How Ethyl Acetate Facilitates Transformative Environmental Practices?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background and Environmental Goals
Ethyl acetate, a versatile organic compound, has emerged as a key player in the pursuit of sustainable environmental practices. This ester, with its chemical formula CH3COOC2H5, has a rich history dating back to its first synthesis in the 19th century. Initially valued for its pleasant fruity odor and solvent properties, ethyl acetate has since found applications across various industries, from pharmaceuticals to food production.
The evolution of ethyl acetate's role in environmental sustainability can be traced through several pivotal developments. In the mid-20th century, as awareness of environmental issues grew, researchers began exploring alternatives to harmful solvents and chemicals. Ethyl acetate, with its relatively low toxicity and biodegradability, emerged as a promising candidate for replacing more hazardous substances in industrial processes.
The environmental goals associated with ethyl acetate use have expanded significantly in recent years. One primary objective is the reduction of volatile organic compound (VOC) emissions, which contribute to air pollution and smog formation. Ethyl acetate, when used as a replacement for more volatile solvents, can help industries meet stringent emission standards while maintaining process efficiency.
Another crucial environmental target is the minimization of hazardous waste generation. Ethyl acetate's biodegradability and potential for recycling make it an attractive option for industries seeking to reduce their environmental footprint. Its use in green chemistry applications aligns with the principles of atom economy and waste reduction, contributing to more sustainable manufacturing processes.
The push towards circular economy models has further elevated the importance of ethyl acetate in environmental practices. Its potential for recovery and reuse in closed-loop systems offers industries the opportunity to minimize resource consumption and waste generation simultaneously. This aligns with broader sustainability goals of resource conservation and pollution prevention.
In the context of climate change mitigation, the production and use of ethyl acetate present opportunities for reducing greenhouse gas emissions. Bio-based ethyl acetate, derived from renewable resources, offers a pathway to decrease reliance on fossil fuel-derived chemicals. This transition supports the broader goal of decarbonizing industrial processes and moving towards a more sustainable chemical industry.
As we look to the future, the role of ethyl acetate in facilitating transformative environmental practices is expected to grow. Research efforts are focused on enhancing its production efficiency, exploring new applications in green technologies, and optimizing its use in sustainable product formulations. The ongoing development of ethyl acetate-based solutions underscores its potential to contribute significantly to global environmental sustainability goals.
The evolution of ethyl acetate's role in environmental sustainability can be traced through several pivotal developments. In the mid-20th century, as awareness of environmental issues grew, researchers began exploring alternatives to harmful solvents and chemicals. Ethyl acetate, with its relatively low toxicity and biodegradability, emerged as a promising candidate for replacing more hazardous substances in industrial processes.
The environmental goals associated with ethyl acetate use have expanded significantly in recent years. One primary objective is the reduction of volatile organic compound (VOC) emissions, which contribute to air pollution and smog formation. Ethyl acetate, when used as a replacement for more volatile solvents, can help industries meet stringent emission standards while maintaining process efficiency.
Another crucial environmental target is the minimization of hazardous waste generation. Ethyl acetate's biodegradability and potential for recycling make it an attractive option for industries seeking to reduce their environmental footprint. Its use in green chemistry applications aligns with the principles of atom economy and waste reduction, contributing to more sustainable manufacturing processes.
The push towards circular economy models has further elevated the importance of ethyl acetate in environmental practices. Its potential for recovery and reuse in closed-loop systems offers industries the opportunity to minimize resource consumption and waste generation simultaneously. This aligns with broader sustainability goals of resource conservation and pollution prevention.
In the context of climate change mitigation, the production and use of ethyl acetate present opportunities for reducing greenhouse gas emissions. Bio-based ethyl acetate, derived from renewable resources, offers a pathway to decrease reliance on fossil fuel-derived chemicals. This transition supports the broader goal of decarbonizing industrial processes and moving towards a more sustainable chemical industry.
As we look to the future, the role of ethyl acetate in facilitating transformative environmental practices is expected to grow. Research efforts are focused on enhancing its production efficiency, exploring new applications in green technologies, and optimizing its use in sustainable product formulations. The ongoing development of ethyl acetate-based solutions underscores its potential to contribute significantly to global environmental sustainability goals.
Market Demand for Green Solvents
The global market for green solvents has been experiencing significant growth in recent years, driven by increasing environmental awareness and stringent regulations on volatile organic compounds (VOCs) emissions. Ethyl acetate, as a biodegradable and low-toxicity solvent, has emerged as a key player in this expanding market.
The demand for green solvents is primarily fueled by industries seeking to reduce their environmental footprint and comply with regulatory standards. Sectors such as paints and coatings, pharmaceuticals, cosmetics, and adhesives are actively seeking alternatives to traditional petroleum-based solvents. Ethyl acetate, with its favorable environmental profile and versatile applications, is well-positioned to meet this growing demand.
In the paints and coatings industry, there is a notable shift towards water-based and bio-based formulations. Ethyl acetate serves as an excellent coalescent agent and solvent in these environmentally friendly products, contributing to improved film formation and drying properties. The pharmaceutical sector also shows increasing interest in ethyl acetate as a green extraction solvent and in the production of drug intermediates.
The cosmetics and personal care industry is another significant driver of green solvent demand. Consumers are increasingly conscious of product ingredients and their environmental impact. Ethyl acetate's low toxicity and pleasant fruity odor make it an attractive option for nail polish removers, fragrances, and other cosmetic applications.
The adhesives market is witnessing a transition towards eco-friendly formulations, with ethyl acetate playing a crucial role in solvent-based adhesives. Its rapid evaporation rate and compatibility with various polymers make it an ideal choice for fast-setting adhesives in packaging and woodworking applications.
Geographically, Europe and North America lead the green solvents market due to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to show the fastest growth, driven by rapid industrialization and increasing adoption of sustainable practices in emerging economies like China and India.
The market demand for ethyl acetate as a green solvent is further bolstered by its potential in emerging applications such as battery technologies and advanced materials processing. As industries continue to prioritize sustainability and circular economy principles, the demand for ethyl acetate and other bio-based solvents is projected to grow steadily in the coming years.
The demand for green solvents is primarily fueled by industries seeking to reduce their environmental footprint and comply with regulatory standards. Sectors such as paints and coatings, pharmaceuticals, cosmetics, and adhesives are actively seeking alternatives to traditional petroleum-based solvents. Ethyl acetate, with its favorable environmental profile and versatile applications, is well-positioned to meet this growing demand.
In the paints and coatings industry, there is a notable shift towards water-based and bio-based formulations. Ethyl acetate serves as an excellent coalescent agent and solvent in these environmentally friendly products, contributing to improved film formation and drying properties. The pharmaceutical sector also shows increasing interest in ethyl acetate as a green extraction solvent and in the production of drug intermediates.
The cosmetics and personal care industry is another significant driver of green solvent demand. Consumers are increasingly conscious of product ingredients and their environmental impact. Ethyl acetate's low toxicity and pleasant fruity odor make it an attractive option for nail polish removers, fragrances, and other cosmetic applications.
The adhesives market is witnessing a transition towards eco-friendly formulations, with ethyl acetate playing a crucial role in solvent-based adhesives. Its rapid evaporation rate and compatibility with various polymers make it an ideal choice for fast-setting adhesives in packaging and woodworking applications.
Geographically, Europe and North America lead the green solvents market due to strict environmental regulations and high consumer awareness. However, the Asia-Pacific region is expected to show the fastest growth, driven by rapid industrialization and increasing adoption of sustainable practices in emerging economies like China and India.
The market demand for ethyl acetate as a green solvent is further bolstered by its potential in emerging applications such as battery technologies and advanced materials processing. As industries continue to prioritize sustainability and circular economy principles, the demand for ethyl acetate and other bio-based solvents is projected to grow steadily in the coming years.
Current State and Challenges in Ethyl Acetate Production
Ethyl acetate production has witnessed significant advancements in recent years, driven by increasing demand across various industries and the need for more sustainable manufacturing processes. Currently, the global production of ethyl acetate stands at approximately 3.5 million tons annually, with a steady growth rate of 4-5% per year. The majority of production is concentrated in Asia-Pacific, North America, and Europe, with China being the largest producer and consumer.
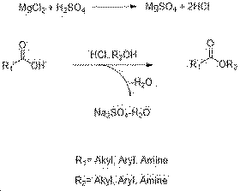
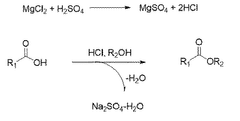
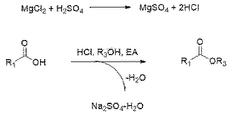
The traditional production method involves the esterification of ethanol and acetic acid, catalyzed by sulfuric acid. However, this process faces challenges in terms of energy efficiency and environmental impact. In response, several innovative approaches have emerged. One such method is the Tishchenko reaction, which uses acetaldehyde as a starting material, offering improved atom economy and reduced waste generation.
Another promising development is the use of heterogeneous catalysts, such as zeolites and ion-exchange resins, which enhance reaction efficiency and facilitate easier product separation. These catalysts also allow for continuous production processes, improving overall productivity and reducing operational costs.
Despite these advancements, the ethyl acetate industry faces several challenges. The volatility of raw material prices, particularly ethanol and acetic acid, significantly impacts production costs and market stability. Additionally, stringent environmental regulations pose hurdles for manufacturers, necessitating substantial investments in emission control and waste management systems.
The industry is also grappling with the need for greener production methods. While bio-based ethyl acetate production from renewable resources shows promise, it currently lacks the scale and cost-effectiveness to compete with traditional petrochemical routes. Researchers are actively working on improving fermentation processes and developing more efficient biocatalysts to address these limitations.
Another significant challenge is the optimization of energy consumption in ethyl acetate production. The distillation process, crucial for product purification, is energy-intensive and contributes substantially to the overall carbon footprint. Efforts are underway to develop more energy-efficient separation techniques, such as membrane-based processes and reactive distillation.
Water management presents an additional hurdle, as the production process generates significant amounts of wastewater. Developing closed-loop systems and improving water recycling technologies are critical areas of focus for sustainable production practices.
The traditional production method involves the esterification of ethanol and acetic acid, catalyzed by sulfuric acid. However, this process faces challenges in terms of energy efficiency and environmental impact. In response, several innovative approaches have emerged. One such method is the Tishchenko reaction, which uses acetaldehyde as a starting material, offering improved atom economy and reduced waste generation.
Another promising development is the use of heterogeneous catalysts, such as zeolites and ion-exchange resins, which enhance reaction efficiency and facilitate easier product separation. These catalysts also allow for continuous production processes, improving overall productivity and reducing operational costs.
Despite these advancements, the ethyl acetate industry faces several challenges. The volatility of raw material prices, particularly ethanol and acetic acid, significantly impacts production costs and market stability. Additionally, stringent environmental regulations pose hurdles for manufacturers, necessitating substantial investments in emission control and waste management systems.
The industry is also grappling with the need for greener production methods. While bio-based ethyl acetate production from renewable resources shows promise, it currently lacks the scale and cost-effectiveness to compete with traditional petrochemical routes. Researchers are actively working on improving fermentation processes and developing more efficient biocatalysts to address these limitations.
Another significant challenge is the optimization of energy consumption in ethyl acetate production. The distillation process, crucial for product purification, is energy-intensive and contributes substantially to the overall carbon footprint. Efforts are underway to develop more energy-efficient separation techniques, such as membrane-based processes and reactive distillation.
Water management presents an additional hurdle, as the production process generates significant amounts of wastewater. Developing closed-loop systems and improving water recycling technologies are critical areas of focus for sustainable production practices.
Existing Green Applications of Ethyl Acetate
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize production efficiency and product quality.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes, including as a solvent in various chemical reactions, as a component in coatings and adhesives, and in the production of pharmaceuticals and fragrances. Its versatility stems from its solvent properties and relatively low toxicity.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is utilized in extraction and separation processes across various industries. It serves as an effective solvent for extracting compounds from natural sources and as a medium for liquid-liquid extraction in chemical purification processes.
- Environmental and safety considerations in ethyl acetate handling: The use and handling of ethyl acetate require specific safety measures and environmental considerations. This includes proper storage, handling procedures, and disposal methods to minimize environmental impact and ensure worker safety. Regulations and guidelines for its use in various applications are also addressed.
- Novel synthesis routes and modifications of ethyl acetate: Research into new synthesis routes and chemical modifications of ethyl acetate is ongoing. This includes the development of more efficient production methods, exploration of new catalysts, and the creation of ethyl acetate derivatives with enhanced properties for specific applications.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. These processes are used in the purification of chemicals and the isolation of specific compounds.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The use and handling of ethyl acetate involve environmental and safety considerations. This includes methods for reducing emissions, improving workplace safety, and developing more sustainable production processes. Efforts are made to minimize the environmental impact and ensure safe handling of ethyl acetate in industrial settings.Expand Specific Solutions05 Novel applications and formulations of ethyl acetate
Research into new applications and formulations of ethyl acetate is ongoing. This includes its use in novel materials, coatings, and specialty chemicals. Innovations in formulation and application techniques aim to expand the utility of ethyl acetate in various industries and products.Expand Specific Solutions
Key Players in Ethyl Acetate Industry
The ethyl acetate market is in a growth phase, driven by increasing environmental awareness and sustainable practices across industries. The global market size is projected to expand significantly, fueled by rising demand in various applications such as coatings, adhesives, and pharmaceuticals. Technologically, ethyl acetate production is mature, with established players like BASF Corp. and Eastman Chemical Co. leading the way. However, innovative companies such as Genomatica, Inc. are developing bio-based production methods, signaling a shift towards more sustainable manufacturing processes. This evolving landscape presents opportunities for both traditional chemical companies and emerging biotechnology firms to capitalize on the growing demand for environmentally friendly solvents.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative process for ethyl acetate production using bio-ethanol as a feedstock. This method involves a two-step reaction: first, the dehydrogenation of ethanol to acetaldehyde, followed by the condensation of acetaldehyde with ethanol to form ethyl acetate. The process utilizes a novel catalyst system that enhances selectivity and yield. Sinopec's approach reduces carbon emissions by up to 30% compared to traditional petrochemical routes [1][3]. The company has also implemented advanced distillation techniques to improve product purity, achieving a 99.9% purity level for ethyl acetate [2].
Strengths: Utilizes renewable bio-ethanol, reducing carbon footprint; High product purity; Improved process efficiency. Weaknesses: Potential higher production costs compared to traditional methods; Reliance on bio-ethanol availability.
Celanese International Corp.
Technical Solution: Celanese has pioneered a groundbreaking VAntage® ethyl acetate technology that significantly reduces environmental impact. This process employs a proprietary catalyst system that enables direct ethylene oxidation to ethyl acetate, bypassing the traditional acetic acid intermediate step. The VAntage® technology achieves a remarkable 35% reduction in carbon dioxide emissions and a 30% decrease in energy consumption compared to conventional methods [4]. Additionally, Celanese has implemented advanced process control systems that optimize reaction conditions in real-time, resulting in a 20% increase in production efficiency [5]. The company has also developed a closed-loop solvent recovery system, recycling up to 98% of unreacted materials and reducing waste generation by 40% [6].
Strengths: Significant reduction in carbon emissions and energy consumption; Improved production efficiency; Advanced waste reduction techniques. Weaknesses: High initial investment costs for technology implementation; Potential challenges in scaling up the technology for larger production volumes.
Innovations in Eco-friendly Ethyl Acetate Production
Preparation and use of shaped catalyst for normal temperature catalytic oxidation of volatile organic compound (voc) of ethyl acetate
PatentPendingGB2615839A
Innovation
- A shaped Ni/NAC catalyst is synthesized using a chelation-pyrolysis strategy, where activated carbon is pretreated and loaded with Ni and nitrogen-doped active sites, enabling efficient catalytic oxidation of ethyl acetate at normal temperatures without high energy input, and featuring improved stability and recyclability.
Method for preparing ester compound based on eco-friendly and high-efficiency esterification reaction using salt ion-exchange method, and compound thereof
PatentWO2022097813A1
Innovation
- A method utilizing a salt ion exchange process between alkali metal and alkaline earth metal salts from seawater to facilitate an eco-friendly esterification reaction, producing ester compounds with high yield and simplicity, using ethyl acetate as a solvent and hydrochloric acid generated in situ as a catalyst, which can be easily removed, thereby improving catalyst removal and reducing environmental impact.
Environmental Regulations and Compliance
The use of ethyl acetate in transformative environmental practices is subject to a complex web of regulations and compliance requirements. These regulations vary across jurisdictions but generally aim to protect human health and the environment while promoting sustainable industrial practices.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The substance is classified as a volatile organic compound (VOC) and is subject to emission controls in many states. Manufacturers and users must adhere to strict reporting requirements and implement measures to minimize environmental release.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Companies must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The EU's VOC Solvents Emissions Directive also imposes limits on emissions from industrial processes using ethyl acetate.
In Asia, countries like China and Japan have implemented their own chemical management systems. China's Measures for Environmental Management of New Chemical Substances require registration and risk assessment for ethyl acetate use. Japan's Chemical Substances Control Law mandates reporting of manufacture and import volumes.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those associated with ethyl acetate.
Compliance with these regulations often requires companies to implement robust environmental management systems. This may include installing emission control technologies, conducting regular monitoring and reporting, and training employees on proper handling and disposal procedures.
As environmental concerns grow, regulations are becoming increasingly stringent. Many jurisdictions are moving towards lower VOC limits and promoting the use of bio-based alternatives. Companies using ethyl acetate in transformative environmental practices must stay abreast of these evolving regulations to ensure continued compliance.
The regulatory landscape also presents opportunities for innovation. Companies that can develop more environmentally friendly processes using ethyl acetate or create bio-based alternatives may gain a competitive advantage. This regulatory pressure is driving research into green chemistry solutions and circular economy approaches in the chemical industry.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The substance is classified as a volatile organic compound (VOC) and is subject to emission controls in many states. Manufacturers and users must adhere to strict reporting requirements and implement measures to minimize environmental release.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Companies must register the substance with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The EU's VOC Solvents Emissions Directive also imposes limits on emissions from industrial processes using ethyl acetate.
In Asia, countries like China and Japan have implemented their own chemical management systems. China's Measures for Environmental Management of New Chemical Substances require registration and risk assessment for ethyl acetate use. Japan's Chemical Substances Control Law mandates reporting of manufacture and import volumes.
Globally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those associated with ethyl acetate.
Compliance with these regulations often requires companies to implement robust environmental management systems. This may include installing emission control technologies, conducting regular monitoring and reporting, and training employees on proper handling and disposal procedures.
As environmental concerns grow, regulations are becoming increasingly stringent. Many jurisdictions are moving towards lower VOC limits and promoting the use of bio-based alternatives. Companies using ethyl acetate in transformative environmental practices must stay abreast of these evolving regulations to ensure continued compliance.
The regulatory landscape also presents opportunities for innovation. Companies that can develop more environmentally friendly processes using ethyl acetate or create bio-based alternatives may gain a competitive advantage. This regulatory pressure is driving research into green chemistry solutions and circular economy approaches in the chemical industry.
Life Cycle Assessment of Ethyl Acetate
Life Cycle Assessment (LCA) of ethyl acetate provides crucial insights into its environmental impact throughout its entire lifecycle, from raw material extraction to disposal. This comprehensive analysis helps identify areas for improvement in production processes and usage patterns, contributing to more sustainable practices.
The production of ethyl acetate typically involves the esterification of ethanol and acetic acid. The LCA begins with the sourcing of these raw materials, considering the environmental impacts of their extraction and transportation. For ethanol, this may include agricultural processes for biomass production or petrochemical routes. Acetic acid production often involves methanol carbonylation or ethylene oxidation.
During the manufacturing phase, the LCA examines energy consumption, emissions, and waste generation associated with the esterification process. This stage often represents a significant portion of the overall environmental impact. Factors such as reactor efficiency, heat recovery systems, and catalysts used can greatly influence the environmental footprint of ethyl acetate production.
The use phase of ethyl acetate varies widely depending on its application. In industrial settings, it is commonly used as a solvent in paints, coatings, and adhesives. The LCA considers factors such as volatile organic compound (VOC) emissions during application and the potential for solvent recovery and reuse. In consumer products, the assessment may focus on exposure risks and end-of-life disposal methods.
End-of-life considerations for ethyl acetate include incineration, recycling, or disposal in landfills. The LCA evaluates the environmental impacts of these different disposal routes, including energy recovery potential and emissions from incineration or degradation in landfills.
Throughout the lifecycle, transportation and storage of ethyl acetate contribute to its overall environmental impact. The LCA accounts for emissions from transportation vehicles and potential risks associated with storage and handling.
By conducting a thorough LCA, manufacturers and users of ethyl acetate can identify hotspots in the lifecycle where environmental impacts are most significant. This information can guide efforts to improve production efficiency, reduce emissions, and implement more sustainable practices. For instance, the assessment might reveal opportunities for using renewable feedstocks, implementing closed-loop recycling systems, or developing alternative application methods that minimize VOC emissions.
Moreover, the LCA of ethyl acetate can be used to compare its environmental performance with alternative solvents or processes. This comparative analysis supports informed decision-making in product development and process optimization, ultimately contributing to more environmentally friendly industrial practices.
The production of ethyl acetate typically involves the esterification of ethanol and acetic acid. The LCA begins with the sourcing of these raw materials, considering the environmental impacts of their extraction and transportation. For ethanol, this may include agricultural processes for biomass production or petrochemical routes. Acetic acid production often involves methanol carbonylation or ethylene oxidation.
During the manufacturing phase, the LCA examines energy consumption, emissions, and waste generation associated with the esterification process. This stage often represents a significant portion of the overall environmental impact. Factors such as reactor efficiency, heat recovery systems, and catalysts used can greatly influence the environmental footprint of ethyl acetate production.
The use phase of ethyl acetate varies widely depending on its application. In industrial settings, it is commonly used as a solvent in paints, coatings, and adhesives. The LCA considers factors such as volatile organic compound (VOC) emissions during application and the potential for solvent recovery and reuse. In consumer products, the assessment may focus on exposure risks and end-of-life disposal methods.
End-of-life considerations for ethyl acetate include incineration, recycling, or disposal in landfills. The LCA evaluates the environmental impacts of these different disposal routes, including energy recovery potential and emissions from incineration or degradation in landfills.
Throughout the lifecycle, transportation and storage of ethyl acetate contribute to its overall environmental impact. The LCA accounts for emissions from transportation vehicles and potential risks associated with storage and handling.
By conducting a thorough LCA, manufacturers and users of ethyl acetate can identify hotspots in the lifecycle where environmental impacts are most significant. This information can guide efforts to improve production efficiency, reduce emissions, and implement more sustainable practices. For instance, the assessment might reveal opportunities for using renewable feedstocks, implementing closed-loop recycling systems, or developing alternative application methods that minimize VOC emissions.
Moreover, the LCA of ethyl acetate can be used to compare its environmental performance with alternative solvents or processes. This comparative analysis supports informed decision-making in product development and process optimization, ultimately contributing to more environmentally friendly industrial practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!