How to Champion Environmental Advancements with Ethyl Acetate?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has gained significant attention in recent years due to its potential for environmental advancements. This colorless liquid, characterized by its fruity odor, is widely used in various industries, including pharmaceuticals, food and beverages, and coatings.
The compound is primarily produced through the esterification of ethanol and acetic acid, a process that has been refined over the years to improve efficiency and reduce environmental impact. Its popularity stems from its relatively low toxicity, biodegradability, and favorable physical properties, making it an attractive alternative to more harmful solvents.
In the context of environmental advancements, ethyl acetate presents several promising applications. Its use as a green solvent in chemical processes has gained traction, offering a more environmentally friendly option compared to traditional petroleum-based solvents. This shift aligns with the growing global emphasis on sustainable chemistry and reduced carbon footprints in industrial operations.
Furthermore, ethyl acetate plays a crucial role in the development of eco-friendly products. Its application in the formulation of low-VOC (Volatile Organic Compound) paints and coatings contributes to improved air quality and reduced environmental pollution. The compound's ability to dissolve a wide range of substances while maintaining low toxicity makes it an ideal candidate for use in green consumer products.
In the realm of waste management and recycling, ethyl acetate has shown promise in the recovery of valuable materials from electronic waste. Its selective solvent properties enable the extraction of precious metals and other components, potentially revolutionizing the e-waste recycling industry and promoting a circular economy.
The agricultural sector has also benefited from ethyl acetate's properties. Its use in pest control formulations offers a more environmentally benign alternative to conventional pesticides, reducing the ecological impact of crop protection measures. Additionally, its role in the production of biofuels and bio-based materials aligns with the global push towards renewable resources and reduced dependence on fossil fuels.
As research and development in green chemistry continue to advance, ethyl acetate is expected to play an increasingly important role in environmental innovations. Its potential applications in areas such as carbon capture, water treatment, and sustainable packaging materials are subjects of ongoing investigation, promising further contributions to environmental sustainability.
The compound is primarily produced through the esterification of ethanol and acetic acid, a process that has been refined over the years to improve efficiency and reduce environmental impact. Its popularity stems from its relatively low toxicity, biodegradability, and favorable physical properties, making it an attractive alternative to more harmful solvents.
In the context of environmental advancements, ethyl acetate presents several promising applications. Its use as a green solvent in chemical processes has gained traction, offering a more environmentally friendly option compared to traditional petroleum-based solvents. This shift aligns with the growing global emphasis on sustainable chemistry and reduced carbon footprints in industrial operations.
Furthermore, ethyl acetate plays a crucial role in the development of eco-friendly products. Its application in the formulation of low-VOC (Volatile Organic Compound) paints and coatings contributes to improved air quality and reduced environmental pollution. The compound's ability to dissolve a wide range of substances while maintaining low toxicity makes it an ideal candidate for use in green consumer products.
In the realm of waste management and recycling, ethyl acetate has shown promise in the recovery of valuable materials from electronic waste. Its selective solvent properties enable the extraction of precious metals and other components, potentially revolutionizing the e-waste recycling industry and promoting a circular economy.
The agricultural sector has also benefited from ethyl acetate's properties. Its use in pest control formulations offers a more environmentally benign alternative to conventional pesticides, reducing the ecological impact of crop protection measures. Additionally, its role in the production of biofuels and bio-based materials aligns with the global push towards renewable resources and reduced dependence on fossil fuels.
As research and development in green chemistry continue to advance, ethyl acetate is expected to play an increasingly important role in environmental innovations. Its potential applications in areas such as carbon capture, water treatment, and sustainable packaging materials are subjects of ongoing investigation, promising further contributions to environmental sustainability.
Green Market Demand
The global market for environmentally friendly products and services has been experiencing significant growth in recent years, driven by increasing awareness of environmental issues and a shift towards sustainable consumption. This trend has created a substantial demand for green alternatives in various industries, including chemicals and solvents. Ethyl acetate, a versatile organic compound, has emerged as a potential champion in this green market due to its relatively low toxicity and biodegradability compared to many traditional solvents.
Consumer preferences have been shifting towards eco-friendly products, with many individuals willing to pay a premium for sustainable alternatives. This shift has been particularly pronounced in developed economies, where environmental concerns are often at the forefront of consumer decision-making. As a result, companies across various sectors are actively seeking ways to incorporate greener materials and processes into their operations, creating a robust market for environmentally friendly solvents like ethyl acetate.
The industrial sector has also been a significant driver of demand for green solvents. Stringent environmental regulations and corporate sustainability goals have pushed manufacturers to seek alternatives to harmful chemicals. Ethyl acetate's low volatility and reduced environmental impact make it an attractive option for industries such as paints and coatings, adhesives, and pharmaceuticals. The automotive and electronics industries have also shown increasing interest in green solvents for cleaning and degreasing applications.
In the packaging industry, there is a growing demand for bio-based and recyclable materials. Ethyl acetate, which can be derived from renewable sources, aligns well with this trend. Its use in the production of flexible packaging and as a component in eco-friendly inks has opened up new market opportunities. The food and beverage industry has also shown interest in ethyl acetate as a natural flavoring agent and in the production of low-emission packaging materials.
The agricultural sector presents another significant market for ethyl acetate. As the demand for organic and sustainable farming practices grows, there is an increasing need for bio-based pesticides and herbicides. Ethyl acetate's potential as a green solvent in the formulation of these products positions it well to capture a share of this expanding market.
Geographically, the demand for green solvents like ethyl acetate is particularly strong in regions with strict environmental regulations, such as the European Union and North America. However, emerging economies in Asia-Pacific and Latin America are also showing rapid growth in this sector as they implement more stringent environmental policies and as consumer awareness increases.
The market potential for ethyl acetate as a green solvent is further enhanced by ongoing research and development efforts aimed at improving its production efficiency and expanding its applications. As technological advancements continue to reduce production costs and enhance performance, the competitiveness of ethyl acetate against traditional solvents is expected to improve, potentially leading to wider adoption across various industries.
Consumer preferences have been shifting towards eco-friendly products, with many individuals willing to pay a premium for sustainable alternatives. This shift has been particularly pronounced in developed economies, where environmental concerns are often at the forefront of consumer decision-making. As a result, companies across various sectors are actively seeking ways to incorporate greener materials and processes into their operations, creating a robust market for environmentally friendly solvents like ethyl acetate.
The industrial sector has also been a significant driver of demand for green solvents. Stringent environmental regulations and corporate sustainability goals have pushed manufacturers to seek alternatives to harmful chemicals. Ethyl acetate's low volatility and reduced environmental impact make it an attractive option for industries such as paints and coatings, adhesives, and pharmaceuticals. The automotive and electronics industries have also shown increasing interest in green solvents for cleaning and degreasing applications.
In the packaging industry, there is a growing demand for bio-based and recyclable materials. Ethyl acetate, which can be derived from renewable sources, aligns well with this trend. Its use in the production of flexible packaging and as a component in eco-friendly inks has opened up new market opportunities. The food and beverage industry has also shown interest in ethyl acetate as a natural flavoring agent and in the production of low-emission packaging materials.
The agricultural sector presents another significant market for ethyl acetate. As the demand for organic and sustainable farming practices grows, there is an increasing need for bio-based pesticides and herbicides. Ethyl acetate's potential as a green solvent in the formulation of these products positions it well to capture a share of this expanding market.
Geographically, the demand for green solvents like ethyl acetate is particularly strong in regions with strict environmental regulations, such as the European Union and North America. However, emerging economies in Asia-Pacific and Latin America are also showing rapid growth in this sector as they implement more stringent environmental policies and as consumer awareness increases.
The market potential for ethyl acetate as a green solvent is further enhanced by ongoing research and development efforts aimed at improving its production efficiency and expanding its applications. As technological advancements continue to reduce production costs and enhance performance, the competitiveness of ethyl acetate against traditional solvents is expected to improve, potentially leading to wider adoption across various industries.
Eco-Friendly Challenges
The adoption of ethyl acetate as an eco-friendly solvent presents several challenges that need to be addressed to champion environmental advancements. One of the primary obstacles is the current reliance on petrochemical-based production methods, which contribute to carbon emissions and environmental degradation. Transitioning to bio-based ethyl acetate production requires significant investment in research and development, as well as the establishment of new supply chains and manufacturing processes.
Another challenge lies in the energy-intensive nature of ethyl acetate production, regardless of the feedstock source. Improving energy efficiency and incorporating renewable energy sources into the manufacturing process are crucial steps towards reducing the overall environmental impact. This transition may require substantial modifications to existing infrastructure and equipment, posing both technical and financial hurdles for industry stakeholders.
The disposal and recycling of ethyl acetate-based products also present environmental concerns. While ethyl acetate is biodegradable, improper disposal can still lead to soil and water contamination. Developing efficient recycling systems and promoting circular economy principles in the use of ethyl acetate are essential for minimizing waste and maximizing resource utilization.
Regulatory compliance and standardization pose additional challenges. As environmental regulations become more stringent, manufacturers must adapt their processes to meet evolving standards. This may involve investing in new technologies for emissions control and waste management, as well as navigating complex regulatory landscapes across different regions.
The perception and acceptance of ethyl acetate as an eco-friendly alternative among consumers and industries are also critical challenges. Overcoming skepticism and educating stakeholders about the environmental benefits of ethyl acetate compared to traditional solvents require comprehensive communication strategies and demonstrable proof of its sustainability credentials.
Furthermore, the scalability of eco-friendly ethyl acetate production remains a significant hurdle. Ensuring a stable and sufficient supply of bio-based feedstocks, without compromising food security or causing land-use changes, is crucial for large-scale adoption. This challenge necessitates careful planning and collaboration across agricultural, industrial, and environmental sectors.
Lastly, the economic viability of eco-friendly ethyl acetate production in comparison to conventional methods presents a persistent challenge. Achieving cost competitiveness while maintaining environmental benefits requires ongoing innovation in production technologies and processes. Balancing economic considerations with environmental goals is essential for the widespread adoption of ethyl acetate as a sustainable solvent solution.
Another challenge lies in the energy-intensive nature of ethyl acetate production, regardless of the feedstock source. Improving energy efficiency and incorporating renewable energy sources into the manufacturing process are crucial steps towards reducing the overall environmental impact. This transition may require substantial modifications to existing infrastructure and equipment, posing both technical and financial hurdles for industry stakeholders.
The disposal and recycling of ethyl acetate-based products also present environmental concerns. While ethyl acetate is biodegradable, improper disposal can still lead to soil and water contamination. Developing efficient recycling systems and promoting circular economy principles in the use of ethyl acetate are essential for minimizing waste and maximizing resource utilization.
Regulatory compliance and standardization pose additional challenges. As environmental regulations become more stringent, manufacturers must adapt their processes to meet evolving standards. This may involve investing in new technologies for emissions control and waste management, as well as navigating complex regulatory landscapes across different regions.
The perception and acceptance of ethyl acetate as an eco-friendly alternative among consumers and industries are also critical challenges. Overcoming skepticism and educating stakeholders about the environmental benefits of ethyl acetate compared to traditional solvents require comprehensive communication strategies and demonstrable proof of its sustainability credentials.
Furthermore, the scalability of eco-friendly ethyl acetate production remains a significant hurdle. Ensuring a stable and sufficient supply of bio-based feedstocks, without compromising food security or causing land-use changes, is crucial for large-scale adoption. This challenge necessitates careful planning and collaboration across agricultural, industrial, and environmental sectors.
Lastly, the economic viability of eco-friendly ethyl acetate production in comparison to conventional methods presents a persistent challenge. Achieving cost competitiveness while maintaining environmental benefits requires ongoing innovation in production technologies and processes. Balancing economic considerations with environmental goals is essential for the widespread adoption of ethyl acetate as a sustainable solvent solution.
Green Production Methods
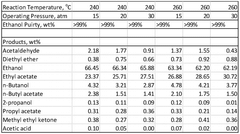
01 Production and purification of ethyl acetate
Various methods are employed for the production and purification of ethyl acetate, including esterification, distillation, and extraction processes. These techniques aim to improve yield, purity, and efficiency in the manufacturing of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve yield, efficiency, and purity of the final product.
- Applications of ethyl acetate in industrial processes: Ethyl acetate is widely used in various industrial applications, such as solvents, coatings, adhesives, and as a raw material in chemical synthesis. Its properties make it suitable for use in diverse fields including pharmaceuticals, electronics, and food processing.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is utilized in extraction and separation processes for various compounds, including natural products, pharmaceuticals, and other chemicals. Its solvent properties make it effective in liquid-liquid extraction and chromatography techniques.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.
- Novel formulations and compositions containing ethyl acetate: Innovative formulations and compositions incorporating ethyl acetate are being developed for various applications. These include specialized coatings, cleaning solutions, and pharmaceutical preparations that leverage the unique properties of ethyl acetate.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is widely used as a solvent and reagent in various chemical processes. It finds applications in organic synthesis, extraction procedures, and as a component in formulations for different industries such as pharmaceuticals, coatings, and adhesives.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of polymers, as a solvent for resins, and in the development of novel materials with specific properties for various industrial uses.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on addressing environmental and safety concerns related to ethyl acetate use. This includes developing eco-friendly production methods, improving handling and storage practices, and exploring alternatives to reduce environmental impact.Expand Specific Solutions05 Analytical methods for ethyl acetate detection and quantification
Various analytical techniques are employed for the detection and quantification of ethyl acetate in different matrices. These methods are crucial for quality control, process monitoring, and ensuring compliance with regulatory standards in industries using ethyl acetate.Expand Specific Solutions
Industry Leaders
The competition landscape for championing environmental advancements with ethyl acetate is evolving rapidly, reflecting the industry's transition towards sustainable practices. The market is experiencing significant growth, driven by increasing demand for eco-friendly solvents across various sectors. While the technology is maturing, it's not yet fully established, with key players like Celanese, BASF, and Eastman Chemical leading innovation. Emerging companies such as LanzaTech and Viridis Chemical are introducing novel bio-based production methods, challenging traditional petrochemical approaches. Research institutions like Dalian Institute of Chemical Physics and universities are contributing to technological advancements, indicating a collaborative ecosystem. The involvement of major oil companies like Shell suggests a broader industry shift towards greener alternatives.
BASF Corp.
Technical Solution: BASF has developed a novel process for producing ethyl acetate from ethylene and acetic acid using heterogeneous catalysts. This method offers improved selectivity and yield compared to traditional processes. The company has also invested in bio-based ethyl acetate production, utilizing renewable feedstocks to reduce carbon footprint. BASF's approach includes the use of advanced catalysts and process intensification techniques to optimize energy efficiency and minimize waste generation[1][3]. Additionally, BASF has implemented closed-loop recycling systems in their ethyl acetate production facilities, significantly reducing water consumption and emissions.
Strengths: Advanced catalyst technology, process efficiency, and commitment to sustainability. Weaknesses: Potential higher initial investment costs for bio-based production and reliance on volatile raw material prices.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has pioneered a proprietary technology for producing ethyl acetate through a low-energy, high-yield process. Their method involves the direct esterification of ethanol and acetic acid using innovative reactive distillation techniques. This approach reduces energy consumption by up to 30% compared to conventional methods[2]. Eastman has also developed a bio-based ethyl acetate product line, sourcing raw materials from sustainable agricultural feedstocks. The company's commitment to circular economy principles is evident in their implementation of solvent recycling programs, which allow customers to return used ethyl acetate for purification and reuse[4].
Strengths: Energy-efficient production process, bio-based product offerings, and circular economy initiatives. Weaknesses: Potential limitations in scaling up bio-based production to meet large-scale industrial demand.
Innovative Applications
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
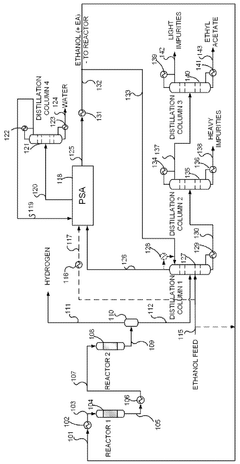
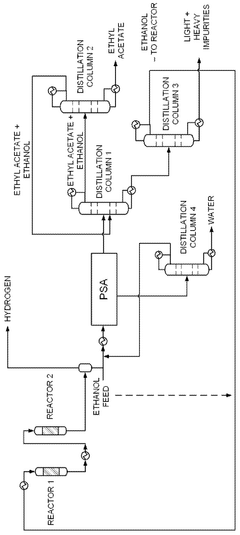
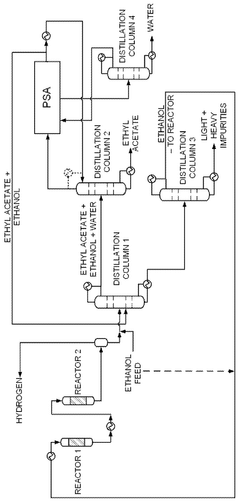
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Integrated system for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, integrated process for producing ethyl acetate, acetaldehyde, hydrogen and ethylene, and products thereby produced
PatentWO2013029129A1
Innovation
- An integrated system utilizing a fixed-bed reactor with a calcined hydrotalcite catalyst for dehydrogenation and dehydration of ethanol, followed by a series of distillation columns for efficient separation and purification, employing ethylene glycol as a solvent to separate ethyl acetate from water, thereby reducing energy expenditure and minimizing azeotropy issues.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in championing environmental advancements. As a solvent widely used in various industries, ethyl acetate is subject to comprehensive regulations aimed at ensuring its safe production, handling, and disposal. In the United States, the Environmental Protection Agency (EPA) oversees the regulation of ethyl acetate under the Toxic Substances Control Act (TSCA). This act requires manufacturers and importers to report production volumes, uses, and potential environmental and health impacts of the chemical.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, companies must register the substance and provide detailed information on its properties, hazards, and safe use. This regulatory framework encourages the development of safer alternatives and promotes the responsible use of ethyl acetate in industrial processes.
In terms of workplace safety, the Occupational Safety and Health Administration (OSHA) in the U.S. has established permissible exposure limits for ethyl acetate to protect workers from potential health hazards. Similar regulations exist in other countries, with varying exposure limits and safety requirements. These regulations drive the development of improved ventilation systems and personal protective equipment in industrial settings.
Environmental regulations also address the potential impact of ethyl acetate on air and water quality. Many countries have implemented volatile organic compound (VOC) emission standards that apply to ethyl acetate, promoting the development of low-VOC and VOC-free alternatives. Additionally, wastewater discharge regulations require proper treatment and disposal of ethyl acetate-containing effluents, encouraging the adoption of more efficient recovery and recycling technologies.
The regulatory landscape for ethyl acetate is continuously evolving, with a growing emphasis on sustainability and circular economy principles. This has led to increased focus on the development of bio-based ethyl acetate production methods and the exploration of more environmentally friendly alternatives. As regulations become more stringent, industries are incentivized to invest in research and development of greener processes and products, ultimately driving environmental advancements in the use and management of ethyl acetate.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, companies must register the substance and provide detailed information on its properties, hazards, and safe use. This regulatory framework encourages the development of safer alternatives and promotes the responsible use of ethyl acetate in industrial processes.
In terms of workplace safety, the Occupational Safety and Health Administration (OSHA) in the U.S. has established permissible exposure limits for ethyl acetate to protect workers from potential health hazards. Similar regulations exist in other countries, with varying exposure limits and safety requirements. These regulations drive the development of improved ventilation systems and personal protective equipment in industrial settings.
Environmental regulations also address the potential impact of ethyl acetate on air and water quality. Many countries have implemented volatile organic compound (VOC) emission standards that apply to ethyl acetate, promoting the development of low-VOC and VOC-free alternatives. Additionally, wastewater discharge regulations require proper treatment and disposal of ethyl acetate-containing effluents, encouraging the adoption of more efficient recovery and recycling technologies.
The regulatory landscape for ethyl acetate is continuously evolving, with a growing emphasis on sustainability and circular economy principles. This has led to increased focus on the development of bio-based ethyl acetate production methods and the exploration of more environmentally friendly alternatives. As regulations become more stringent, industries are incentivized to invest in research and development of greener processes and products, ultimately driving environmental advancements in the use and management of ethyl acetate.
Life Cycle Assessment
Life Cycle Assessment (LCA) plays a crucial role in championing environmental advancements with ethyl acetate. This comprehensive approach evaluates the environmental impacts associated with all stages of ethyl acetate's life cycle, from raw material extraction to disposal or recycling. By conducting a thorough LCA, stakeholders can identify areas for improvement and make informed decisions to enhance the sustainability of ethyl acetate production and use.
The LCA process for ethyl acetate typically begins with the extraction and processing of raw materials, primarily ethanol and acetic acid. This stage examines the environmental impacts of sourcing these materials, including energy consumption, water usage, and greenhouse gas emissions. The production phase follows, where the synthesis of ethyl acetate through esterification is analyzed. This step often involves significant energy inputs and potential emissions, making it a key focus for environmental optimization.
Transportation and distribution of ethyl acetate to end-users constitute another important phase in the LCA. The environmental footprint of various transportation modes, packaging materials, and storage requirements are evaluated to identify opportunities for reducing carbon emissions and improving logistics efficiency. The use phase of ethyl acetate, which varies depending on its application in industries such as coatings, pharmaceuticals, or food processing, is then assessed to determine its environmental impact during actual application.
End-of-life considerations form a critical component of the LCA for ethyl acetate. This includes analyzing disposal methods, potential for recycling or recovery, and the environmental consequences of each option. Given ethyl acetate's volatility, special attention is paid to emissions during use and disposal, as well as potential for recovery and reuse in closed-loop systems.
Throughout the LCA process, key environmental indicators are measured and analyzed. These typically include global warming potential, ozone depletion potential, acidification, eutrophication, and resource depletion. By quantifying these impacts across the entire life cycle, researchers and industry professionals can identify hotspots where environmental improvements can be most effectively implemented.
The results of a comprehensive LCA for ethyl acetate provide valuable insights for developing more sustainable production methods, improving energy efficiency, and exploring alternative raw materials or synthesis routes. This data-driven approach enables the formulation of targeted strategies to reduce the overall environmental footprint of ethyl acetate, aligning its production and use with broader sustainability goals and regulatory requirements.
Moreover, LCA findings can drive innovation in green chemistry practices, encouraging the development of bio-based alternatives to traditional ethyl acetate production methods. This may include exploring renewable feedstocks or implementing novel catalytic processes that reduce energy consumption and minimize waste generation. By leveraging LCA insights, stakeholders can champion environmental advancements that not only improve the sustainability profile of ethyl acetate but also contribute to the broader transition towards a more circular and environmentally responsible chemical industry.
The LCA process for ethyl acetate typically begins with the extraction and processing of raw materials, primarily ethanol and acetic acid. This stage examines the environmental impacts of sourcing these materials, including energy consumption, water usage, and greenhouse gas emissions. The production phase follows, where the synthesis of ethyl acetate through esterification is analyzed. This step often involves significant energy inputs and potential emissions, making it a key focus for environmental optimization.
Transportation and distribution of ethyl acetate to end-users constitute another important phase in the LCA. The environmental footprint of various transportation modes, packaging materials, and storage requirements are evaluated to identify opportunities for reducing carbon emissions and improving logistics efficiency. The use phase of ethyl acetate, which varies depending on its application in industries such as coatings, pharmaceuticals, or food processing, is then assessed to determine its environmental impact during actual application.
End-of-life considerations form a critical component of the LCA for ethyl acetate. This includes analyzing disposal methods, potential for recycling or recovery, and the environmental consequences of each option. Given ethyl acetate's volatility, special attention is paid to emissions during use and disposal, as well as potential for recovery and reuse in closed-loop systems.
Throughout the LCA process, key environmental indicators are measured and analyzed. These typically include global warming potential, ozone depletion potential, acidification, eutrophication, and resource depletion. By quantifying these impacts across the entire life cycle, researchers and industry professionals can identify hotspots where environmental improvements can be most effectively implemented.
The results of a comprehensive LCA for ethyl acetate provide valuable insights for developing more sustainable production methods, improving energy efficiency, and exploring alternative raw materials or synthesis routes. This data-driven approach enables the formulation of targeted strategies to reduce the overall environmental footprint of ethyl acetate, aligning its production and use with broader sustainability goals and regulatory requirements.
Moreover, LCA findings can drive innovation in green chemistry practices, encouraging the development of bio-based alternatives to traditional ethyl acetate production methods. This may include exploring renewable feedstocks or implementing novel catalytic processes that reduce energy consumption and minimize waste generation. By leveraging LCA insights, stakeholders can champion environmental advancements that not only improve the sustainability profile of ethyl acetate but also contribute to the broader transition towards a more circular and environmentally responsible chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!