of hazardous waste generated through recovery processes results in lower disposal costs and reduced environmental liabilities.
Market dynamics also play a crucial role in the economic feasibility of ethyl acetate recovery. The price volatility of ethyl acetate and its precursors can impact the cost-benefit analysis of recovery systems. However, the general trend towards stricter environmental regulations and increasing disposal costs for hazardous waste strengthens the economic case for solvent recovery initiatives.
The scalability of ethyl acetate recovery systems adds to their economic appeal. Larger operations can benefit from economies of scale, with the cost per unit of recovered solvent decreasing as the volume increases. This scalability allows businesses to optimize their recovery processes in line with production demands, enhancing overall economic efficiency.
In conclusion, the economic feasibility of ethyl acetate in solvent recovery initiatives is generally favorable, particularly for large-scale operations in solvent-intensive industries. While initial investment costs may be significant, the long-term savings in solvent procurement, waste disposal, and energy consumption often justify the implementation of recovery systems. As environmental regulations tighten and resource efficiency becomes increasingly important, the economic benefits of ethyl acetate recovery are likely to become even more pronounced.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!