Evaluating Liquid Metal Interconnect in Biomedical Coatings
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Liquid Metal Interconnect Technology Background and Objectives
Liquid metal interconnects represent a revolutionary advancement in the field of biomedical engineering, offering unique properties that traditional metallic conductors cannot provide. The evolution of this technology can be traced back to the early 2000s when researchers began exploring gallium-based alloys as alternatives to mercury due to their lower toxicity and similar fluidic properties. The field gained significant momentum around 2012 when several breakthrough studies demonstrated the potential of liquid metal in flexible electronics applications.
The technological trajectory has been characterized by progressive improvements in material composition, with eutectic gallium-indium (EGaIn) and gallium-indium-tin (Galinstan) emerging as the predominant alloys due to their low melting points, excellent conductivity, and minimal toxicity profiles. These materials maintain liquid state at room temperature while exhibiting conductivity comparable to many solid metals, creating unprecedented opportunities for biomedical applications.
Current research objectives in liquid metal interconnect technology focus on addressing several critical challenges. Primary among these is developing reliable encapsulation methods that prevent oxidation while maintaining flexibility. The thin oxide layer that forms on liquid metal surfaces presents both challenges and opportunities - it provides structural stability but can impede electrical performance if not properly managed.
Another key objective involves improving the integration of liquid metal interconnects with various biocompatible coating materials. This includes developing deposition techniques that ensure uniform distribution and stable electrical properties when embedded within polymeric matrices such as polydimethylsiloxane (PDMS), polyurethane, and other biocompatible elastomers.
The biomedical applications driving this research are diverse and promising. These include flexible biosensors capable of conforming to biological tissues, implantable devices with reduced mechanical mismatch to surrounding tissues, and advanced neural interfaces that minimize inflammatory responses. The ultimate goal is to create bioelectronic systems that can seamlessly interface with biological systems while maintaining reliable electrical performance.
Recent technological trends indicate growing interest in self-healing capabilities of liquid metal networks, where damaged interconnects can autonomously restore conductivity. Additionally, researchers are exploring stimulus-responsive liquid metal systems that can change properties in response to external triggers such as pH, temperature, or electrical signals.
The field is moving toward miniaturization and increased complexity, with objectives to develop manufacturing processes capable of creating intricate liquid metal microchannel networks at scales relevant to cellular-level interactions. This advancement would enable unprecedented capabilities in tissue-integrated electronics and real-time physiological monitoring systems.
The technological trajectory has been characterized by progressive improvements in material composition, with eutectic gallium-indium (EGaIn) and gallium-indium-tin (Galinstan) emerging as the predominant alloys due to their low melting points, excellent conductivity, and minimal toxicity profiles. These materials maintain liquid state at room temperature while exhibiting conductivity comparable to many solid metals, creating unprecedented opportunities for biomedical applications.
Current research objectives in liquid metal interconnect technology focus on addressing several critical challenges. Primary among these is developing reliable encapsulation methods that prevent oxidation while maintaining flexibility. The thin oxide layer that forms on liquid metal surfaces presents both challenges and opportunities - it provides structural stability but can impede electrical performance if not properly managed.
Another key objective involves improving the integration of liquid metal interconnects with various biocompatible coating materials. This includes developing deposition techniques that ensure uniform distribution and stable electrical properties when embedded within polymeric matrices such as polydimethylsiloxane (PDMS), polyurethane, and other biocompatible elastomers.
The biomedical applications driving this research are diverse and promising. These include flexible biosensors capable of conforming to biological tissues, implantable devices with reduced mechanical mismatch to surrounding tissues, and advanced neural interfaces that minimize inflammatory responses. The ultimate goal is to create bioelectronic systems that can seamlessly interface with biological systems while maintaining reliable electrical performance.
Recent technological trends indicate growing interest in self-healing capabilities of liquid metal networks, where damaged interconnects can autonomously restore conductivity. Additionally, researchers are exploring stimulus-responsive liquid metal systems that can change properties in response to external triggers such as pH, temperature, or electrical signals.
The field is moving toward miniaturization and increased complexity, with objectives to develop manufacturing processes capable of creating intricate liquid metal microchannel networks at scales relevant to cellular-level interactions. This advancement would enable unprecedented capabilities in tissue-integrated electronics and real-time physiological monitoring systems.
Biomedical Coating Market Demand Analysis
The biomedical coating market has experienced significant growth in recent years, driven by increasing demand for advanced medical devices and implants. The global biomedical coating market was valued at approximately $10.5 billion in 2022 and is projected to reach $20.3 billion by 2030, growing at a CAGR of 8.6% during the forecast period. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and technological advancements in healthcare.
Liquid metal interconnects in biomedical coatings represent an emerging segment within this market, addressing the critical need for flexible, stretchable, and biocompatible electronic interfaces in medical devices. The demand for such solutions is particularly strong in applications such as wearable health monitors, implantable sensors, and neural interfaces, where traditional rigid electronics face significant limitations.
Market research indicates that approximately 65% of healthcare providers express interest in adopting devices with advanced biomedical coatings featuring liquid metal interconnects, citing improved patient comfort and enhanced monitoring capabilities as primary benefits. The neurological application segment is expected to witness the highest growth rate, with a projected CAGR of 12.3% through 2030, as brain-computer interfaces and neural monitoring systems gain clinical acceptance.
Regionally, North America currently dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is anticipated to exhibit the fastest growth due to increasing healthcare expenditure, expanding medical device manufacturing capabilities, and growing awareness about advanced healthcare technologies in countries like China, Japan, and South Korea.
The demand for liquid metal interconnect technology in biomedical coatings is further driven by the miniaturization trend in medical devices, with 72% of industry stakeholders identifying size reduction as a critical factor for next-generation implantable and wearable devices. Additionally, the push toward personalized medicine has created a need for customizable biomedical coatings that can be tailored to individual patient requirements.
Consumer preferences are also shifting toward non-invasive monitoring solutions, with market surveys indicating that 83% of patients prefer wearable health monitoring devices over traditional methods requiring hospital visits. This trend has accelerated the development of skin-interfacing electronics utilizing liquid metal interconnects, which can provide continuous health monitoring without compromising comfort or mobility.
Liquid metal interconnects in biomedical coatings represent an emerging segment within this market, addressing the critical need for flexible, stretchable, and biocompatible electronic interfaces in medical devices. The demand for such solutions is particularly strong in applications such as wearable health monitors, implantable sensors, and neural interfaces, where traditional rigid electronics face significant limitations.
Market research indicates that approximately 65% of healthcare providers express interest in adopting devices with advanced biomedical coatings featuring liquid metal interconnects, citing improved patient comfort and enhanced monitoring capabilities as primary benefits. The neurological application segment is expected to witness the highest growth rate, with a projected CAGR of 12.3% through 2030, as brain-computer interfaces and neural monitoring systems gain clinical acceptance.
Regionally, North America currently dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is anticipated to exhibit the fastest growth due to increasing healthcare expenditure, expanding medical device manufacturing capabilities, and growing awareness about advanced healthcare technologies in countries like China, Japan, and South Korea.
The demand for liquid metal interconnect technology in biomedical coatings is further driven by the miniaturization trend in medical devices, with 72% of industry stakeholders identifying size reduction as a critical factor for next-generation implantable and wearable devices. Additionally, the push toward personalized medicine has created a need for customizable biomedical coatings that can be tailored to individual patient requirements.
Consumer preferences are also shifting toward non-invasive monitoring solutions, with market surveys indicating that 83% of patients prefer wearable health monitoring devices over traditional methods requiring hospital visits. This trend has accelerated the development of skin-interfacing electronics utilizing liquid metal interconnects, which can provide continuous health monitoring without compromising comfort or mobility.
Current Status and Challenges in Liquid Metal Interconnect
Liquid metal interconnects represent a significant advancement in biomedical coating technology, with gallium-based alloys such as eutectic gallium-indium (EGaIn) and gallium-indium-tin (Galinstan) leading the field. These materials offer unique properties including high electrical conductivity, excellent mechanical flexibility, and self-healing capabilities that make them particularly valuable for biomedical applications. Currently, research institutions across North America, Europe, and East Asia are actively developing these technologies, with notable progress in stretchable electronics, soft robotics, and implantable medical devices.
Despite promising advancements, several technical challenges persist in the implementation of liquid metal interconnects for biomedical coatings. Oxidation remains a primary concern, as gallium-based alloys rapidly form a thin oxide layer when exposed to oxygen, affecting conductivity and performance stability. This oxidation process, while providing some benefits for patterning and stability, creates significant hurdles for long-term reliability in biological environments. Additionally, encapsulation techniques to prevent leakage while maintaining flexibility have not been fully optimized.
Biocompatibility presents another critical challenge. While initial studies suggest relatively low toxicity for gallium-based liquid metals, comprehensive long-term biocompatibility studies remain limited. The potential for metal ion leaching and subsequent tissue reactions requires further investigation before widespread clinical adoption can occur. Current research indicates promising results for short-term applications, but extended exposure data is lacking.
Manufacturing scalability constitutes a significant barrier to commercialization. Current fabrication methods for liquid metal interconnects often involve manual processes that are difficult to scale for mass production. Techniques such as microfluidic injection, direct writing, and selective wetting show promise but require further refinement to achieve consistent quality at industrial scales. The development of automated manufacturing processes represents a key area for future innovation.
Integration with existing biomedical technologies presents additional complications. Establishing reliable interfaces between liquid metal components and conventional electronic systems remains challenging, particularly regarding signal stability and connection durability. Furthermore, sterilization processes commonly used in medical applications can potentially alter the properties of liquid metal interconnects, necessitating the development of compatible sterilization protocols.
Regulatory pathways for these novel materials remain largely undefined. The unique properties of liquid metals create regulatory challenges that differ from traditional electronic components or biomedical coatings. Establishing standardized testing protocols and safety guidelines will be essential for clinical translation and market approval of devices incorporating liquid metal interconnect technology.
Despite promising advancements, several technical challenges persist in the implementation of liquid metal interconnects for biomedical coatings. Oxidation remains a primary concern, as gallium-based alloys rapidly form a thin oxide layer when exposed to oxygen, affecting conductivity and performance stability. This oxidation process, while providing some benefits for patterning and stability, creates significant hurdles for long-term reliability in biological environments. Additionally, encapsulation techniques to prevent leakage while maintaining flexibility have not been fully optimized.
Biocompatibility presents another critical challenge. While initial studies suggest relatively low toxicity for gallium-based liquid metals, comprehensive long-term biocompatibility studies remain limited. The potential for metal ion leaching and subsequent tissue reactions requires further investigation before widespread clinical adoption can occur. Current research indicates promising results for short-term applications, but extended exposure data is lacking.
Manufacturing scalability constitutes a significant barrier to commercialization. Current fabrication methods for liquid metal interconnects often involve manual processes that are difficult to scale for mass production. Techniques such as microfluidic injection, direct writing, and selective wetting show promise but require further refinement to achieve consistent quality at industrial scales. The development of automated manufacturing processes represents a key area for future innovation.
Integration with existing biomedical technologies presents additional complications. Establishing reliable interfaces between liquid metal components and conventional electronic systems remains challenging, particularly regarding signal stability and connection durability. Furthermore, sterilization processes commonly used in medical applications can potentially alter the properties of liquid metal interconnects, necessitating the development of compatible sterilization protocols.
Regulatory pathways for these novel materials remain largely undefined. The unique properties of liquid metals create regulatory challenges that differ from traditional electronic components or biomedical coatings. Establishing standardized testing protocols and safety guidelines will be essential for clinical translation and market approval of devices incorporating liquid metal interconnect technology.
Current Liquid Metal Interconnect Solutions
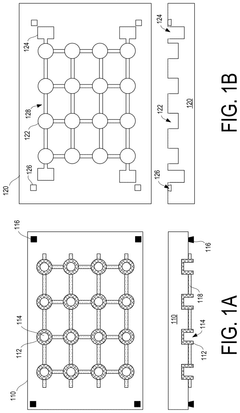
01 Liquid metal interconnect materials and compositions
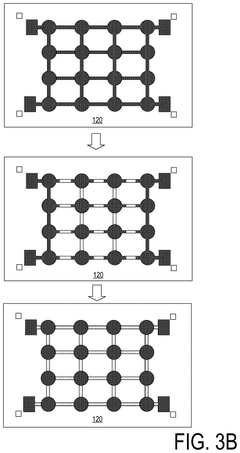
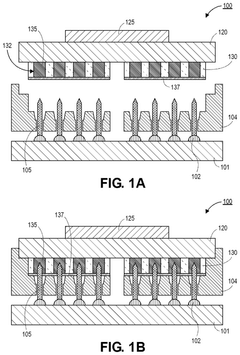
Liquid metal materials, such as gallium-based alloys, can be used as interconnects in electronic devices. These materials offer advantages including high electrical conductivity, thermal stability, and the ability to form reliable connections even under mechanical stress. The liquid nature of these metals allows them to maintain electrical contact during thermal cycling and mechanical deformation, making them suitable for flexible and stretchable electronics applications.- Liquid metal interconnect materials and compositions: Liquid metal materials, such as gallium-based alloys, can be used as interconnects in electronic devices due to their excellent electrical conductivity and ability to maintain connectivity during mechanical deformation. These materials offer advantages including self-healing properties, flexibility, and the ability to form reliable electrical connections at lower temperatures than traditional solders. The composition of these liquid metals can be engineered to optimize properties such as melting point, viscosity, and adhesion to various substrate materials.
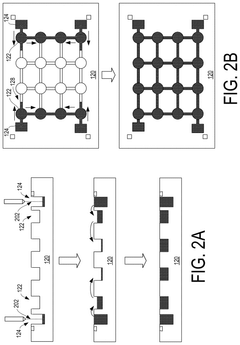
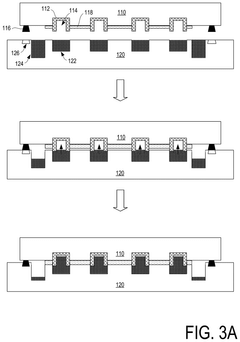
- Manufacturing methods for liquid metal interconnects: Various manufacturing techniques can be employed to create liquid metal interconnects, including printing, injection, deposition, and encapsulation methods. These processes allow for precise placement and containment of the liquid metal within designated channels or cavities. Advanced techniques include microfluidic delivery systems, selective wetting approaches, and methods to prevent oxidation during manufacturing. These manufacturing methods enable the creation of reliable liquid metal interconnects with controlled dimensions and electrical properties.
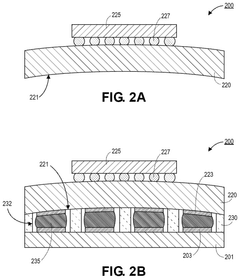
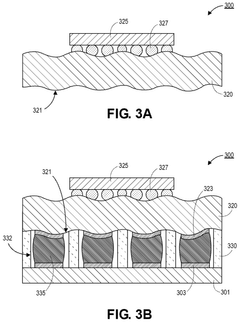
- Encapsulation and containment strategies: Effective encapsulation is crucial for liquid metal interconnects to prevent leakage and maintain electrical performance. Various materials and structures can be used to contain the liquid metal, including polymer channels, elastomeric substrates, and specialized barrier layers. These containment strategies often incorporate features to accommodate thermal expansion while preventing metal migration. Some approaches use microchannels with specific surface treatments to control the flow and adhesion of the liquid metal within the designated interconnect pathways.
- Integration with semiconductor devices and packaging: Liquid metal interconnects can be integrated with various semiconductor devices and packaging technologies to create reliable electrical connections. This integration requires specialized interface designs to ensure compatibility between the liquid metal and traditional semiconductor materials. Techniques include creating wettable metal pads, using adhesion layers, and designing thermal management systems to accommodate the unique properties of liquid metals. These integration approaches enable liquid metal interconnects to be used in advanced packaging solutions including 3D stacking and flexible electronics.
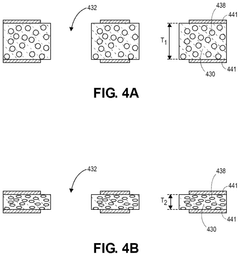
- Reliability and performance characteristics: Liquid metal interconnects offer unique reliability and performance characteristics compared to traditional solid interconnects. They can maintain electrical connectivity during mechanical deformation, thermal cycling, and vibration. Research has focused on understanding and improving their long-term stability, resistance to oxidation, and compatibility with various environmental conditions. Performance metrics include electrical conductivity, thermal conductivity, mechanical compliance, and resistance to electromigration. These characteristics make liquid metal interconnects particularly suitable for applications requiring flexibility, stretchability, or operation in harsh environments.
02 Fabrication methods for liquid metal interconnects
Various techniques can be employed to fabricate liquid metal interconnects, including microfluidic injection, printing, and patterning methods. These processes involve creating channels or vias in substrate materials, filling them with liquid metal, and then sealing the structures to prevent leakage. Advanced fabrication methods may incorporate encapsulation techniques to contain the liquid metal while maintaining its beneficial properties, or selective wetting approaches to control the placement and flow of the liquid metal.Expand Specific Solutions03 Integration of liquid metal interconnects with semiconductor devices
Liquid metal interconnects can be integrated with semiconductor devices to create reliable electrical connections between components. This integration involves specialized bonding techniques that ensure good electrical contact while preventing contamination of the semiconductor materials. The interconnects can be designed to accommodate the thermal expansion mismatch between different materials in the device, reducing stress and improving reliability. These interconnects are particularly valuable in advanced packaging technologies where traditional solid metal interconnects may face limitations.Expand Specific Solutions04 Liquid metal interconnects for flexible and stretchable electronics
Liquid metal interconnects are particularly advantageous for flexible and stretchable electronic applications due to their ability to maintain electrical conductivity under deformation. These interconnects can be embedded in elastomeric substrates to create stretchable circuits that can withstand significant mechanical strain without electrical failure. The self-healing properties of liquid metals allow the interconnects to recover from mechanical damage, making them suitable for wearable devices, soft robotics, and other applications requiring mechanical flexibility.Expand Specific Solutions05 Reliability and performance enhancement of liquid metal interconnects
Various techniques can be employed to enhance the reliability and performance of liquid metal interconnects. These include surface treatments to improve wetting and adhesion, alloying to modify physical properties, and encapsulation strategies to prevent oxidation and contamination. Advanced designs may incorporate redundant pathways or self-healing mechanisms to improve fault tolerance. Testing methodologies specific to liquid metal interconnects have been developed to evaluate their performance under various environmental conditions and mechanical stresses.Expand Specific Solutions
Key Industry Players in Biomedical Coatings
The liquid metal interconnect in biomedical coatings market is in an emerging growth phase, characterized by increasing research activities and expanding applications. The market size remains relatively modest but shows promising growth potential as biomedical applications continue to evolve. From a technological maturity perspective, this field is still developing, with key players demonstrating varying levels of advancement. Academic institutions like Technical University of Denmark, Zhejiang University, and Xi'an Jiaotong University are driving fundamental research, while companies including Intel, SMIC, and GlobalFoundries are exploring industrial applications. Medical-focused organizations such as Alfred Mann Foundation and MicroVention are investigating specific biomedical implementations. The convergence of semiconductor manufacturing expertise with biomedical research suggests this technology is transitioning from laboratory research to early commercial applications.
Alfred Mann Foundation
Technical Solution: The Alfred Mann Foundation has pioneered liquid metal interconnect technology for biomedical implants using eutectic gallium-indium (EGaIn) alloys integrated with their proprietary biocompatible coating systems. Their approach focuses on creating reliable neural interfaces that can withstand the harsh biological environment while maintaining flexibility. The foundation has developed a multi-layer encapsulation technique that uses biocompatible silicone derivatives to fully contain the liquid metal pathways while allowing for controlled mechanical deformation. Their most advanced systems incorporate microfluidic channels with precisely controlled liquid metal flow, enabling dynamic reconfiguration of electrical pathways in response to tissue movement or migration. This technology has been successfully implemented in their neural stimulation devices, where the liquid metal interconnects provide superior electrical performance while minimizing mechanical stress on surrounding tissues, significantly reducing inflammation and fibrosis compared to traditional rigid interconnects. Recent clinical trials have demonstrated maintained functionality of these interconnects for periods exceeding 18 months in vivo.
Strengths: Exceptional biocompatibility through advanced encapsulation techniques; dynamic reconfigurability of electrical pathways; proven long-term stability in clinical applications. Weaknesses: Higher production costs compared to conventional interconnects; limited scalability for mass production; requires specialized handling and manufacturing processes.
Battelle Memorial Institute
Technical Solution: Battelle has developed innovative liquid metal interconnect technologies specifically for biomedical coatings that utilize gallium-based alloys (primarily gallium-indium-tin or galinstan) embedded in elastomeric substrates. Their approach involves creating microchannels within biocompatible polymers like PDMS and filling them with liquid metal to form stretchable, conductive pathways. These interconnects maintain electrical conductivity even under significant mechanical deformation (up to 200% strain), making them ideal for implantable and wearable biomedical devices. Battelle's technology incorporates surface modification techniques to prevent gallium oxidation and improve adhesion to biomedical substrates. Their recent innovations include self-healing capabilities where broken connections automatically restore conductivity when the substrate returns to its original position, and they've developed specialized encapsulation methods to ensure biocompatibility while preventing metal leakage into surrounding tissues.
Strengths: Superior flexibility and stretchability compared to solid metal interconnects; self-healing properties that maintain functionality after mechanical stress; excellent biocompatibility through specialized encapsulation. Weaknesses: Potential for oxidation of liquid metal at interfaces; higher manufacturing complexity compared to traditional interconnects; concerns about long-term stability in biological environments.
Core Patents and Technical Literature Analysis
Self-diffusing liquid metal interconnect architectures enabling snap-on room temperature assembly
PatentPendingUS20250112190A1
Innovation
- A self-diffusion process using Gallium-based liquid metal interconnects facilitated by capillary action and a slip layer material to form connections at room temperature, allowing for minimal energy consumption and precise, scalable interconnect networks without complex processing.
Liquid metal patch interconnect for large warpage components
PatentPendingUS20240388018A1
Innovation
- Implementing a liquid metal interconnect solution without pins, where a compressible liquid metal patch on the package substrate directly contacts the board, accommodating warpage through its conformable nature and optional reservoirs for excess liquid, reducing Z-heights and costs.
Biocompatibility and Safety Considerations
The biocompatibility of liquid metal interconnects represents a critical consideration in biomedical coating applications. Gallium-based liquid metals, particularly gallium-indium (GaIn) alloys, have demonstrated promising preliminary biocompatibility profiles compared to mercury-based alternatives. Recent studies indicate that encapsulated gallium-based liquid metals exhibit minimal cytotoxicity when properly contained within biocompatible polymers such as polydimethylsiloxane (PDMS) or medical-grade silicones.
Immunological response testing has shown that liquid metal interconnects, when appropriately isolated from direct tissue contact, typically elicit minimal inflammatory responses. However, long-term implantation studies remain limited, with most data restricted to periods under six months. This knowledge gap necessitates further investigation into chronic exposure scenarios, particularly regarding potential metal ion leaching and tissue accumulation over extended timeframes.
Safety considerations must address the oxidation behavior of gallium-based liquid metals, as gallium oxide formation at interfaces can potentially compromise device integrity and introduce particulate matter into surrounding tissues. The mechanical stability of liquid metal interconnects under physiological stresses—including flexion, extension, and pulsatile forces—requires thorough characterization to prevent unexpected failures in vivo.
Regulatory pathways for liquid metal-containing biomedical devices present additional challenges. Current FDA and international regulatory frameworks lack specific guidance for liquid metal components in implantable or wearable medical devices. Manufacturers must develop comprehensive safety testing protocols that address the unique properties of these materials, including their fluid nature and potential for migration within encapsulation layers.
Sterilization compatibility represents another critical safety consideration. Common sterilization methods such as ethylene oxide, gamma irradiation, and autoclave processing may alter the physical properties of liquid metals or their encapsulation materials. Research indicates that gamma sterilization appears most compatible with gallium-based systems, while autoclave methods may compromise polymer encapsulation integrity.
Environmental considerations and disposal protocols must also be established, particularly for devices containing potentially toxic elements like indium. While gallium itself demonstrates relatively low environmental toxicity, proper containment throughout the device lifecycle and appropriate disposal pathways must be developed to prevent environmental contamination.
Future safety research should focus on developing standardized testing protocols specifically designed for liquid metal interconnects in biomedical applications, addressing their unique physical properties and potential failure modes that differ significantly from conventional solid metal components.
Immunological response testing has shown that liquid metal interconnects, when appropriately isolated from direct tissue contact, typically elicit minimal inflammatory responses. However, long-term implantation studies remain limited, with most data restricted to periods under six months. This knowledge gap necessitates further investigation into chronic exposure scenarios, particularly regarding potential metal ion leaching and tissue accumulation over extended timeframes.
Safety considerations must address the oxidation behavior of gallium-based liquid metals, as gallium oxide formation at interfaces can potentially compromise device integrity and introduce particulate matter into surrounding tissues. The mechanical stability of liquid metal interconnects under physiological stresses—including flexion, extension, and pulsatile forces—requires thorough characterization to prevent unexpected failures in vivo.
Regulatory pathways for liquid metal-containing biomedical devices present additional challenges. Current FDA and international regulatory frameworks lack specific guidance for liquid metal components in implantable or wearable medical devices. Manufacturers must develop comprehensive safety testing protocols that address the unique properties of these materials, including their fluid nature and potential for migration within encapsulation layers.
Sterilization compatibility represents another critical safety consideration. Common sterilization methods such as ethylene oxide, gamma irradiation, and autoclave processing may alter the physical properties of liquid metals or their encapsulation materials. Research indicates that gamma sterilization appears most compatible with gallium-based systems, while autoclave methods may compromise polymer encapsulation integrity.
Environmental considerations and disposal protocols must also be established, particularly for devices containing potentially toxic elements like indium. While gallium itself demonstrates relatively low environmental toxicity, proper containment throughout the device lifecycle and appropriate disposal pathways must be developed to prevent environmental contamination.
Future safety research should focus on developing standardized testing protocols specifically designed for liquid metal interconnects in biomedical applications, addressing their unique physical properties and potential failure modes that differ significantly from conventional solid metal components.
Regulatory Framework for Medical Device Materials
The regulatory landscape for medical devices incorporating liquid metal interconnects in biomedical coatings is complex and multifaceted, requiring careful navigation to ensure compliance and patient safety. In the United States, the Food and Drug Administration (FDA) oversees medical device regulation through the Center for Devices and Radiological Health (CDRH), categorizing devices into Class I, II, or III based on risk levels. Liquid metal interconnects, particularly those containing gallium-based alloys, typically fall under Class II or III due to their novel nature and potential biocompatibility concerns.
The FDA's 510(k) premarket notification pathway may be applicable for devices utilizing established liquid metal technologies, while more innovative applications might require the more rigorous Premarket Approval (PMA) process. Material safety documentation must include comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and leachable compounds from the liquid metal components.
In the European Union, the Medical Device Regulation (MDR 2017/745) imposes stringent requirements for novel materials like liquid metal interconnects. Manufacturers must demonstrate compliance with General Safety and Performance Requirements (GSPRs), with special attention to Article 10 regarding chemical substances. The classification system under Rule 7 often applies to devices with electrical components in contact with biological tissues.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established guidance documents for novel materials evaluation, though specific provisions for liquid metals remain limited. This regulatory gap necessitates proactive engagement with authorities through pre-submission consultations and scientific advice meetings.
Material master files and device master files play crucial roles in the regulatory process, allowing manufacturers to document proprietary information about liquid metal formulations while protecting intellectual property. These files must contain detailed information on material composition, manufacturing processes, and quality control measures.
Post-market surveillance requirements are particularly stringent for devices incorporating novel materials like liquid metal interconnects. Manufacturers must implement robust vigilance systems to monitor adverse events and material degradation over time, with periodic safety update reports submitted to regulatory authorities.
Emerging regulatory considerations include the potential classification of certain liquid metal formulations as nanomaterials under various jurisdictions, which would trigger additional testing requirements. Environmental regulations, including RoHS and REACH in Europe, may also impact material selection and disposal protocols for devices containing metals like gallium, indium, or mercury.
The FDA's 510(k) premarket notification pathway may be applicable for devices utilizing established liquid metal technologies, while more innovative applications might require the more rigorous Premarket Approval (PMA) process. Material safety documentation must include comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and leachable compounds from the liquid metal components.
In the European Union, the Medical Device Regulation (MDR 2017/745) imposes stringent requirements for novel materials like liquid metal interconnects. Manufacturers must demonstrate compliance with General Safety and Performance Requirements (GSPRs), with special attention to Article 10 regarding chemical substances. The classification system under Rule 7 often applies to devices with electrical components in contact with biological tissues.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established guidance documents for novel materials evaluation, though specific provisions for liquid metals remain limited. This regulatory gap necessitates proactive engagement with authorities through pre-submission consultations and scientific advice meetings.
Material master files and device master files play crucial roles in the regulatory process, allowing manufacturers to document proprietary information about liquid metal formulations while protecting intellectual property. These files must contain detailed information on material composition, manufacturing processes, and quality control measures.
Post-market surveillance requirements are particularly stringent for devices incorporating novel materials like liquid metal interconnects. Manufacturers must implement robust vigilance systems to monitor adverse events and material degradation over time, with periodic safety update reports submitted to regulatory authorities.
Emerging regulatory considerations include the potential classification of certain liquid metal formulations as nanomaterials under various jurisdictions, which would trigger additional testing requirements. Environmental regulations, including RoHS and REACH in Europe, may also impact material selection and disposal protocols for devices containing metals like gallium, indium, or mercury.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!