How Ethyl Acetate Addresses Interdisciplinary Industry Needs?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has emerged as a crucial substance in various industries due to its unique properties and wide-ranging applications. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its low toxicity, high solvency, and moderate volatility make it an ideal choice for numerous industrial processes.
In the chemical industry, ethyl acetate serves as a key intermediate in the synthesis of various compounds, including pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an excellent solvent for paints, coatings, and adhesives, contributing to its significant role in the manufacturing sector. The electronics industry also benefits from ethyl acetate's properties, utilizing it in the production of circuit boards and as a cleaning agent for delicate components.
The pharmaceutical sector relies heavily on ethyl acetate for drug formulation and as an extraction solvent in the production of antibiotics and vitamins. Its low toxicity and high purity standards make it suitable for use in medications and dietary supplements. In the food industry, ethyl acetate is employed as a flavoring agent and in the decaffeination of coffee and tea, showcasing its versatility across different applications.
Environmental considerations have further boosted the demand for ethyl acetate. As a more environmentally friendly alternative to many traditional solvents, it aligns with the growing trend towards sustainable industrial practices. Its biodegradability and lower environmental impact compared to some petroleum-based solvents have made it increasingly popular in green chemistry initiatives and eco-friendly product formulations.
The global market for ethyl acetate has been experiencing steady growth, driven by increasing demand from end-use industries and emerging applications. Asia-Pacific region, particularly China and India, has become a major consumer and producer of ethyl acetate, reflecting the shift in global manufacturing trends. The compound's role in addressing interdisciplinary industry needs is evident in its diverse applications, from high-tech electronics to everyday consumer products, demonstrating its significance in modern industrial processes and product development.
In the chemical industry, ethyl acetate serves as a key intermediate in the synthesis of various compounds, including pharmaceuticals, plastics, and other specialty chemicals. Its ability to dissolve a wide range of organic substances makes it an excellent solvent for paints, coatings, and adhesives, contributing to its significant role in the manufacturing sector. The electronics industry also benefits from ethyl acetate's properties, utilizing it in the production of circuit boards and as a cleaning agent for delicate components.
The pharmaceutical sector relies heavily on ethyl acetate for drug formulation and as an extraction solvent in the production of antibiotics and vitamins. Its low toxicity and high purity standards make it suitable for use in medications and dietary supplements. In the food industry, ethyl acetate is employed as a flavoring agent and in the decaffeination of coffee and tea, showcasing its versatility across different applications.
Environmental considerations have further boosted the demand for ethyl acetate. As a more environmentally friendly alternative to many traditional solvents, it aligns with the growing trend towards sustainable industrial practices. Its biodegradability and lower environmental impact compared to some petroleum-based solvents have made it increasingly popular in green chemistry initiatives and eco-friendly product formulations.
The global market for ethyl acetate has been experiencing steady growth, driven by increasing demand from end-use industries and emerging applications. Asia-Pacific region, particularly China and India, has become a major consumer and producer of ethyl acetate, reflecting the shift in global manufacturing trends. The compound's role in addressing interdisciplinary industry needs is evident in its diverse applications, from high-tech electronics to everyday consumer products, demonstrating its significance in modern industrial processes and product development.
Market Analysis
The market for ethyl acetate has shown significant growth and diversification across multiple industries in recent years. This versatile chemical compound has found applications in various sectors, including coatings, adhesives, pharmaceuticals, food and beverages, and electronics, driving its demand globally.
In the coatings and adhesives industry, ethyl acetate serves as a crucial solvent due to its excellent solvency properties and low toxicity. The expanding construction and automotive sectors have fueled the demand for paints, coatings, and adhesives, consequently boosting the ethyl acetate market. The compound's ability to dissolve a wide range of resins and polymers makes it indispensable in these applications.
The pharmaceutical industry has also emerged as a significant consumer of ethyl acetate. Its use as a solvent in drug formulation and as an extraction medium in the production of antibiotics has increased its importance in this sector. The growing emphasis on healthcare and the rising demand for medicines worldwide have contributed to the expansion of this market segment.
In the food and beverage industry, ethyl acetate finds application as a flavoring agent and in the decaffeination of coffee and tea. The increasing consumer preference for flavored food products and the rising popularity of decaffeinated beverages have created new opportunities for ethyl acetate in this sector.
The electronics industry utilizes ethyl acetate in the production of printed circuit boards and in cleaning processes. With the rapid growth of the electronics sector, particularly in emerging economies, the demand for ethyl acetate in this application has seen a steady increase.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by the robust growth of end-use industries in countries like China and India. North America and Europe follow, with steady demand from established industries and increasing focus on eco-friendly solvents.
The market is characterized by the presence of several key players, including Celanese Corporation, Eastman Chemical Company, and INEOS, among others. These companies are focusing on capacity expansion and technological advancements to meet the growing demand and maintain their market position.
Looking ahead, the ethyl acetate market is expected to continue its growth trajectory. Factors such as increasing industrialization in developing countries, growing demand for eco-friendly solvents, and expanding applications in various industries are likely to drive market growth. However, challenges such as volatile raw material prices and stringent environmental regulations may impact market dynamics in the coming years.
In the coatings and adhesives industry, ethyl acetate serves as a crucial solvent due to its excellent solvency properties and low toxicity. The expanding construction and automotive sectors have fueled the demand for paints, coatings, and adhesives, consequently boosting the ethyl acetate market. The compound's ability to dissolve a wide range of resins and polymers makes it indispensable in these applications.
The pharmaceutical industry has also emerged as a significant consumer of ethyl acetate. Its use as a solvent in drug formulation and as an extraction medium in the production of antibiotics has increased its importance in this sector. The growing emphasis on healthcare and the rising demand for medicines worldwide have contributed to the expansion of this market segment.
In the food and beverage industry, ethyl acetate finds application as a flavoring agent and in the decaffeination of coffee and tea. The increasing consumer preference for flavored food products and the rising popularity of decaffeinated beverages have created new opportunities for ethyl acetate in this sector.
The electronics industry utilizes ethyl acetate in the production of printed circuit boards and in cleaning processes. With the rapid growth of the electronics sector, particularly in emerging economies, the demand for ethyl acetate in this application has seen a steady increase.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by the robust growth of end-use industries in countries like China and India. North America and Europe follow, with steady demand from established industries and increasing focus on eco-friendly solvents.
The market is characterized by the presence of several key players, including Celanese Corporation, Eastman Chemical Company, and INEOS, among others. These companies are focusing on capacity expansion and technological advancements to meet the growing demand and maintain their market position.
Looking ahead, the ethyl acetate market is expected to continue its growth trajectory. Factors such as increasing industrialization in developing countries, growing demand for eco-friendly solvents, and expanding applications in various industries are likely to drive market growth. However, challenges such as volatile raw material prices and stringent environmental regulations may impact market dynamics in the coming years.
Technical Challenges
Ethyl acetate, despite its widespread use across various industries, faces several technical challenges that hinder its full potential. One of the primary issues is its high volatility, which leads to significant emissions during production, storage, and application processes. This volatility not only poses environmental concerns but also affects the efficiency and cost-effectiveness of its use in many applications.
Another challenge lies in the production process of ethyl acetate. The traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid requires high temperatures and the use of strong acid catalysts. This energy-intensive process results in increased production costs and environmental impact. Furthermore, the corrosive nature of the catalysts used can lead to equipment degradation, necessitating frequent maintenance and replacement.
The purification of ethyl acetate presents additional technical hurdles. The formation of azeotropes with water and other solvents during the production process makes separation and purification complex and energy-intensive. This challenge is particularly significant in industries requiring high-purity ethyl acetate, such as pharmaceuticals and electronics manufacturing.
In terms of application, ethyl acetate's limited solubility in water restricts its use in certain water-based formulations. This limitation is particularly problematic in industries seeking to develop more environmentally friendly, water-based products. Additionally, the flammability of ethyl acetate poses safety concerns in storage and handling, requiring specialized equipment and safety protocols.
The interdisciplinary nature of ethyl acetate's applications also presents challenges in optimizing its properties for diverse industry needs. For instance, while its fast evaporation rate is beneficial in some applications, it can be a drawback in others, necessitating the development of tailored formulations for specific uses.
Regulatory challenges further complicate the landscape for ethyl acetate. Increasing environmental regulations and restrictions on volatile organic compound (VOC) emissions are pushing industries to seek alternatives or develop more efficient containment and recovery systems. This regulatory pressure drives the need for innovation in production methods and application technologies to reduce emissions and improve overall sustainability.
Lastly, the fluctuating availability and cost of raw materials used in ethyl acetate production, primarily ethanol and acetic acid, pose challenges for consistent supply and pricing. This volatility affects the entire value chain, from manufacturers to end-users, and necessitates the development of more stable and sustainable production pathways.
Another challenge lies in the production process of ethyl acetate. The traditional method of synthesizing ethyl acetate through the esterification of ethanol and acetic acid requires high temperatures and the use of strong acid catalysts. This energy-intensive process results in increased production costs and environmental impact. Furthermore, the corrosive nature of the catalysts used can lead to equipment degradation, necessitating frequent maintenance and replacement.
The purification of ethyl acetate presents additional technical hurdles. The formation of azeotropes with water and other solvents during the production process makes separation and purification complex and energy-intensive. This challenge is particularly significant in industries requiring high-purity ethyl acetate, such as pharmaceuticals and electronics manufacturing.
In terms of application, ethyl acetate's limited solubility in water restricts its use in certain water-based formulations. This limitation is particularly problematic in industries seeking to develop more environmentally friendly, water-based products. Additionally, the flammability of ethyl acetate poses safety concerns in storage and handling, requiring specialized equipment and safety protocols.
The interdisciplinary nature of ethyl acetate's applications also presents challenges in optimizing its properties for diverse industry needs. For instance, while its fast evaporation rate is beneficial in some applications, it can be a drawback in others, necessitating the development of tailored formulations for specific uses.
Regulatory challenges further complicate the landscape for ethyl acetate. Increasing environmental regulations and restrictions on volatile organic compound (VOC) emissions are pushing industries to seek alternatives or develop more efficient containment and recovery systems. This regulatory pressure drives the need for innovation in production methods and application technologies to reduce emissions and improve overall sustainability.
Lastly, the fluctuating availability and cost of raw materials used in ethyl acetate production, primarily ethanol and acetic acid, pose challenges for consistent supply and pricing. This volatility affects the entire value chain, from manufacturers to end-users, and necessitates the development of more stable and sustainable production pathways.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include distillation processes, reactive distillation, and the use of specific catalysts to improve yield and purity. The techniques aim to optimize the production process and enhance the quality of the final product.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, chromatography, and other separation techniques. These processes are used in the purification of natural products, pharmaceuticals, and other chemicals.
- Ethyl acetate as a green solvent: The use of ethyl acetate as an environmentally friendly solvent is explored. Its relatively low toxicity and biodegradability make it a suitable alternative to more harmful solvents in various applications. Research focuses on developing sustainable processes using ethyl acetate.
- Recovery and recycling of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes are developed. These techniques aim to improve the efficiency and sustainability of processes using ethyl acetate by reducing waste and minimizing environmental impact. Various separation and purification methods are employed to achieve this goal.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. These processes are used in the purification of chemicals and the isolation of specific compounds.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The use and handling of ethyl acetate involve environmental and safety considerations. This includes methods for reducing emissions, improving workplace safety, and developing more sustainable production processes. Efforts are made to minimize the environmental impact and ensure safe handling of the compound.Expand Specific Solutions05 Novel applications and formulations of ethyl acetate
Research into new applications and formulations of ethyl acetate is ongoing. This includes its use in novel materials, coatings, and specialized chemical processes. The development of new formulations aims to expand the utility of ethyl acetate in various industries and applications.Expand Specific Solutions
Industry Leaders
The ethyl acetate market is in a mature stage, characterized by steady growth and established applications across various industries. The global market size is estimated to be around $3-4 billion, with a projected CAGR of 5-6% over the next five years. Technologically, ethyl acetate production is well-established, with major players like Celanese, INEOS, and Eastman Chemical employing advanced processes. However, there's ongoing research into more sustainable production methods, as evidenced by collaborations between industry leaders and academic institutions such as Nanjing Normal University and the University of Campinas. The competitive landscape is dominated by large chemical corporations, with emerging players from Asia, particularly China, gaining market share through cost-effective production and expanding applications.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary process for ethyl acetate production using advanced catalysts and reactor designs. Their method employs a single-step ethanol dehydrogenation process, which offers improved yield and selectivity compared to traditional methods[1]. The company has also implemented energy integration techniques to reduce overall energy consumption in the production process. Celanese's ethyl acetate is used in a wide range of applications, including coatings, adhesives, and pharmaceuticals, addressing interdisciplinary industry needs[2]. The company has invested in research to develop bio-based ethyl acetate, using renewable ethanol sources to meet growing sustainability demands[3].
Strengths: High-efficiency production process, wide range of applications, and focus on sustainability. Weaknesses: Dependence on ethanol feedstock prices and potential competition from alternative solvents.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel approach to ethyl acetate production using its proprietary STRATCO® Alkylation Technology. This process allows for the efficient production of high-purity ethyl acetate from ethanol and acetic acid[1]. The company has also invested in research to improve the sustainability of ethyl acetate production, focusing on bio-based feedstocks and green chemistry principles[2]. DuPont's ethyl acetate finds applications in various industries, including electronics, pharmaceuticals, and food packaging, demonstrating its interdisciplinary utility[3]. The company has recently patented a method for producing ethyl acetate from biomass-derived acetic acid, addressing the growing demand for renewable chemicals[4].
Strengths: Advanced production technology, focus on sustainability, and diverse application portfolio. Weaknesses: Potential competition from specialized chemical companies and dependence on raw material costs.
Key Innovations
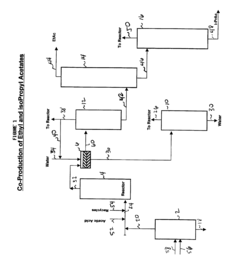
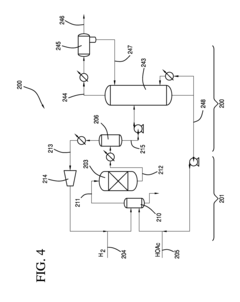
Process for the simultaneous coproduction and purification of ethyl acetate and isopropyl acetate
PatentInactiveUS6765110B2
Innovation
- A process involving the reaction of a mixed alcohol stream of ethanol and isopropanol with acetic acid in the liquid phase using an acidic catalyst at elevated temperatures and pressures, with optional purification of the Fischer Tropsch alcohol mixture before use, followed by a series of distillation towers to separate and purify the ester products, achieving high purity levels of ethyl and isopropyl acetate.
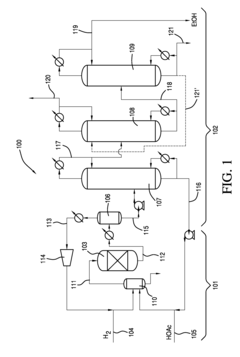
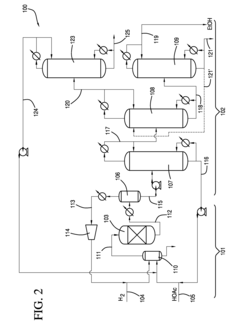
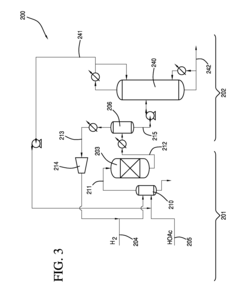
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
Environmental Impact
Ethyl acetate, a versatile organic compound, has gained significant attention due to its potential to address various environmental concerns across multiple industries. As a solvent with relatively low toxicity and high biodegradability, ethyl acetate offers a more environmentally friendly alternative to many traditional petrochemical-based solvents.
In the realm of air quality, ethyl acetate demonstrates lower volatile organic compound (VOC) emissions compared to many conventional solvents. This characteristic is particularly valuable in industries such as paints, coatings, and adhesives, where solvent emissions can contribute significantly to air pollution. By substituting ethyl acetate for more harmful solvents, manufacturers can reduce their overall environmental footprint and comply with increasingly stringent air quality regulations.
Water pollution is another area where ethyl acetate shows promise. Unlike some persistent organic pollutants, ethyl acetate readily breaks down in aquatic environments. Its rapid biodegradation helps minimize long-term impacts on aquatic ecosystems, making it a preferred choice for industries that generate wastewater containing solvents. This property is especially beneficial in sectors like pharmaceuticals and personal care products, where solvent residues in effluents can pose environmental risks.
From a lifecycle perspective, ethyl acetate offers advantages in terms of resource conservation and waste reduction. Its production can utilize renewable feedstocks, such as ethanol derived from biomass, potentially reducing reliance on fossil fuel-based raw materials. Additionally, the compound's high recovery and recycling potential in many applications contribute to circular economy principles, minimizing waste generation and resource depletion.
In the context of climate change mitigation, ethyl acetate's role is multifaceted. Its lower energy-intensive production process, compared to some alternative solvents, can lead to reduced greenhouse gas emissions during manufacturing. Furthermore, its use in energy-efficient formulations, such as low-VOC coatings, can indirectly contribute to energy savings and emissions reductions in end-use applications.
Biodiversity conservation also benefits from the adoption of ethyl acetate. By replacing more toxic solvents, the compound helps reduce the risk of harmful chemical releases into ecosystems. This is particularly relevant in agricultural applications, where ethyl acetate-based formulations can offer effective pest control with lower environmental impact on non-target organisms.
However, it is important to note that while ethyl acetate presents numerous environmental advantages, its use is not without challenges. Proper handling, storage, and disposal practices are essential to fully realize its environmental benefits and prevent unintended consequences. Ongoing research and development efforts are focused on further optimizing ethyl acetate's environmental profile, exploring new applications, and addressing any potential long-term ecological effects that may arise from its increased use across industries.
In the realm of air quality, ethyl acetate demonstrates lower volatile organic compound (VOC) emissions compared to many conventional solvents. This characteristic is particularly valuable in industries such as paints, coatings, and adhesives, where solvent emissions can contribute significantly to air pollution. By substituting ethyl acetate for more harmful solvents, manufacturers can reduce their overall environmental footprint and comply with increasingly stringent air quality regulations.
Water pollution is another area where ethyl acetate shows promise. Unlike some persistent organic pollutants, ethyl acetate readily breaks down in aquatic environments. Its rapid biodegradation helps minimize long-term impacts on aquatic ecosystems, making it a preferred choice for industries that generate wastewater containing solvents. This property is especially beneficial in sectors like pharmaceuticals and personal care products, where solvent residues in effluents can pose environmental risks.
From a lifecycle perspective, ethyl acetate offers advantages in terms of resource conservation and waste reduction. Its production can utilize renewable feedstocks, such as ethanol derived from biomass, potentially reducing reliance on fossil fuel-based raw materials. Additionally, the compound's high recovery and recycling potential in many applications contribute to circular economy principles, minimizing waste generation and resource depletion.
In the context of climate change mitigation, ethyl acetate's role is multifaceted. Its lower energy-intensive production process, compared to some alternative solvents, can lead to reduced greenhouse gas emissions during manufacturing. Furthermore, its use in energy-efficient formulations, such as low-VOC coatings, can indirectly contribute to energy savings and emissions reductions in end-use applications.
Biodiversity conservation also benefits from the adoption of ethyl acetate. By replacing more toxic solvents, the compound helps reduce the risk of harmful chemical releases into ecosystems. This is particularly relevant in agricultural applications, where ethyl acetate-based formulations can offer effective pest control with lower environmental impact on non-target organisms.
However, it is important to note that while ethyl acetate presents numerous environmental advantages, its use is not without challenges. Proper handling, storage, and disposal practices are essential to fully realize its environmental benefits and prevent unintended consequences. Ongoing research and development efforts are focused on further optimizing ethyl acetate's environmental profile, exploring new applications, and addressing any potential long-term ecological effects that may arise from its increased use across industries.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in its application across various industries. As a widely used solvent and chemical intermediate, ethyl acetate is subject to a complex web of regulations that govern its production, distribution, and use. These regulations are designed to ensure safety, environmental protection, and quality standards are met throughout the product lifecycle.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA Inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace settings, ensuring worker safety during handling and processing.
The Food and Drug Administration (FDA) regulates the use of ethyl acetate in food-related applications. It is classified as a Generally Recognized as Safe (GRAS) substance when used as a synthetic flavoring agent or adjuvant. This designation allows for its use in food products, subject to good manufacturing practices and specific concentration limits.
In the European Union, ethyl acetate falls under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register the substance with the European Chemicals Agency (ECHA) and provide safety data. The Classification, Labeling, and Packaging (CLP) Regulation also applies, ensuring proper hazard communication throughout the supply chain.
For pharmaceutical applications, ethyl acetate must comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards define the purity and quality requirements for ethyl acetate used in drug formulations and manufacturing processes.
The transport of ethyl acetate is regulated by international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging, labeling, and documentation requirements for safe transportation.
As sustainability concerns grow, regulations are evolving to address the environmental impact of ethyl acetate production and use. Many jurisdictions have implemented volatile organic compound (VOC) emission limits, encouraging the development of low-VOC or VOC-free alternatives in certain applications.
Understanding and navigating this complex regulatory landscape is essential for companies utilizing ethyl acetate in their products or processes. Compliance with these regulations not only ensures legal operation but also promotes safety, quality, and environmental responsibility across industries.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA Inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace settings, ensuring worker safety during handling and processing.
The Food and Drug Administration (FDA) regulates the use of ethyl acetate in food-related applications. It is classified as a Generally Recognized as Safe (GRAS) substance when used as a synthetic flavoring agent or adjuvant. This designation allows for its use in food products, subject to good manufacturing practices and specific concentration limits.
In the European Union, ethyl acetate falls under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register the substance with the European Chemicals Agency (ECHA) and provide safety data. The Classification, Labeling, and Packaging (CLP) Regulation also applies, ensuring proper hazard communication throughout the supply chain.
For pharmaceutical applications, ethyl acetate must comply with pharmacopeia standards, such as those set by the United States Pharmacopeia (USP) and the European Pharmacopoeia (Ph. Eur.). These standards define the purity and quality requirements for ethyl acetate used in drug formulations and manufacturing processes.
The transport of ethyl acetate is regulated by international agreements such as the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) and the International Maritime Dangerous Goods (IMDG) Code. These regulations specify packaging, labeling, and documentation requirements for safe transportation.
As sustainability concerns grow, regulations are evolving to address the environmental impact of ethyl acetate production and use. Many jurisdictions have implemented volatile organic compound (VOC) emission limits, encouraging the development of low-VOC or VOC-free alternatives in certain applications.
Understanding and navigating this complex regulatory landscape is essential for companies utilizing ethyl acetate in their products or processes. Compliance with these regulations not only ensures legal operation but also promotes safety, quality, and environmental responsibility across industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!