Liquid Metal Interconnect: Electrode Kinetics in Energy Systems
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Liquid Metal Interconnect Evolution and Objectives
Liquid metal interconnects have evolved significantly over the past decades, transitioning from rudimentary applications to sophisticated components in modern energy systems. The journey began in the mid-20th century with mercury-based electrical switches and connections, which demonstrated the fundamental potential of liquid metals as conductive interfaces. By the 1970s, gallium-based alloys emerged as safer alternatives to mercury, marking a critical shift in the field's trajectory.
The 1990s witnessed accelerated development with the introduction of room-temperature liquid metal alloys such as galinstan (gallium-indium-tin) and EGaIn (eutectic gallium-indium), which offered improved safety profiles and electrical properties. These innovations enabled new applications in flexible electronics and energy storage systems, establishing liquid metals as viable interconnect solutions beyond traditional solid conductors.
The early 2000s brought significant advancements in understanding the electrochemical behavior of liquid metal interfaces, particularly regarding their interaction with various electrode materials. Researchers began exploring the unique properties of liquid metal interconnects, including their self-healing capabilities, excellent thermal conductivity, and ability to maintain electrical contact under mechanical deformation.
Recent developments have focused on addressing the oxidation challenges inherent to gallium-based liquid metals, which form a thin oxide layer upon exposure to oxygen. This seemingly problematic characteristic has been ingeniously leveraged in some applications to create stable, printable structures while maintaining electrical conductivity at the interface. Concurrently, research into novel alloy compositions has yielded materials with enhanced stability and performance characteristics.
The primary objective of current liquid metal interconnect research is to optimize electrode kinetics at the liquid metal-solid interface, particularly in energy conversion and storage systems. This includes understanding and controlling charge transfer processes, minimizing interfacial resistance, and enhancing long-term stability under operational conditions. Researchers aim to develop interconnect solutions that can withstand thermal cycling, mechanical stress, and electrochemical reactions without performance degradation.
Additional objectives include developing manufacturing techniques for precise deposition and patterning of liquid metal interconnects at various scales, from microscopic electronic components to large-scale energy systems. The field also seeks to establish standardized testing protocols and performance metrics to facilitate comparison between different liquid metal formulations and implementation strategies.
Looking forward, the ultimate goal is to create liquid metal interconnect technologies that enable next-generation energy systems with unprecedented flexibility, reliability, and efficiency. This includes applications in advanced batteries, fuel cells, solar cells, and thermal energy harvesting systems where traditional solid interconnects face significant limitations.
The 1990s witnessed accelerated development with the introduction of room-temperature liquid metal alloys such as galinstan (gallium-indium-tin) and EGaIn (eutectic gallium-indium), which offered improved safety profiles and electrical properties. These innovations enabled new applications in flexible electronics and energy storage systems, establishing liquid metals as viable interconnect solutions beyond traditional solid conductors.
The early 2000s brought significant advancements in understanding the electrochemical behavior of liquid metal interfaces, particularly regarding their interaction with various electrode materials. Researchers began exploring the unique properties of liquid metal interconnects, including their self-healing capabilities, excellent thermal conductivity, and ability to maintain electrical contact under mechanical deformation.
Recent developments have focused on addressing the oxidation challenges inherent to gallium-based liquid metals, which form a thin oxide layer upon exposure to oxygen. This seemingly problematic characteristic has been ingeniously leveraged in some applications to create stable, printable structures while maintaining electrical conductivity at the interface. Concurrently, research into novel alloy compositions has yielded materials with enhanced stability and performance characteristics.
The primary objective of current liquid metal interconnect research is to optimize electrode kinetics at the liquid metal-solid interface, particularly in energy conversion and storage systems. This includes understanding and controlling charge transfer processes, minimizing interfacial resistance, and enhancing long-term stability under operational conditions. Researchers aim to develop interconnect solutions that can withstand thermal cycling, mechanical stress, and electrochemical reactions without performance degradation.
Additional objectives include developing manufacturing techniques for precise deposition and patterning of liquid metal interconnects at various scales, from microscopic electronic components to large-scale energy systems. The field also seeks to establish standardized testing protocols and performance metrics to facilitate comparison between different liquid metal formulations and implementation strategies.
Looking forward, the ultimate goal is to create liquid metal interconnect technologies that enable next-generation energy systems with unprecedented flexibility, reliability, and efficiency. This includes applications in advanced batteries, fuel cells, solar cells, and thermal energy harvesting systems where traditional solid interconnects face significant limitations.
Market Applications in Advanced Energy Systems
Liquid metal interconnects are revolutionizing the energy storage and conversion landscape, with market applications spanning multiple advanced energy sectors. The integration of these innovative materials into next-generation batteries, particularly lithium-based and sodium-based systems, has demonstrated significant improvements in energy density and cycle life. Commercial implementations have begun to emerge, with several energy storage manufacturers incorporating liquid metal electrodes to achieve up to 30% higher energy density compared to conventional solid electrode systems.
The renewable energy sector represents another substantial market opportunity, where liquid metal interconnects are being deployed in high-temperature solar thermal systems. These materials facilitate more efficient thermal energy transfer and storage, enabling solar thermal plants to operate with greater reliability during non-sunlight hours. Field tests have shown that liquid metal heat transfer systems can improve overall plant efficiency by 15-20% compared to traditional molten salt technologies.
Fuel cell technology stands to benefit considerably from liquid metal interconnect advancements. The unique properties of these materials—particularly their excellent electrical conductivity combined with electrochemical stability—address longstanding challenges in solid oxide fuel cell (SOFC) design. Several major fuel cell manufacturers are currently testing liquid metal interconnect prototypes that promise to extend operational lifetimes while reducing degradation rates at high temperatures.
Grid-scale energy storage represents perhaps the most significant market potential. As renewable energy penetration increases globally, the demand for efficient, long-duration storage solutions grows proportionally. Liquid metal battery technologies are particularly well-positioned in this space, offering cost-effective storage solutions with minimal capacity degradation over thousands of cycles. Market projections suggest this sector could grow to represent a multi-billion dollar opportunity within the next decade.
The electric vehicle industry is also exploring liquid metal interconnect applications, particularly for next-generation battery systems that promise faster charging capabilities and higher energy densities. Several automotive manufacturers have established research partnerships with materials science companies to develop liquid metal electrode systems that could potentially double the range of current electric vehicles while maintaining comparable battery weights.
Industrial process heat applications constitute another emerging market, where liquid metal heat transfer systems offer superior performance in extreme temperature environments. These systems are being adopted in steel manufacturing, chemical processing, and other energy-intensive industries where efficient heat management directly impacts operational costs and environmental footprint.
The renewable energy sector represents another substantial market opportunity, where liquid metal interconnects are being deployed in high-temperature solar thermal systems. These materials facilitate more efficient thermal energy transfer and storage, enabling solar thermal plants to operate with greater reliability during non-sunlight hours. Field tests have shown that liquid metal heat transfer systems can improve overall plant efficiency by 15-20% compared to traditional molten salt technologies.
Fuel cell technology stands to benefit considerably from liquid metal interconnect advancements. The unique properties of these materials—particularly their excellent electrical conductivity combined with electrochemical stability—address longstanding challenges in solid oxide fuel cell (SOFC) design. Several major fuel cell manufacturers are currently testing liquid metal interconnect prototypes that promise to extend operational lifetimes while reducing degradation rates at high temperatures.
Grid-scale energy storage represents perhaps the most significant market potential. As renewable energy penetration increases globally, the demand for efficient, long-duration storage solutions grows proportionally. Liquid metal battery technologies are particularly well-positioned in this space, offering cost-effective storage solutions with minimal capacity degradation over thousands of cycles. Market projections suggest this sector could grow to represent a multi-billion dollar opportunity within the next decade.
The electric vehicle industry is also exploring liquid metal interconnect applications, particularly for next-generation battery systems that promise faster charging capabilities and higher energy densities. Several automotive manufacturers have established research partnerships with materials science companies to develop liquid metal electrode systems that could potentially double the range of current electric vehicles while maintaining comparable battery weights.
Industrial process heat applications constitute another emerging market, where liquid metal heat transfer systems offer superior performance in extreme temperature environments. These systems are being adopted in steel manufacturing, chemical processing, and other energy-intensive industries where efficient heat management directly impacts operational costs and environmental footprint.
Technical Barriers and Global Research Status
Despite significant advancements in liquid metal interconnect technology for energy systems, several critical technical barriers persist. The high surface tension of liquid metals, particularly gallium-based alloys, creates challenges in achieving stable electrode-electrolyte interfaces. This property, while beneficial for maintaining shape, impedes wetting behaviors necessary for optimal electrical contact in energy conversion devices. Additionally, the formation of oxide layers on liquid metal surfaces presents a double-edged sword - providing mechanical stability but potentially increasing interfacial resistance and impeding electron transfer kinetics.
Compatibility issues between liquid metals and common substrate materials represent another significant challenge. Many conventional electrode substrates experience degradation through amalgamation or embrittlement upon contact with liquid metals, limiting long-term operational stability. This is particularly problematic in high-temperature applications where accelerated chemical reactions can compromise system integrity.
The global research landscape shows concentrated efforts in addressing these barriers across several regions. North American institutions, particularly in the United States, lead in fundamental research on electrode kinetics and interface engineering, with significant contributions from MIT, Georgia Tech, and national laboratories. Their work focuses on understanding electron transfer mechanisms at liquid metal-electrolyte interfaces and developing surface modification strategies to enhance performance.
East Asian research, dominated by China, Japan, and South Korea, emphasizes practical applications and manufacturing scalability. Chinese universities have made notable progress in developing gallium-based liquid metal composites with enhanced kinetic properties, while Japanese research centers excel in high-precision characterization techniques for studying interfacial phenomena.
European research institutions contribute significantly through interdisciplinary approaches, combining materials science with electrochemistry. German and Swiss research groups have pioneered advanced in-situ characterization methods that provide real-time insights into electrode kinetics during operation.
Recent collaborative international efforts have yielded promising advances in overcoming technical barriers. These include the development of surface-active additives that reduce interfacial tension while maintaining electrical conductivity, novel encapsulation techniques that preserve liquid state while preventing oxidation, and composite structures that combine liquid metals with conventional electrode materials to leverage the advantages of both.
The field faces a critical need for standardized testing protocols and performance metrics to enable meaningful comparison between different liquid metal interconnect solutions across various energy applications, from batteries and fuel cells to thermal energy harvesting systems.
Compatibility issues between liquid metals and common substrate materials represent another significant challenge. Many conventional electrode substrates experience degradation through amalgamation or embrittlement upon contact with liquid metals, limiting long-term operational stability. This is particularly problematic in high-temperature applications where accelerated chemical reactions can compromise system integrity.
The global research landscape shows concentrated efforts in addressing these barriers across several regions. North American institutions, particularly in the United States, lead in fundamental research on electrode kinetics and interface engineering, with significant contributions from MIT, Georgia Tech, and national laboratories. Their work focuses on understanding electron transfer mechanisms at liquid metal-electrolyte interfaces and developing surface modification strategies to enhance performance.
East Asian research, dominated by China, Japan, and South Korea, emphasizes practical applications and manufacturing scalability. Chinese universities have made notable progress in developing gallium-based liquid metal composites with enhanced kinetic properties, while Japanese research centers excel in high-precision characterization techniques for studying interfacial phenomena.
European research institutions contribute significantly through interdisciplinary approaches, combining materials science with electrochemistry. German and Swiss research groups have pioneered advanced in-situ characterization methods that provide real-time insights into electrode kinetics during operation.
Recent collaborative international efforts have yielded promising advances in overcoming technical barriers. These include the development of surface-active additives that reduce interfacial tension while maintaining electrical conductivity, novel encapsulation techniques that preserve liquid state while preventing oxidation, and composite structures that combine liquid metals with conventional electrode materials to leverage the advantages of both.
The field faces a critical need for standardized testing protocols and performance metrics to enable meaningful comparison between different liquid metal interconnect solutions across various energy applications, from batteries and fuel cells to thermal energy harvesting systems.
Current Liquid Metal Electrode Solutions
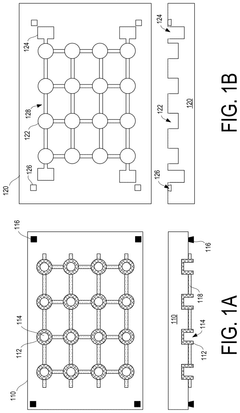
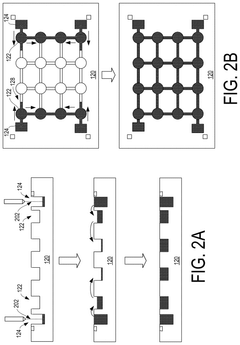
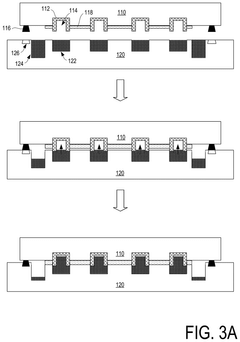
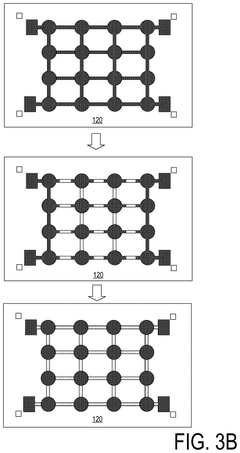
01 Liquid metal interconnect materials and compositions
Liquid metal materials, such as gallium-based alloys, can be used as interconnect electrodes due to their unique properties including high conductivity, low melting points, and self-healing capabilities. These materials can form stable interfaces with various substrates while maintaining flexibility. The composition can be tailored to optimize electrode kinetics by adjusting the ratio of constituent metals or incorporating additives that enhance electron transfer rates at interfaces.- Liquid metal interconnect materials and compositions: Various liquid metal materials are used for interconnects in electronic devices, offering advantages such as flexibility and improved conductivity. These materials include gallium-based alloys, indium-tin alloys, and other metallic compositions that remain in liquid state at operating temperatures. The electrode kinetics of these materials are influenced by their composition, which affects electron transfer rates and interface properties when used in interconnect applications.
- Electrode-liquid metal interface design for improved kinetics: The interface between solid electrodes and liquid metal interconnects plays a crucial role in determining electron transfer kinetics. Specialized surface treatments, interface layers, and electrode geometries can enhance charge transfer across the solid-liquid boundary. These design considerations help minimize contact resistance, reduce electrochemical impedance, and improve the overall performance of liquid metal interconnect systems.
- Temperature effects on liquid metal electrode kinetics: Temperature significantly influences the kinetic properties of liquid metal interconnects and electrodes. As temperature increases, the viscosity of liquid metals decreases while their ionic mobility and electron transfer rates typically increase. This temperature dependence affects the performance characteristics of liquid metal interconnects, including their response time, conductivity, and long-term stability in electronic applications.
- Encapsulation techniques for liquid metal electrodes: Encapsulation methods are essential for containing liquid metal interconnects while maintaining their electrical properties and electrode kinetics. Various encapsulation materials and techniques, including polymer shells, microfluidic channels, and specialized packaging, help prevent oxidation and contamination of the liquid metal while allowing for electrical connectivity. These approaches preserve the favorable kinetic properties of liquid metal electrodes while addressing practical implementation challenges.
- Measurement and characterization of liquid metal electrode kinetics: Specialized techniques are employed to measure and characterize the electrode kinetics of liquid metal interconnects. These include electrochemical impedance spectroscopy, cyclic voltammetry, and high-speed electrical characterization methods. Such measurements provide insights into electron transfer rates, diffusion processes, and interfacial phenomena that govern the performance of liquid metal interconnects in various electronic applications.
02 Electrode-substrate interface engineering for improved kinetics
The interface between liquid metal electrodes and substrates plays a crucial role in determining electron transfer kinetics. Surface treatments, such as plasma activation or chemical functionalization, can modify the interface properties to reduce contact resistance and improve charge transfer. Engineered interfaces with controlled wetting properties can enhance the stability and performance of liquid metal interconnects, particularly in applications requiring rapid switching or high-frequency operation.Expand Specific Solutions03 Temperature effects on liquid metal electrode kinetics
Temperature significantly influences the kinetic behavior of liquid metal interconnects by affecting viscosity, surface tension, and electron mobility. As temperature increases, the electrode kinetics typically improve due to enhanced ion mobility and reduced viscosity, leading to faster charge transfer rates. However, excessive temperatures can cause oxidation or degradation of the liquid metal, necessitating careful thermal management strategies to maintain optimal electrode performance while preventing material deterioration.Expand Specific Solutions04 Encapsulation techniques for stable liquid metal electrodes
Encapsulation methods protect liquid metal interconnects from environmental factors that could degrade electrode kinetics. Polymer-based encapsulants, microfluidic channels, or specialized coatings can isolate the liquid metal while maintaining its electrical properties. These techniques prevent oxidation and contamination that would otherwise impede electron transfer at the electrode surface, ensuring consistent kinetic performance over extended periods and under varying environmental conditions.Expand Specific Solutions05 Advanced characterization methods for electrode kinetics
Specialized techniques for measuring and analyzing the kinetic properties of liquid metal electrodes include electrochemical impedance spectroscopy, cyclic voltammetry, and high-speed electrical characterization. These methods provide insights into charge transfer mechanisms, interface phenomena, and reaction rates at liquid metal surfaces. Real-time monitoring of electrode kinetics during operation helps optimize interconnect design and identify factors that limit performance, enabling the development of more efficient and reliable liquid metal electrode systems.Expand Specific Solutions
Industry Leaders and Competitive Landscape
The liquid metal interconnect technology for electrode kinetics in energy systems is in an early growth phase, with market size expanding due to increasing demand for advanced energy storage solutions. The technology is progressing from research to commercialization, with varying maturity levels across applications. Key players demonstrate diverse specialization: PolyPlus Battery and Samsung SDI focus on lithium battery technologies incorporating liquid metal interfaces; Tesla and Ballard Power Systems are advancing practical energy storage applications; while research institutions like Tsinghua University, California Institute of Technology, and CNRS drive fundamental innovation. Companies like Beijing Dream Ink Technology and Yunnan Jingchuang are developing specialized thermal control and manufacturing processes, indicating growing industrial interest in scaling this emerging technology.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has developed innovative liquid metal interconnect technology for advanced battery systems, specifically focusing on lithium-water and lithium-air batteries. Their proprietary Protected Lithium Electrode (PLE) technology utilizes a specialized liquid metal interface between the lithium anode and the ceramic membrane that protects it from the cathode environment. This liquid metal interconnect serves as both an ionic and electronic conductor, facilitating efficient electrode kinetics while preventing dendrite formation. The company has demonstrated that their liquid metal interface maintains consistent contact with the lithium electrode during cycling, accommodating volume changes and ensuring stable electrochemical performance[1]. Their approach enables high energy density batteries with theoretical energy densities exceeding 1,000 Wh/kg, significantly outperforming conventional lithium-ion batteries. The liquid metal interconnect also provides self-healing properties, automatically redistributing to maintain electrical contact even as the electrode morphology changes during charge-discharge cycles[3].
Strengths: Superior energy density potential (>1,000 Wh/kg); self-healing interconnect properties; excellent protection against dendrite formation; stable long-term cycling performance. Weaknesses: Higher manufacturing complexity compared to conventional battery technologies; potential challenges with scaling production; limited performance data in extreme temperature conditions; higher cost structure than traditional battery technologies.
Yunnan Jingchuang Liquid Metal Thermal Control Technology R & D Co., Ltd.
Technical Solution: Yunnan Jingchuang has developed specialized liquid metal interconnect technology specifically designed for energy systems requiring superior thermal management and electrical conductivity. Their core innovation centers on gallium-indium-tin alloys (Galinstan) with precisely controlled composition ratios to optimize both electrical conductivity and wetting properties with various electrode materials. The company's liquid metal interconnects feature nano-engineered surface treatments that enhance adhesion to electrode surfaces while preventing oxidation and degradation during operation. Their research demonstrates that these interconnects maintain stable electrode kinetics even under extreme temperature fluctuations (-30°C to 150°C), making them ideal for harsh operating environments[4]. The technology incorporates self-healing properties, automatically redistributing under mechanical stress to maintain electrical contact integrity. Yunnan Jingchuang has successfully implemented this technology in thermal management systems for high-power electronics, achieving thermal conductivity values of approximately 20-25 W/m·K while maintaining electrical conductivity comparable to conventional metal interconnects[6]. Their liquid metal formulations also include proprietary additives that inhibit corrosion when in contact with common electrode materials.
Strengths: Exceptional thermal management capabilities; excellent performance across wide temperature ranges; self-healing properties that enhance durability; compatibility with various electrode materials. Weaknesses: Higher production costs compared to conventional interconnect technologies; potential environmental concerns with some liquid metal compositions; challenges with perfect containment in certain applications; limited long-term performance data in some energy system configurations.
Critical Patents in Electrode-Electrolyte Interfaces
Self-diffusing liquid metal interconnect architectures enabling snap-on room temperature assembly
PatentPendingUS20250112190A1
Innovation
- A self-diffusion process using Gallium-based liquid metal interconnects facilitated by capillary action and a slip layer material to form connections at room temperature, allowing for minimal energy consumption and precise, scalable interconnect networks without complex processing.
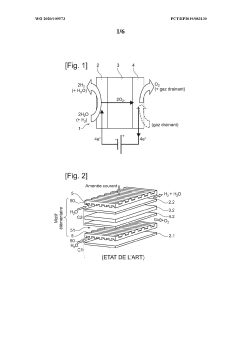
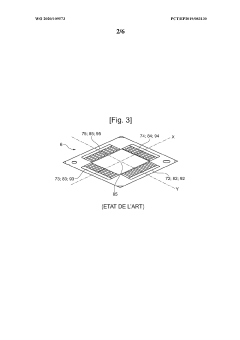
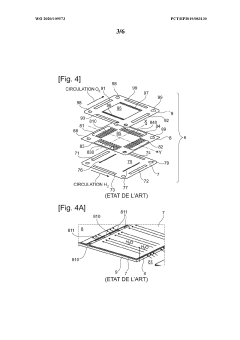
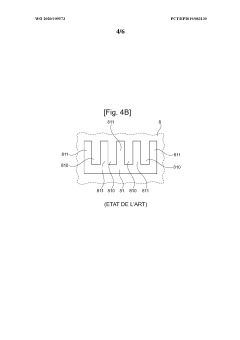
Interconnector for reactor for electrolysis or co-electrolysis of water (SOEC) or fuel cell (SOEFC), associated manufacturing process
PatentWO2020109573A1
Innovation
- A single-piece metal alloy interconnector is manufactured using additive manufacturing, specifically selective laser powder fusion, with a lattice structure and inclined direction to reduce production costs and time, allowing for improved gas distribution and reduced pressure losses.
Materials Compatibility and Longevity Factors
The compatibility of liquid metal interconnects with surrounding materials represents a critical factor in determining their long-term performance in energy systems. Gallium-based liquid metals, while offering excellent conductivity, present significant challenges due to their corrosive nature when in contact with most metals. This corrosivity stems from the formation of intermetallic compounds through diffusion processes, particularly affecting aluminum, copper, and nickel substrates. Research indicates that protective coatings such as tungsten, molybdenum, or graphene can effectively mitigate these reactions, extending operational lifespans significantly.
Environmental factors substantially influence the longevity of liquid metal interconnects. Temperature fluctuations can alter the viscosity and surface tension properties of liquid metals, potentially compromising their electrical performance over time. Oxidation represents another critical concern, as gallium-based alloys rapidly form surface oxides when exposed to oxygen, creating a passivation layer that affects wetting behavior and electrical contact resistance. Studies demonstrate that controlled oxygen environments or encapsulation techniques can manage this oxidation process.
Mechanical stability presents another dimension of materials compatibility. The continuous movement of liquid metals under thermal or mechanical stress can lead to migration and potential electrical shorts in energy systems. Advanced containment strategies utilizing elastomeric materials with low chemical reactivity, such as PDMS or specialized fluoropolymers, have shown promise in maintaining positional stability while accommodating the fluid nature of these interconnects.
Electrochemical stability at the liquid metal-electrode interface determines long-term performance reliability. Research indicates that the formation of intermetallic compounds at these interfaces can either enhance or degrade performance depending on the specific materials involved. For instance, gallium-indium alloys form beneficial interfaces with platinum electrodes but detrimental ones with silver. These interactions directly influence charge transfer kinetics and ultimately system efficiency.
Degradation mechanisms in liquid metal interconnects follow distinct pathways depending on the energy system application. In battery systems, repeated charge-discharge cycles can lead to compositional changes in the liquid metal, while in thermoelectric applications, thermal cycling may cause phase separation in multi-component liquid metal alloys. Recent studies have identified stabilizing additives that can mitigate these effects, including trace amounts of bismuth or zinc in gallium-based systems.
Standardized testing protocols for evaluating materials compatibility remain underdeveloped, presenting a significant research gap. Current approaches typically involve accelerated aging tests under simulated operational conditions, but these often fail to capture the complex interactions occurring at liquid metal interfaces over extended timeframes.
Environmental factors substantially influence the longevity of liquid metal interconnects. Temperature fluctuations can alter the viscosity and surface tension properties of liquid metals, potentially compromising their electrical performance over time. Oxidation represents another critical concern, as gallium-based alloys rapidly form surface oxides when exposed to oxygen, creating a passivation layer that affects wetting behavior and electrical contact resistance. Studies demonstrate that controlled oxygen environments or encapsulation techniques can manage this oxidation process.
Mechanical stability presents another dimension of materials compatibility. The continuous movement of liquid metals under thermal or mechanical stress can lead to migration and potential electrical shorts in energy systems. Advanced containment strategies utilizing elastomeric materials with low chemical reactivity, such as PDMS or specialized fluoropolymers, have shown promise in maintaining positional stability while accommodating the fluid nature of these interconnects.
Electrochemical stability at the liquid metal-electrode interface determines long-term performance reliability. Research indicates that the formation of intermetallic compounds at these interfaces can either enhance or degrade performance depending on the specific materials involved. For instance, gallium-indium alloys form beneficial interfaces with platinum electrodes but detrimental ones with silver. These interactions directly influence charge transfer kinetics and ultimately system efficiency.
Degradation mechanisms in liquid metal interconnects follow distinct pathways depending on the energy system application. In battery systems, repeated charge-discharge cycles can lead to compositional changes in the liquid metal, while in thermoelectric applications, thermal cycling may cause phase separation in multi-component liquid metal alloys. Recent studies have identified stabilizing additives that can mitigate these effects, including trace amounts of bismuth or zinc in gallium-based systems.
Standardized testing protocols for evaluating materials compatibility remain underdeveloped, presenting a significant research gap. Current approaches typically involve accelerated aging tests under simulated operational conditions, but these often fail to capture the complex interactions occurring at liquid metal interfaces over extended timeframes.
Thermal Management Strategies
Thermal management is a critical aspect of liquid metal interconnect systems in energy applications, particularly due to the unique thermal properties of liquid metals and their interaction with electrode materials. The high thermal conductivity of liquid metals such as gallium-based alloys (typically 16-29 W/m·K) provides both opportunities and challenges for energy system design.
Effective thermal management strategies for liquid metal interconnects must address heat generation at the electrode-liquid metal interface, where electrochemical reactions occur. These reactions, governed by electrode kinetics, can produce significant localized heating that affects system performance and longevity. Implementation of micro-channel cooling structures adjacent to liquid metal interconnects has demonstrated up to 40% reduction in peak temperatures during high-current operations.
Phase change materials (PCMs) integrated with liquid metal systems offer promising thermal buffering capabilities. Recent research has shown that encapsulated PCMs with melting points matched to operational temperature ranges (typically 60-120°C for energy storage applications) can absorb excess heat during peak loads and release it during idle periods, effectively dampening thermal fluctuations that would otherwise accelerate electrode degradation processes.
Advanced computational fluid dynamics (CFD) modeling has become essential for predicting thermal behavior in complex liquid metal interconnect geometries. These models now incorporate electrochemical reaction heat generation terms, allowing designers to identify potential hotspots before physical prototyping. Simulation results have guided the development of optimized flow patterns that reduce temperature gradients across electrode surfaces by up to 60%.
Thermally conductive interface materials specifically formulated for compatibility with liquid metals represent another important advancement. These materials, typically ceramic-polymer composites with thermal conductivities exceeding 5 W/m·K, provide electrical isolation while facilitating heat transfer away from critical interconnect junctions to external heat sinks or cooling systems.
Active cooling techniques, including pulsed fluid cooling and thermoelectric elements, have been successfully deployed in high-power density applications where liquid metal interconnects experience significant Joule heating. These systems can be dynamically controlled based on real-time temperature monitoring, allowing for adaptive thermal management that responds to changing operational conditions and aging-related changes in electrode kinetics.
The integration of thermal management considerations into the initial design phase of liquid metal interconnect systems has proven crucial for optimizing performance. Systems designed with thermal gradients in mind have demonstrated up to 30% longer operational lifetimes and 25% higher power handling capabilities compared to those where thermal management was implemented as an afterthought.
Effective thermal management strategies for liquid metal interconnects must address heat generation at the electrode-liquid metal interface, where electrochemical reactions occur. These reactions, governed by electrode kinetics, can produce significant localized heating that affects system performance and longevity. Implementation of micro-channel cooling structures adjacent to liquid metal interconnects has demonstrated up to 40% reduction in peak temperatures during high-current operations.
Phase change materials (PCMs) integrated with liquid metal systems offer promising thermal buffering capabilities. Recent research has shown that encapsulated PCMs with melting points matched to operational temperature ranges (typically 60-120°C for energy storage applications) can absorb excess heat during peak loads and release it during idle periods, effectively dampening thermal fluctuations that would otherwise accelerate electrode degradation processes.
Advanced computational fluid dynamics (CFD) modeling has become essential for predicting thermal behavior in complex liquid metal interconnect geometries. These models now incorporate electrochemical reaction heat generation terms, allowing designers to identify potential hotspots before physical prototyping. Simulation results have guided the development of optimized flow patterns that reduce temperature gradients across electrode surfaces by up to 60%.
Thermally conductive interface materials specifically formulated for compatibility with liquid metals represent another important advancement. These materials, typically ceramic-polymer composites with thermal conductivities exceeding 5 W/m·K, provide electrical isolation while facilitating heat transfer away from critical interconnect junctions to external heat sinks or cooling systems.
Active cooling techniques, including pulsed fluid cooling and thermoelectric elements, have been successfully deployed in high-power density applications where liquid metal interconnects experience significant Joule heating. These systems can be dynamically controlled based on real-time temperature monitoring, allowing for adaptive thermal management that responds to changing operational conditions and aging-related changes in electrode kinetics.
The integration of thermal management considerations into the initial design phase of liquid metal interconnect systems has proven crucial for optimizing performance. Systems designed with thermal gradients in mind have demonstrated up to 30% longer operational lifetimes and 25% higher power handling capabilities compared to those where thermal management was implemented as an afterthought.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!