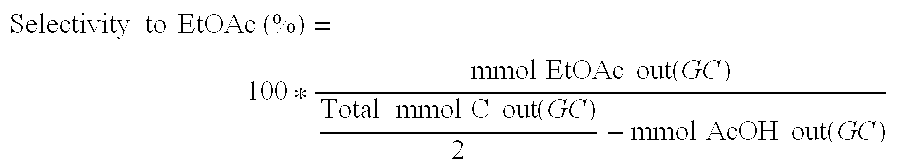Exploring the Extensive Uses of Ethyl Acetate in New Industries
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has been a staple in various industries for decades. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its unique properties, including low toxicity, high solvency, and rapid evaporation rate, have made it an indispensable component in numerous applications.
Traditionally, ethyl acetate has been widely used in the production of paints, coatings, and adhesives due to its excellent solvent properties. It effectively dissolves a wide range of resins and polymers, making it an ideal choice for these industries. In the pharmaceutical sector, ethyl acetate plays a crucial role in the extraction and purification of various drugs and active ingredients, owing to its ability to selectively dissolve certain compounds.
The food industry has long relied on ethyl acetate as a flavoring agent and extraction solvent. Its natural presence in fruits and wines contributes to their characteristic aromas. In the production of decaffeinated coffee and tea, ethyl acetate serves as an efficient and safe solvent for removing caffeine from the beans and leaves.
In recent years, the demand for ethyl acetate has expanded into new and emerging industries. The electronics sector, for instance, has found applications for ethyl acetate in the manufacturing of printed circuit boards and in cleaning processes for delicate electronic components. Its low residue properties make it particularly suitable for these precision-oriented tasks.
The growing emphasis on sustainability has also opened up new avenues for ethyl acetate usage. As a bio-based solvent, it is increasingly being considered as an environmentally friendly alternative to petroleum-derived solvents in various applications. This shift aligns with the global trend towards greener and more sustainable industrial processes.
Furthermore, the cosmetics and personal care industry has been exploring innovative uses of ethyl acetate. Its mild nature and pleasant scent make it an attractive ingredient in nail polish removers, perfumes, and other beauty products. The compound's ability to blend well with other ingredients while maintaining stability has contributed to its rising popularity in this sector.
As industries continue to evolve and new technologies emerge, the potential applications for ethyl acetate are likely to expand further. Its versatility, coupled with ongoing research into its properties and potential modifications, positions ethyl acetate as a compound of significant interest for future industrial innovations and sustainable practices.
Traditionally, ethyl acetate has been widely used in the production of paints, coatings, and adhesives due to its excellent solvent properties. It effectively dissolves a wide range of resins and polymers, making it an ideal choice for these industries. In the pharmaceutical sector, ethyl acetate plays a crucial role in the extraction and purification of various drugs and active ingredients, owing to its ability to selectively dissolve certain compounds.
The food industry has long relied on ethyl acetate as a flavoring agent and extraction solvent. Its natural presence in fruits and wines contributes to their characteristic aromas. In the production of decaffeinated coffee and tea, ethyl acetate serves as an efficient and safe solvent for removing caffeine from the beans and leaves.
In recent years, the demand for ethyl acetate has expanded into new and emerging industries. The electronics sector, for instance, has found applications for ethyl acetate in the manufacturing of printed circuit boards and in cleaning processes for delicate electronic components. Its low residue properties make it particularly suitable for these precision-oriented tasks.
The growing emphasis on sustainability has also opened up new avenues for ethyl acetate usage. As a bio-based solvent, it is increasingly being considered as an environmentally friendly alternative to petroleum-derived solvents in various applications. This shift aligns with the global trend towards greener and more sustainable industrial processes.
Furthermore, the cosmetics and personal care industry has been exploring innovative uses of ethyl acetate. Its mild nature and pleasant scent make it an attractive ingredient in nail polish removers, perfumes, and other beauty products. The compound's ability to blend well with other ingredients while maintaining stability has contributed to its rising popularity in this sector.
As industries continue to evolve and new technologies emerge, the potential applications for ethyl acetate are likely to expand further. Its versatility, coupled with ongoing research into its properties and potential modifications, positions ethyl acetate as a compound of significant interest for future industrial innovations and sustainable practices.
Market Demand Analysis
The market demand for ethyl acetate has been experiencing significant growth across various industries, driven by its versatile properties and expanding applications. In the coatings and paints sector, ethyl acetate's excellent solvency and fast evaporation rate have made it a preferred choice for manufacturers seeking to improve product performance and reduce environmental impact. This trend is particularly evident in the automotive and construction industries, where demand for high-quality, low-VOC coatings continues to rise.
The pharmaceutical industry represents another major growth area for ethyl acetate. Its use as a solvent in drug formulation and as an extraction medium in the production of antibiotics has led to increased demand. The global push for more efficient and environmentally friendly pharmaceutical manufacturing processes has further bolstered ethyl acetate's position in this sector.
In the food and beverage industry, ethyl acetate's role as a flavoring agent and extraction solvent has seen steady growth. The rising consumer preference for natural and clean-label products has driven manufacturers to explore ethyl acetate as a safer alternative to synthetic additives. Its application in decaffeination processes for coffee and tea has also contributed to market expansion.
The electronics industry has emerged as a promising new market for ethyl acetate. Its use in the production of flexible printed circuits and as a cleaning agent for electronic components has grown in tandem with the increasing demand for consumer electronics and smart devices. The ongoing miniaturization trend in electronics manufacturing has further emphasized the need for high-purity solvents like ethyl acetate.
The adhesives and sealants market has also shown increased adoption of ethyl acetate, particularly in the packaging industry. Its fast-drying properties and compatibility with various substrates make it an attractive option for manufacturers looking to improve production efficiency and product performance.
Geographically, Asia-Pacific has emerged as the fastest-growing market for ethyl acetate, driven by rapid industrialization and increasing manufacturing activities in countries like China and India. North America and Europe continue to be significant markets, with growth primarily fueled by innovations in sustainable and bio-based ethyl acetate production.
The global ethyl acetate market is projected to maintain a steady growth trajectory, with analysts forecasting a compound annual growth rate (CAGR) between 5% and 7% over the next five years. This growth is expected to be driven by ongoing innovations in application technologies, increasing demand from emerging economies, and the shift towards more sustainable and eco-friendly industrial processes across various sectors.
The pharmaceutical industry represents another major growth area for ethyl acetate. Its use as a solvent in drug formulation and as an extraction medium in the production of antibiotics has led to increased demand. The global push for more efficient and environmentally friendly pharmaceutical manufacturing processes has further bolstered ethyl acetate's position in this sector.
In the food and beverage industry, ethyl acetate's role as a flavoring agent and extraction solvent has seen steady growth. The rising consumer preference for natural and clean-label products has driven manufacturers to explore ethyl acetate as a safer alternative to synthetic additives. Its application in decaffeination processes for coffee and tea has also contributed to market expansion.
The electronics industry has emerged as a promising new market for ethyl acetate. Its use in the production of flexible printed circuits and as a cleaning agent for electronic components has grown in tandem with the increasing demand for consumer electronics and smart devices. The ongoing miniaturization trend in electronics manufacturing has further emphasized the need for high-purity solvents like ethyl acetate.
The adhesives and sealants market has also shown increased adoption of ethyl acetate, particularly in the packaging industry. Its fast-drying properties and compatibility with various substrates make it an attractive option for manufacturers looking to improve production efficiency and product performance.
Geographically, Asia-Pacific has emerged as the fastest-growing market for ethyl acetate, driven by rapid industrialization and increasing manufacturing activities in countries like China and India. North America and Europe continue to be significant markets, with growth primarily fueled by innovations in sustainable and bio-based ethyl acetate production.
The global ethyl acetate market is projected to maintain a steady growth trajectory, with analysts forecasting a compound annual growth rate (CAGR) between 5% and 7% over the next five years. This growth is expected to be driven by ongoing innovations in application technologies, increasing demand from emerging economies, and the shift towards more sustainable and eco-friendly industrial processes across various sectors.
Technical Challenges
Despite the widespread use of ethyl acetate in various industries, several technical challenges persist in expanding its applications to new sectors. One of the primary obstacles is the compound's high volatility, which can lead to significant losses during storage and transportation. This characteristic also poses safety concerns, as ethyl acetate is highly flammable and can form explosive mixtures with air.
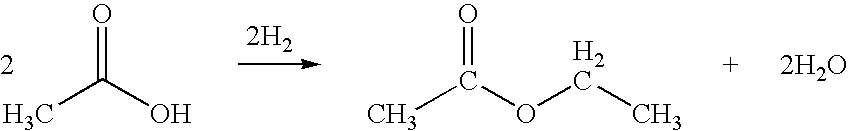
Another challenge lies in the production process of ethyl acetate. The traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid is energy-intensive and requires the use of strong acid catalysts. This process often results in unwanted side reactions and the formation of byproducts, which can affect the purity of the final product and increase production costs.
The purification of ethyl acetate presents additional technical hurdles. Conventional distillation methods are not always effective in separating ethyl acetate from its azeotropic mixtures with water and other solvents. This limitation can impact the quality of the product and its suitability for certain high-purity applications in new industries.
Environmental concerns also pose significant challenges to the expanded use of ethyl acetate. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone. Developing eco-friendly production methods and implementing effective emission control strategies are crucial for its sustainable use in new industries.
The stability of ethyl acetate in various formulations and under different environmental conditions is another area of concern. In some applications, especially those involving long-term storage or exposure to extreme temperatures, ethyl acetate may degrade or react with other components, potentially compromising the integrity of the final product.
Furthermore, the development of new applications for ethyl acetate in industries such as pharmaceuticals, electronics, and advanced materials requires overcoming compatibility issues with diverse substrates and materials. Ensuring that ethyl acetate does not adversely affect the properties or performance of these materials is a complex challenge that demands extensive research and testing.
Lastly, the regulatory landscape surrounding the use of ethyl acetate in new industries presents a significant hurdle. Compliance with varying international standards and regulations regarding its use, handling, and disposal adds complexity to its adoption in novel applications. Addressing these regulatory challenges requires ongoing efforts in toxicological studies, safety assessments, and the development of industry-specific guidelines.
Another challenge lies in the production process of ethyl acetate. The traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid is energy-intensive and requires the use of strong acid catalysts. This process often results in unwanted side reactions and the formation of byproducts, which can affect the purity of the final product and increase production costs.
The purification of ethyl acetate presents additional technical hurdles. Conventional distillation methods are not always effective in separating ethyl acetate from its azeotropic mixtures with water and other solvents. This limitation can impact the quality of the product and its suitability for certain high-purity applications in new industries.
Environmental concerns also pose significant challenges to the expanded use of ethyl acetate. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone. Developing eco-friendly production methods and implementing effective emission control strategies are crucial for its sustainable use in new industries.
The stability of ethyl acetate in various formulations and under different environmental conditions is another area of concern. In some applications, especially those involving long-term storage or exposure to extreme temperatures, ethyl acetate may degrade or react with other components, potentially compromising the integrity of the final product.
Furthermore, the development of new applications for ethyl acetate in industries such as pharmaceuticals, electronics, and advanced materials requires overcoming compatibility issues with diverse substrates and materials. Ensuring that ethyl acetate does not adversely affect the properties or performance of these materials is a complex challenge that demands extensive research and testing.
Lastly, the regulatory landscape surrounding the use of ethyl acetate in new industries presents a significant hurdle. Compliance with varying international standards and regulations regarding its use, handling, and disposal adds complexity to its adoption in novel applications. Addressing these regulatory challenges requires ongoing efforts in toxicological studies, safety assessments, and the development of industry-specific guidelines.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve yield, efficiency, and purity of the final product.- Production and purification of ethyl acetate: Various methods are employed for the production and purification of ethyl acetate, including esterification reactions, distillation processes, and separation techniques. These methods aim to improve yield, purity, and efficiency in the manufacturing of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is widely used as a solvent and reagent in various chemical processes. It finds applications in extraction, synthesis, and as a reaction medium in different industries, including pharmaceuticals, coatings, and adhesives.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of polymers, as a solvent for resins, and in the development of advanced materials with specific properties.
- Environmental and safety considerations for ethyl acetate: Research focuses on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, enhancing recovery and recycling processes, and implementing safety measures in handling and storage.
- Novel applications and derivatives of ethyl acetate: Ongoing research explores new applications and derivatives of ethyl acetate. This includes its use in emerging technologies, development of novel compounds based on ethyl acetate, and its potential in sustainable chemistry applications.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other compounds. Its versatility makes it valuable in industries such as pharmaceuticals, polymers, and fine chemicals.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various substances, including natural products, pharmaceuticals, and other organic compounds. Its properties make it suitable for liquid-liquid extraction and chromatographic techniques.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.Expand Specific Solutions05 Novel applications and formulations containing ethyl acetate
Innovative applications and formulations incorporating ethyl acetate are being developed across various industries. These include its use in coatings, adhesives, cleaning products, and specialized industrial processes, leveraging its unique properties.Expand Specific Solutions
Key Industry Players
The market for ethyl acetate applications in new industries is experiencing dynamic growth, driven by increasing demand across various sectors. The industry is in a transitional phase, with established players like Celanese International Corp., Eastman Chemical Co., and BP Chemicals Ltd. leading the market. However, emerging companies and research institutions are actively exploring novel applications, indicating a maturing but still evolving market. The global ethyl acetate market size is projected to expand significantly, fueled by its versatility in industries such as pharmaceuticals, electronics, and sustainable materials. Technological advancements by key players like BASF Corp. and DuPont de Nemours, Inc. are enhancing the product's efficiency and expanding its potential uses, signaling a moderate to high level of technological maturity in the field.
Celanese International Corp.
Technical Solution: Celanese has developed advanced production methods for ethyl acetate, focusing on sustainability and efficiency. Their AcetylMax™ technology enables the direct production of ethyl acetate from ethylene and acetic acid, reducing energy consumption by up to 30% compared to traditional processes[1]. The company has also explored new applications in biodegradable plastics, where ethyl acetate serves as a key solvent in the production of polylactic acid (PLA) films[2]. Additionally, Celanese has invested in research for using ethyl acetate in advanced battery technologies, particularly as an electrolyte additive to improve the performance of lithium-ion batteries[3].
Strengths: Innovative production technology, diverse application research, strong market position. Weaknesses: Dependence on petrochemical feedstocks, potential regulatory challenges in some applications.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has focused on developing high-purity ethyl acetate for specialized industries. Their patented process produces ultra-low impurity ethyl acetate suitable for electronic and pharmaceutical applications[4]. The company has also pioneered the use of ethyl acetate in eco-friendly coating technologies, developing water-based formulations that reduce VOC emissions by up to 50% compared to traditional solvent-based coatings[5]. Eastman's research extends to using ethyl acetate as a green extraction solvent in the food and nutraceutical industries, offering a safer alternative to hexane in processes such as decaffeination and oil extraction[6].
Strengths: High-purity product offerings, focus on sustainable applications, strong presence in specialty markets. Weaknesses: Higher production costs for specialized grades, potential competition from bio-based alternatives.
Innovative Formulations
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentInactiveUS20100029980A1
Innovation
- A process utilizing a hydrogenating catalyst composed of metals like nickel, platinum, or palladium in combination with molybdenum, rhenium, zirconium, copper, or cobalt supported on catalysts such as silica or zeolites, which selectively converts acetic acid to ethyl acetate with high yield and selectivity.
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Environmental Impact
The environmental impact of ethyl acetate in new industries is a critical consideration as its usage expands. While ethyl acetate is generally considered less harmful than many other solvents, its increased production and application across diverse sectors raise important environmental concerns.
Atmospheric emissions are a primary environmental issue associated with ethyl acetate. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone and photochemical smog when released into the air. This can have detrimental effects on air quality, particularly in urban and industrial areas where ethyl acetate usage is concentrated.
Water pollution is another potential environmental risk. Although ethyl acetate is biodegradable and has low toxicity to aquatic life, large-scale industrial releases into water bodies can still pose threats to ecosystems. Proper wastewater treatment and management practices are essential to mitigate these risks.
The production process of ethyl acetate also carries environmental implications. Traditional methods often involve the use of sulfuric acid as a catalyst, which can lead to the generation of acidic waste streams. However, newer, greener production techniques are being developed to reduce these impacts, such as reactive distillation and enzymatic processes.
Soil contamination is a lesser concern but still relevant, particularly in cases of accidental spills or improper disposal. Ethyl acetate can potentially leach into groundwater, though its relatively rapid biodegradation helps limit long-term soil impacts.
On a positive note, the increasing use of ethyl acetate as a replacement for more harmful solvents in various industries can lead to overall environmental benefits. For instance, its application in eco-friendly paints and coatings helps reduce the emission of more toxic VOCs traditionally used in these products.
The lifecycle assessment of ethyl acetate is an important aspect of its environmental impact. While its production does require energy and resources, its potential for recycling and recovery in many applications can offset some of these impacts. Additionally, bio-based ethyl acetate derived from renewable resources is gaining attention as a more sustainable alternative to petrochemical-based production.
As new industries adopt ethyl acetate, there is a growing need for comprehensive environmental management strategies. This includes implementing efficient recovery and recycling systems, optimizing production processes to minimize waste and emissions, and developing more sustainable sourcing methods. Regulatory bodies are also likely to play a crucial role in setting and enforcing standards for ethyl acetate usage and disposal across these emerging applications.
Atmospheric emissions are a primary environmental issue associated with ethyl acetate. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone and photochemical smog when released into the air. This can have detrimental effects on air quality, particularly in urban and industrial areas where ethyl acetate usage is concentrated.
Water pollution is another potential environmental risk. Although ethyl acetate is biodegradable and has low toxicity to aquatic life, large-scale industrial releases into water bodies can still pose threats to ecosystems. Proper wastewater treatment and management practices are essential to mitigate these risks.
The production process of ethyl acetate also carries environmental implications. Traditional methods often involve the use of sulfuric acid as a catalyst, which can lead to the generation of acidic waste streams. However, newer, greener production techniques are being developed to reduce these impacts, such as reactive distillation and enzymatic processes.
Soil contamination is a lesser concern but still relevant, particularly in cases of accidental spills or improper disposal. Ethyl acetate can potentially leach into groundwater, though its relatively rapid biodegradation helps limit long-term soil impacts.
On a positive note, the increasing use of ethyl acetate as a replacement for more harmful solvents in various industries can lead to overall environmental benefits. For instance, its application in eco-friendly paints and coatings helps reduce the emission of more toxic VOCs traditionally used in these products.
The lifecycle assessment of ethyl acetate is an important aspect of its environmental impact. While its production does require energy and resources, its potential for recycling and recovery in many applications can offset some of these impacts. Additionally, bio-based ethyl acetate derived from renewable resources is gaining attention as a more sustainable alternative to petrochemical-based production.
As new industries adopt ethyl acetate, there is a growing need for comprehensive environmental management strategies. This includes implementing efficient recovery and recycling systems, optimizing production processes to minimize waste and emissions, and developing more sustainable sourcing methods. Regulatory bodies are also likely to play a crucial role in setting and enforcing standards for ethyl acetate usage and disposal across these emerging applications.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate usage in new industries is complex and multifaceted, reflecting the compound's diverse applications and potential environmental impacts. Globally, regulatory bodies have established guidelines for the production, handling, and use of ethyl acetate, considering its flammability, volatility, and potential health effects.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers, while the Food and Drug Administration (FDA) oversees its use in food-related applications. The European Union, through REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), imposes strict regulations on ethyl acetate's manufacture and import.
As new industries explore ethyl acetate's potential, they must navigate these existing frameworks while anticipating future regulatory changes. The cosmetics industry, for instance, faces scrutiny from the EU Cosmetics Regulation regarding the use of ethyl acetate in personal care products. In the pharmaceutical sector, Good Manufacturing Practice (GMP) guidelines govern its use as a solvent in drug production.
Emerging applications in green chemistry and sustainable manufacturing have prompted regulatory bodies to reassess ethyl acetate's environmental impact. Some jurisdictions are implementing stricter emissions controls and waste management protocols for industries utilizing this compound. The push towards circular economy principles is influencing regulations, encouraging the development of recycling and recovery processes for ethyl acetate.
Regulatory compliance in new industries often requires extensive documentation, safety assessments, and environmental impact studies. Companies must invest in robust quality control systems and employee training programs to meet these requirements. As global awareness of environmental issues grows, industries can expect increasingly stringent regulations on volatile organic compounds (VOCs), including ethyl acetate.
The regulatory landscape also varies significantly across different regions, presenting challenges for companies operating in multiple markets. Harmonization efforts are underway to streamline regulations, but discrepancies remain. This diversity necessitates a flexible approach to product development and market entry strategies for industries exploring new applications of ethyl acetate.
As research continues to uncover both benefits and potential risks associated with ethyl acetate use, regulatory frameworks are likely to evolve. Industries must stay abreast of these changes, engaging proactively with regulatory bodies and participating in the development of new standards. This dynamic regulatory environment underscores the importance of ongoing research and development to ensure compliance while maximizing the potential of ethyl acetate in innovative applications.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for workers, while the Food and Drug Administration (FDA) oversees its use in food-related applications. The European Union, through REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), imposes strict regulations on ethyl acetate's manufacture and import.
As new industries explore ethyl acetate's potential, they must navigate these existing frameworks while anticipating future regulatory changes. The cosmetics industry, for instance, faces scrutiny from the EU Cosmetics Regulation regarding the use of ethyl acetate in personal care products. In the pharmaceutical sector, Good Manufacturing Practice (GMP) guidelines govern its use as a solvent in drug production.
Emerging applications in green chemistry and sustainable manufacturing have prompted regulatory bodies to reassess ethyl acetate's environmental impact. Some jurisdictions are implementing stricter emissions controls and waste management protocols for industries utilizing this compound. The push towards circular economy principles is influencing regulations, encouraging the development of recycling and recovery processes for ethyl acetate.
Regulatory compliance in new industries often requires extensive documentation, safety assessments, and environmental impact studies. Companies must invest in robust quality control systems and employee training programs to meet these requirements. As global awareness of environmental issues grows, industries can expect increasingly stringent regulations on volatile organic compounds (VOCs), including ethyl acetate.
The regulatory landscape also varies significantly across different regions, presenting challenges for companies operating in multiple markets. Harmonization efforts are underway to streamline regulations, but discrepancies remain. This diversity necessitates a flexible approach to product development and market entry strategies for industries exploring new applications of ethyl acetate.
As research continues to uncover both benefits and potential risks associated with ethyl acetate use, regulatory frameworks are likely to evolve. Industries must stay abreast of these changes, engaging proactively with regulatory bodies and participating in the development of new standards. This dynamic regulatory environment underscores the importance of ongoing research and development to ensure compliance while maximizing the potential of ethyl acetate in innovative applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!