How to Implement Ethyl Acetate in Progress-Driven Sectors?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background and Objectives
Ethyl acetate, a versatile organic compound with the formula CH3COOC2H5, has been a cornerstone in various industries for decades. Its journey from a laboratory curiosity to a vital industrial chemical exemplifies the evolution of progress-driven sectors. The compound's unique properties, including its low toxicity, pleasant fruity odor, and excellent solvency, have made it indispensable in numerous applications.
The technological evolution of ethyl acetate production has been marked by significant milestones. Initially synthesized through the esterification of ethanol and acetic acid, the process has undergone substantial refinements. Modern production methods, such as the Tishchenko reaction and the dehydrogenation of ethanol, have improved efficiency and reduced environmental impact. These advancements reflect the industry's commitment to sustainability and cost-effectiveness.
In progress-driven sectors, the implementation of ethyl acetate faces both opportunities and challenges. The primary objective is to optimize its use while minimizing environmental footprint and maximizing economic benefits. This involves developing innovative production techniques, exploring new applications, and enhancing the compound's performance in existing uses.
One key goal is to improve the sustainability of ethyl acetate production. This includes developing bio-based production methods, utilizing renewable feedstocks, and implementing closed-loop recycling systems. Such initiatives align with the global push towards greener chemistry and circular economy principles.
Another critical objective is to expand ethyl acetate's application range. While it is already widely used in coatings, adhesives, and pharmaceuticals, there is potential for growth in emerging fields such as advanced materials and nanotechnology. Research is ongoing to explore its role in these cutting-edge areas, potentially opening new markets and driving innovation.
The implementation of ethyl acetate in progress-driven sectors also aims to enhance process efficiency. This involves optimizing reaction conditions, developing more selective catalysts, and improving separation and purification techniques. Such advancements not only reduce production costs but also minimize waste and energy consumption, contributing to overall sustainability goals.
As industries continue to evolve, the role of ethyl acetate is expected to grow and diversify. The compound's implementation in progress-driven sectors will likely focus on developing smarter, more sustainable, and more versatile applications. This may include its use in smart coatings, advanced drug delivery systems, and eco-friendly consumer products. The ongoing research and development in this field underscore the compound's enduring importance and its potential to drive future technological advancements.
The technological evolution of ethyl acetate production has been marked by significant milestones. Initially synthesized through the esterification of ethanol and acetic acid, the process has undergone substantial refinements. Modern production methods, such as the Tishchenko reaction and the dehydrogenation of ethanol, have improved efficiency and reduced environmental impact. These advancements reflect the industry's commitment to sustainability and cost-effectiveness.
In progress-driven sectors, the implementation of ethyl acetate faces both opportunities and challenges. The primary objective is to optimize its use while minimizing environmental footprint and maximizing economic benefits. This involves developing innovative production techniques, exploring new applications, and enhancing the compound's performance in existing uses.
One key goal is to improve the sustainability of ethyl acetate production. This includes developing bio-based production methods, utilizing renewable feedstocks, and implementing closed-loop recycling systems. Such initiatives align with the global push towards greener chemistry and circular economy principles.
Another critical objective is to expand ethyl acetate's application range. While it is already widely used in coatings, adhesives, and pharmaceuticals, there is potential for growth in emerging fields such as advanced materials and nanotechnology. Research is ongoing to explore its role in these cutting-edge areas, potentially opening new markets and driving innovation.
The implementation of ethyl acetate in progress-driven sectors also aims to enhance process efficiency. This involves optimizing reaction conditions, developing more selective catalysts, and improving separation and purification techniques. Such advancements not only reduce production costs but also minimize waste and energy consumption, contributing to overall sustainability goals.
As industries continue to evolve, the role of ethyl acetate is expected to grow and diversify. The compound's implementation in progress-driven sectors will likely focus on developing smarter, more sustainable, and more versatile applications. This may include its use in smart coatings, advanced drug delivery systems, and eco-friendly consumer products. The ongoing research and development in this field underscore the compound's enduring importance and its potential to drive future technological advancements.
Market Demand Analysis for Ethyl Acetate
The market demand for ethyl acetate in progress-driven sectors has been experiencing significant growth in recent years. This versatile organic compound, known for its low toxicity and pleasant fruity odor, has found widespread applications across various industries, driving its demand upward.
In the coatings and paints industry, ethyl acetate serves as an excellent solvent due to its fast evaporation rate and ability to dissolve a wide range of resins. The construction and automotive sectors, in particular, have been key drivers of demand in this area. As urbanization continues and infrastructure development accelerates globally, the need for high-quality coatings and paints is expected to surge, consequently boosting ethyl acetate consumption.
The pharmaceutical industry represents another major consumer of ethyl acetate. Its use as a solvent in the production of various drugs and as an extraction agent in the manufacture of antibiotics has led to a steady increase in demand. With the global pharmaceutical market projected to grow at a CAGR of 5-6% over the next five years, the demand for ethyl acetate in this sector is anticipated to rise correspondingly.
The food and beverage industry also contributes significantly to ethyl acetate demand. Its application as a flavoring agent and in the decaffeination of coffee and tea has seen consistent growth. As consumer preferences shift towards natural and organic products, the use of ethyl acetate as a more environmentally friendly alternative to other solvents is likely to increase.
In the electronics industry, ethyl acetate plays a crucial role in the production of printed circuit boards and semiconductor devices. The rapid expansion of the electronics sector, driven by technological advancements and increasing digitalization, is expected to fuel the demand for ethyl acetate in this area.
The packaging industry, particularly flexible packaging, has emerged as a promising growth sector for ethyl acetate. Its use in the production of adhesives for lamination in food packaging has seen a notable uptick, driven by changing consumer lifestyles and the growing preference for convenience foods.
Geographically, Asia-Pacific remains the largest consumer of ethyl acetate, with China and India leading the demand. The region's robust industrial growth, particularly in manufacturing and construction, continues to drive consumption. North America and Europe follow, with steady demand from established industries and increasing focus on sustainable solutions.
Looking ahead, the global ethyl acetate market is projected to maintain a healthy growth trajectory. Factors such as increasing industrialization in developing economies, growing environmental concerns leading to the adoption of eco-friendly solvents, and ongoing research into new applications are expected to sustain and potentially accelerate market demand in the coming years.
In the coatings and paints industry, ethyl acetate serves as an excellent solvent due to its fast evaporation rate and ability to dissolve a wide range of resins. The construction and automotive sectors, in particular, have been key drivers of demand in this area. As urbanization continues and infrastructure development accelerates globally, the need for high-quality coatings and paints is expected to surge, consequently boosting ethyl acetate consumption.
The pharmaceutical industry represents another major consumer of ethyl acetate. Its use as a solvent in the production of various drugs and as an extraction agent in the manufacture of antibiotics has led to a steady increase in demand. With the global pharmaceutical market projected to grow at a CAGR of 5-6% over the next five years, the demand for ethyl acetate in this sector is anticipated to rise correspondingly.
The food and beverage industry also contributes significantly to ethyl acetate demand. Its application as a flavoring agent and in the decaffeination of coffee and tea has seen consistent growth. As consumer preferences shift towards natural and organic products, the use of ethyl acetate as a more environmentally friendly alternative to other solvents is likely to increase.
In the electronics industry, ethyl acetate plays a crucial role in the production of printed circuit boards and semiconductor devices. The rapid expansion of the electronics sector, driven by technological advancements and increasing digitalization, is expected to fuel the demand for ethyl acetate in this area.
The packaging industry, particularly flexible packaging, has emerged as a promising growth sector for ethyl acetate. Its use in the production of adhesives for lamination in food packaging has seen a notable uptick, driven by changing consumer lifestyles and the growing preference for convenience foods.
Geographically, Asia-Pacific remains the largest consumer of ethyl acetate, with China and India leading the demand. The region's robust industrial growth, particularly in manufacturing and construction, continues to drive consumption. North America and Europe follow, with steady demand from established industries and increasing focus on sustainable solutions.
Looking ahead, the global ethyl acetate market is projected to maintain a healthy growth trajectory. Factors such as increasing industrialization in developing economies, growing environmental concerns leading to the adoption of eco-friendly solvents, and ongoing research into new applications are expected to sustain and potentially accelerate market demand in the coming years.
Current Status and Challenges in Ethyl Acetate Production
The current status of ethyl acetate production is characterized by a mature industrial process, primarily based on the esterification of ethanol with acetic acid. This method, while well-established, faces challenges in terms of sustainability and efficiency. The global production capacity of ethyl acetate is estimated to be around 3.5 million tons per year, with Asia-Pacific region dominating the market share.
One of the main challenges in ethyl acetate production is the reliance on petrochemical feedstocks. The traditional process uses ethanol and acetic acid derived from fossil fuels, raising concerns about long-term sustainability and environmental impact. This has led to increased research into bio-based production methods, utilizing renewable resources such as biomass-derived ethanol and acetic acid.
Energy efficiency remains a significant challenge in the production process. The esterification reaction requires high temperatures and pressures, resulting in substantial energy consumption. Improving catalysts and reactor designs to reduce energy requirements without compromising yield is an ongoing area of research and development.
Another challenge is the purification of ethyl acetate. The azeotropic nature of the ethyl acetate-water mixture makes separation difficult and energy-intensive. Current distillation techniques are effective but consume significant amounts of energy. There is a growing interest in developing more efficient separation technologies, such as membrane-based processes or advanced distillation configurations.
Water management in ethyl acetate production is also a critical issue. The reaction produces water as a by-product, which needs to be removed to drive the equilibrium towards product formation. Efficient water removal and management strategies are essential for improving overall process efficiency and reducing environmental impact.
The volatility of raw material prices, particularly ethanol and acetic acid, poses a challenge for producers. Fluctuations in feedstock costs can significantly impact production economics, necessitating the development of more flexible and resilient production processes.
In terms of product quality, meeting stringent purity requirements for various applications, especially in high-tech industries, remains a challenge. Trace impurities can significantly affect the performance of ethyl acetate in sensitive applications, driving the need for advanced purification technologies.
Regulatory compliance, particularly regarding environmental and safety standards, presents ongoing challenges. Producers must continually adapt to evolving regulations on emissions, waste management, and product safety, which can necessitate significant investments in process modifications and control systems.
One of the main challenges in ethyl acetate production is the reliance on petrochemical feedstocks. The traditional process uses ethanol and acetic acid derived from fossil fuels, raising concerns about long-term sustainability and environmental impact. This has led to increased research into bio-based production methods, utilizing renewable resources such as biomass-derived ethanol and acetic acid.
Energy efficiency remains a significant challenge in the production process. The esterification reaction requires high temperatures and pressures, resulting in substantial energy consumption. Improving catalysts and reactor designs to reduce energy requirements without compromising yield is an ongoing area of research and development.
Another challenge is the purification of ethyl acetate. The azeotropic nature of the ethyl acetate-water mixture makes separation difficult and energy-intensive. Current distillation techniques are effective but consume significant amounts of energy. There is a growing interest in developing more efficient separation technologies, such as membrane-based processes or advanced distillation configurations.
Water management in ethyl acetate production is also a critical issue. The reaction produces water as a by-product, which needs to be removed to drive the equilibrium towards product formation. Efficient water removal and management strategies are essential for improving overall process efficiency and reducing environmental impact.
The volatility of raw material prices, particularly ethanol and acetic acid, poses a challenge for producers. Fluctuations in feedstock costs can significantly impact production economics, necessitating the development of more flexible and resilient production processes.
In terms of product quality, meeting stringent purity requirements for various applications, especially in high-tech industries, remains a challenge. Trace impurities can significantly affect the performance of ethyl acetate in sensitive applications, driving the need for advanced purification technologies.
Regulatory compliance, particularly regarding environmental and safety standards, presents ongoing challenges. Producers must continually adapt to evolving regulations on emissions, waste management, and product safety, which can necessitate significant investments in process modifications and control systems.
Current Ethyl Acetate Implementation Solutions
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the reaction conditions and separation processes to obtain high-quality ethyl acetate efficiently.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including coatings, adhesives, and pharmaceuticals. The compound is also employed in extraction processes, as a reaction medium, and in the production of other chemicals. Its versatility makes it a valuable component in many manufacturing processes.
- Ethyl acetate in green chemistry and sustainable processes: Research focuses on developing environmentally friendly methods for producing and using ethyl acetate. This includes the use of renewable resources as feedstock, the development of bio-based production routes, and the implementation of energy-efficient processes. These approaches aim to reduce the environmental impact of ethyl acetate production and utilization.
- Recovery and recycling of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes are described. These include adsorption techniques, membrane separation, and advanced distillation processes. The goal is to minimize waste, reduce environmental impact, and improve the overall efficiency of processes involving ethyl acetate.
- Novel derivatives and modifications of ethyl acetate: Research on developing novel derivatives and modifications of ethyl acetate is presented. This includes the synthesis of new compounds based on ethyl acetate, chemical modifications to enhance its properties, and the exploration of ethyl acetate-based materials with improved characteristics for specific applications.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also employed in extraction processes, particularly in the food and beverage industry for flavor extraction.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the production of certain polymers, as a solvent for resins, and in the formulation of coatings and adhesives. The compound's properties make it suitable for various material processing techniques.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental profile and safety aspects of ethyl acetate production and use. This includes developing greener production methods, improving recycling processes, and enhancing safety measures in handling and storage of the compound.Expand Specific Solutions05 Novel synthesis routes and catalysts for ethyl acetate
Innovative approaches to ethyl acetate synthesis are being explored, including the development of new catalysts and reaction pathways. These novel methods aim to improve efficiency, reduce energy consumption, and enhance the overall sustainability of ethyl acetate production.Expand Specific Solutions
Key Players in Ethyl Acetate Industry
The implementation of ethyl acetate in progress-driven sectors is in a mature stage, with a well-established market and diverse applications. The global ethyl acetate market size is projected to reach significant growth in the coming years, driven by increasing demand in various industries. Technologically, the production process is well-developed, with major players like Celanese International Corp., Eastman Chemical Co., and China Petroleum & Chemical Corp. leading the way. These companies, along with others such as Resonac Corp. and SABIC, are continuously innovating to improve production efficiency and product quality. The competitive landscape is characterized by a mix of large multinational corporations and specialized chemical companies, each leveraging their strengths in research, production capacity, and market reach.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary process for ethyl acetate production using advanced catalysts and reactive distillation technology. Their method involves the esterification of ethanol and acetic acid in a reactive distillation column, which combines reaction and separation in a single unit operation. This process achieves high conversion rates and product purity while reducing energy consumption by up to 30% compared to conventional methods[1][3]. Celanese's approach also incorporates a novel heterogeneous catalyst system that enhances selectivity and reduces byproduct formation, resulting in a more efficient and environmentally friendly production process[2].
Strengths: High conversion rates, improved energy efficiency, reduced byproduct formation. Weaknesses: Potentially higher initial capital costs, reliance on proprietary catalyst technology.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has implemented a large-scale ethyl acetate production process using a reactive distillation technology. Their approach integrates reaction and separation processes in a single column, significantly reducing equipment costs and energy consumption. Sinopec's method employs a specially designed structured packing with embedded catalyst, allowing for enhanced mass transfer and reaction efficiency[4]. The process achieves a conversion rate of over 99% and a product purity exceeding 99.5%[5]. Additionally, Sinopec has developed a heat integration system that utilizes waste heat from other refinery processes, further improving overall energy efficiency by up to 25%[6].
Strengths: High conversion and purity rates, reduced equipment footprint, improved energy efficiency. Weaknesses: Complex process control requirements, potential catalyst deactivation issues.
Key Innovations in Ethyl Acetate Technology
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
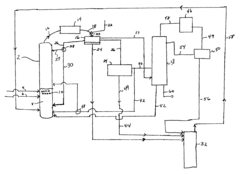
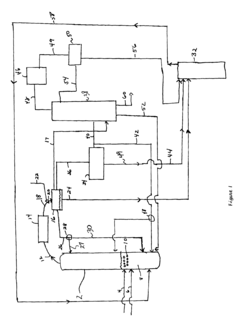
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
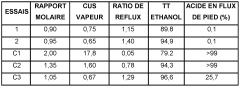
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Environmental Impact of Ethyl Acetate Use
The implementation of ethyl acetate in progress-driven sectors necessitates a thorough examination of its environmental impact. Ethyl acetate, a widely used solvent in various industries, presents both advantages and challenges from an ecological perspective.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. When released into the atmosphere, ethyl acetate can contribute to the formation of ground-level ozone, a key component of smog. This can lead to air quality issues, particularly in urban and industrial areas where its use is more prevalent. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a preferable choice in many applications.
Water pollution is another aspect to consider when assessing the environmental impact of ethyl acetate. Although it is not highly toxic to aquatic life, improper disposal or accidental spills can lead to contamination of water bodies. The compound's high solubility in water means it can spread quickly if released into aquatic environments, potentially affecting ecosystems and water quality.
In terms of biodegradability, ethyl acetate presents a more positive environmental profile. It is readily biodegradable under both aerobic and anaerobic conditions, breaking down relatively quickly in the environment. This characteristic reduces its long-term environmental persistence and mitigates some of the potential negative impacts associated with its release.
The production process of ethyl acetate also warrants consideration in environmental impact assessments. Traditional methods of synthesis often involve petrochemical feedstocks, contributing to carbon emissions and resource depletion. However, recent advancements in green chemistry have led to the development of more sustainable production routes, including bio-based methods using renewable resources. These alternative production techniques can significantly reduce the overall environmental footprint of ethyl acetate.
When implementing ethyl acetate in progress-driven sectors, it is crucial to adopt proper handling, storage, and disposal practices to minimize environmental risks. This includes using appropriate containment systems, implementing efficient recycling and recovery processes, and ensuring proper treatment of waste streams. Additionally, the use of closed-loop systems and vapor recovery technologies can significantly reduce emissions and environmental exposure.
In conclusion, while ethyl acetate presents certain environmental challenges, particularly in terms of VOC emissions and potential water contamination, its biodegradability and the availability of greener production methods offer opportunities for more sustainable use. Progress-driven sectors implementing ethyl acetate should focus on minimizing emissions, optimizing usage efficiency, and exploring eco-friendly alternatives where possible to mitigate environmental impacts.
One of the primary environmental concerns associated with ethyl acetate is its volatile organic compound (VOC) status. When released into the atmosphere, ethyl acetate can contribute to the formation of ground-level ozone, a key component of smog. This can lead to air quality issues, particularly in urban and industrial areas where its use is more prevalent. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential, making it a preferable choice in many applications.
Water pollution is another aspect to consider when assessing the environmental impact of ethyl acetate. Although it is not highly toxic to aquatic life, improper disposal or accidental spills can lead to contamination of water bodies. The compound's high solubility in water means it can spread quickly if released into aquatic environments, potentially affecting ecosystems and water quality.
In terms of biodegradability, ethyl acetate presents a more positive environmental profile. It is readily biodegradable under both aerobic and anaerobic conditions, breaking down relatively quickly in the environment. This characteristic reduces its long-term environmental persistence and mitigates some of the potential negative impacts associated with its release.
The production process of ethyl acetate also warrants consideration in environmental impact assessments. Traditional methods of synthesis often involve petrochemical feedstocks, contributing to carbon emissions and resource depletion. However, recent advancements in green chemistry have led to the development of more sustainable production routes, including bio-based methods using renewable resources. These alternative production techniques can significantly reduce the overall environmental footprint of ethyl acetate.
When implementing ethyl acetate in progress-driven sectors, it is crucial to adopt proper handling, storage, and disposal practices to minimize environmental risks. This includes using appropriate containment systems, implementing efficient recycling and recovery processes, and ensuring proper treatment of waste streams. Additionally, the use of closed-loop systems and vapor recovery technologies can significantly reduce emissions and environmental exposure.
In conclusion, while ethyl acetate presents certain environmental challenges, particularly in terms of VOC emissions and potential water contamination, its biodegradability and the availability of greener production methods offer opportunities for more sustainable use. Progress-driven sectors implementing ethyl acetate should focus on minimizing emissions, optimizing usage efficiency, and exploring eco-friendly alternatives where possible to mitigate environmental impacts.
Regulatory Framework for Ethyl Acetate Application
The regulatory framework for ethyl acetate application in progress-driven sectors is a complex and evolving landscape that requires careful navigation. At the international level, organizations such as the World Health Organization (WHO) and the International Labour Organization (ILO) provide guidelines on the safe handling and use of ethyl acetate in industrial settings. These guidelines often serve as a basis for national regulations and industry standards.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers and mandates safety measures in workplaces where ethyl acetate is used. The Food and Drug Administration (FDA) also regulates its use in food packaging and pharmaceutical applications.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to register ethyl acetate and provide safety data. The Classification, Labeling, and Packaging (CLP) Regulation ensures that the hazards of ethyl acetate are clearly communicated to workers and consumers through standardized classifications and labeling.
In Asia, countries like China and Japan have their own regulatory frameworks. China's Ministry of Ecology and Environment oversees the environmental aspects of ethyl acetate use, while the State Administration for Market Regulation handles product safety. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture and import of ethyl acetate.
Industry-specific regulations also play a crucial role. In the pharmaceutical sector, Good Manufacturing Practice (GMP) guidelines often dictate the use and handling of ethyl acetate in drug production. The electronics industry follows standards set by organizations like the IPC (Association Connecting Electronics Industries) for the use of solvents in manufacturing processes.
Compliance with these regulations often requires companies to implement comprehensive management systems. This includes maintaining detailed documentation, conducting regular risk assessments, providing worker training, and implementing proper waste management procedures. Many organizations opt for third-party certifications, such as ISO 14001 for environmental management, to demonstrate their commitment to regulatory compliance and best practices.
As sustainability becomes increasingly important, regulations are evolving to address the environmental impact of ethyl acetate use. This includes stricter controls on emissions, waste disposal, and the promotion of recycling and recovery processes. Companies in progress-driven sectors must stay abreast of these changing regulations to ensure continued compliance and to maintain their social license to operate.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers and mandates safety measures in workplaces where ethyl acetate is used. The Food and Drug Administration (FDA) also regulates its use in food packaging and pharmaceutical applications.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to register ethyl acetate and provide safety data. The Classification, Labeling, and Packaging (CLP) Regulation ensures that the hazards of ethyl acetate are clearly communicated to workers and consumers through standardized classifications and labeling.
In Asia, countries like China and Japan have their own regulatory frameworks. China's Ministry of Ecology and Environment oversees the environmental aspects of ethyl acetate use, while the State Administration for Market Regulation handles product safety. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture and import of ethyl acetate.
Industry-specific regulations also play a crucial role. In the pharmaceutical sector, Good Manufacturing Practice (GMP) guidelines often dictate the use and handling of ethyl acetate in drug production. The electronics industry follows standards set by organizations like the IPC (Association Connecting Electronics Industries) for the use of solvents in manufacturing processes.
Compliance with these regulations often requires companies to implement comprehensive management systems. This includes maintaining detailed documentation, conducting regular risk assessments, providing worker training, and implementing proper waste management procedures. Many organizations opt for third-party certifications, such as ISO 14001 for environmental management, to demonstrate their commitment to regulatory compliance and best practices.
As sustainability becomes increasingly important, regulations are evolving to address the environmental impact of ethyl acetate use. This includes stricter controls on emissions, waste disposal, and the promotion of recycling and recovery processes. Companies in progress-driven sectors must stay abreast of these changing regulations to ensure continued compliance and to maintain their social license to operate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!