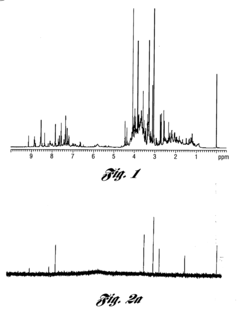
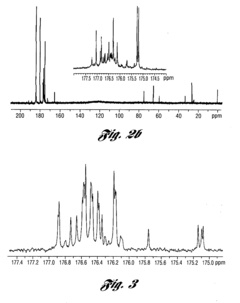
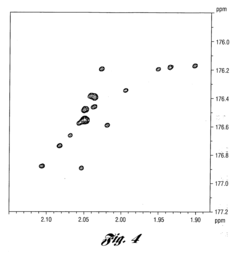
Isotope Labeling for Enhanced NMR Sensitivity
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotope Labeling NMR Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, becoming an indispensable analytical tool in chemistry, biochemistry, and materials science. The technique relies on the magnetic properties of certain atomic nuclei when placed in a magnetic field, providing detailed information about molecular structure, dynamics, and interactions. However, NMR has historically been limited by its inherent low sensitivity compared to other analytical methods.
Isotope labeling emerged as a revolutionary approach to address this fundamental limitation. The technique involves replacing specific atoms in a molecule with their isotopes that possess more favorable NMR properties. The development of isotope labeling strategies began in the 1960s but gained significant momentum in the 1980s and 1990s with advances in molecular biology and organic synthesis methods.
The evolution of isotope labeling techniques has closely followed technological advancements in NMR instrumentation, with each development enabling new applications and expanding the scope of NMR analysis. From early uniform labeling approaches to sophisticated selective and site-specific labeling strategies, the field has continuously refined methodologies to enhance spectral resolution and sensitivity.
Recent years have witnessed remarkable progress in isotope labeling technologies, particularly for biological macromolecules. Techniques such as Stereo-Array Isotope Labeling (SAIL), Segmental Isotope Labeling, and Cell-Free Expression systems have revolutionized structural biology by enabling the study of increasingly complex biomolecular systems.
The primary objective of isotope labeling for enhanced NMR sensitivity is to overcome the technique's inherent sensitivity limitations while maximizing information content. Specific goals include developing cost-effective labeling strategies, improving signal-to-noise ratios, reducing spectral complexity, and enabling the study of larger molecular systems and lower concentration samples.
Current research aims to expand the application of isotope labeling to challenging systems such as membrane proteins, intrinsically disordered proteins, and large protein complexes. Additionally, there is growing interest in combining isotope labeling with other sensitivity enhancement techniques like Dynamic Nuclear Polarization (DNP) and cryogenic probes to achieve multiplicative sensitivity gains.
The field is trending toward more selective and economical labeling approaches, driven by the high cost of isotopically labeled precursors. Emerging technologies focus on minimizing isotope usage while maximizing spectral information, with computational methods playing an increasingly important role in experimental design and data interpretation.
As we look forward, isotope labeling continues to be at the forefront of efforts to expand NMR's capabilities, with the ultimate goal of making this powerful analytical technique accessible to an even broader range of molecular systems and research applications.
Isotope labeling emerged as a revolutionary approach to address this fundamental limitation. The technique involves replacing specific atoms in a molecule with their isotopes that possess more favorable NMR properties. The development of isotope labeling strategies began in the 1960s but gained significant momentum in the 1980s and 1990s with advances in molecular biology and organic synthesis methods.
The evolution of isotope labeling techniques has closely followed technological advancements in NMR instrumentation, with each development enabling new applications and expanding the scope of NMR analysis. From early uniform labeling approaches to sophisticated selective and site-specific labeling strategies, the field has continuously refined methodologies to enhance spectral resolution and sensitivity.
Recent years have witnessed remarkable progress in isotope labeling technologies, particularly for biological macromolecules. Techniques such as Stereo-Array Isotope Labeling (SAIL), Segmental Isotope Labeling, and Cell-Free Expression systems have revolutionized structural biology by enabling the study of increasingly complex biomolecular systems.
The primary objective of isotope labeling for enhanced NMR sensitivity is to overcome the technique's inherent sensitivity limitations while maximizing information content. Specific goals include developing cost-effective labeling strategies, improving signal-to-noise ratios, reducing spectral complexity, and enabling the study of larger molecular systems and lower concentration samples.
Current research aims to expand the application of isotope labeling to challenging systems such as membrane proteins, intrinsically disordered proteins, and large protein complexes. Additionally, there is growing interest in combining isotope labeling with other sensitivity enhancement techniques like Dynamic Nuclear Polarization (DNP) and cryogenic probes to achieve multiplicative sensitivity gains.
The field is trending toward more selective and economical labeling approaches, driven by the high cost of isotopically labeled precursors. Emerging technologies focus on minimizing isotope usage while maximizing spectral information, with computational methods playing an increasingly important role in experimental design and data interpretation.
As we look forward, isotope labeling continues to be at the forefront of efforts to expand NMR's capabilities, with the ultimate goal of making this powerful analytical technique accessible to an even broader range of molecular systems and research applications.
Market Analysis for Isotope-Enhanced NMR Applications
The global market for isotope-enhanced NMR applications has experienced significant growth in recent years, driven primarily by advancements in structural biology, drug discovery, and materials science. The market size for isotope labeling reagents used in NMR spectroscopy was valued at approximately $215 million in 2022, with projections indicating a compound annual growth rate (CAGR) of 8.3% through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 65% of the total demand. This dominance stems from the critical role isotope-enhanced NMR plays in protein structure determination, drug-target interaction studies, and metabolomics research. The increasing focus on precision medicine and biologics has further accelerated demand within these sectors.
Academic and research institutions constitute the second-largest market segment at 25%, where isotope labeling techniques are extensively employed for fundamental research in biochemistry and structural biology. The remaining 10% is distributed across food safety testing, environmental monitoring, and emerging applications in nanotechnology.
Geographically, North America leads the market with a 42% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China, Japan, and India, is witnessing the fastest growth rate at 10.5% annually, driven by increasing R&D investments and the expansion of biotechnology sectors in these countries.
Key market drivers include the growing complexity of biological targets in drug discovery, which necessitates more sensitive analytical techniques. The pharmaceutical industry's shift toward biologics and large molecule therapeutics has created substantial demand for isotope labeling methods that can elucidate complex protein structures and interactions.
Market challenges primarily revolve around the high cost of isotopically labeled compounds and specialized NMR equipment. For instance, uniformly 13C/15N-labeled protein production can cost between $2,000-$10,000 per sample, creating accessibility barriers for smaller research organizations and academic institutions.
Emerging trends include the development of selective labeling strategies that reduce costs while maintaining analytical power, cell-free protein expression systems for efficient isotope incorporation, and the integration of isotope-enhanced NMR with complementary techniques like cryo-electron microscopy for comprehensive structural analysis.
The market is also witnessing increased demand for specialized isotope labeling services, with contract research organizations offering custom labeling solutions experiencing annual revenue growth of 12-15%. This trend reflects the technical complexity of isotope incorporation and the specialized expertise required for successful implementation.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 65% of the total demand. This dominance stems from the critical role isotope-enhanced NMR plays in protein structure determination, drug-target interaction studies, and metabolomics research. The increasing focus on precision medicine and biologics has further accelerated demand within these sectors.
Academic and research institutions constitute the second-largest market segment at 25%, where isotope labeling techniques are extensively employed for fundamental research in biochemistry and structural biology. The remaining 10% is distributed across food safety testing, environmental monitoring, and emerging applications in nanotechnology.
Geographically, North America leads the market with a 42% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region, particularly China, Japan, and India, is witnessing the fastest growth rate at 10.5% annually, driven by increasing R&D investments and the expansion of biotechnology sectors in these countries.
Key market drivers include the growing complexity of biological targets in drug discovery, which necessitates more sensitive analytical techniques. The pharmaceutical industry's shift toward biologics and large molecule therapeutics has created substantial demand for isotope labeling methods that can elucidate complex protein structures and interactions.
Market challenges primarily revolve around the high cost of isotopically labeled compounds and specialized NMR equipment. For instance, uniformly 13C/15N-labeled protein production can cost between $2,000-$10,000 per sample, creating accessibility barriers for smaller research organizations and academic institutions.
Emerging trends include the development of selective labeling strategies that reduce costs while maintaining analytical power, cell-free protein expression systems for efficient isotope incorporation, and the integration of isotope-enhanced NMR with complementary techniques like cryo-electron microscopy for comprehensive structural analysis.
The market is also witnessing increased demand for specialized isotope labeling services, with contract research organizations offering custom labeling solutions experiencing annual revenue growth of 12-15%. This trend reflects the technical complexity of isotope incorporation and the specialized expertise required for successful implementation.
Current Isotope Labeling Techniques and Limitations
Isotope labeling techniques have evolved significantly over the past decades, becoming essential tools for enhancing NMR sensitivity and resolution. Currently, several established methodologies dominate the field, each with specific applications and inherent limitations that researchers must navigate.
Uniform isotopic labeling represents the most traditional approach, where biomolecules are synthesized with complete replacement of naturally abundant isotopes (typically 12C, 14N) with NMR-active nuclei (13C, 15N). This technique is implemented through expression systems grown in media containing isotopically enriched precursors. While providing comprehensive structural information, uniform labeling often suffers from spectral crowding in larger biomolecules, limiting its utility for complex systems.
Selective isotope labeling has emerged as a refined strategy, targeting specific amino acids or nucleotides within a biomolecule. This approach significantly reduces spectral complexity and enables focused investigation of particular molecular regions. However, it requires sophisticated synthetic chemistry expertise and often involves prohibitively expensive precursors, restricting widespread adoption in routine applications.
Site-specific isotope labeling represents the pinnacle of precision, incorporating isotopes at individual atomic positions. Techniques such as expressed protein ligation (EPL) and native chemical ligation (NCL) facilitate this high-resolution approach. Despite its exceptional specificity, the methodology demands extensive synthetic effort and typically yields lower quantities of labeled material, creating challenges for sensitivity-limited experiments.
Segmental isotope labeling has gained prominence for studying domain-specific dynamics in multi-domain proteins. By isotopically labeling discrete segments within a protein, researchers can isolate NMR signals from regions of interest. Technical challenges include efficient ligation of labeled and unlabeled segments and maintaining protein structural integrity throughout the process.
Metabolic precursor strategies leverage cellular metabolism to incorporate isotopes into specific molecular positions. While cost-effective compared to chemical synthesis approaches, these methods often suffer from metabolic scrambling, where isotopes redistribute unpredictably throughout the molecular structure, reducing labeling specificity.
Current limitations across all isotope labeling techniques include prohibitive costs of enriched precursors, especially for 13C sources, which can exceed $1,000 per gram. Technical complexity presents another significant barrier, requiring specialized expertise in synthetic chemistry, molecular biology, and protein expression systems. Additionally, isotope dilution during expression and purification processes frequently results in sub-optimal incorporation rates, diminishing potential sensitivity gains.
Uniform isotopic labeling represents the most traditional approach, where biomolecules are synthesized with complete replacement of naturally abundant isotopes (typically 12C, 14N) with NMR-active nuclei (13C, 15N). This technique is implemented through expression systems grown in media containing isotopically enriched precursors. While providing comprehensive structural information, uniform labeling often suffers from spectral crowding in larger biomolecules, limiting its utility for complex systems.
Selective isotope labeling has emerged as a refined strategy, targeting specific amino acids or nucleotides within a biomolecule. This approach significantly reduces spectral complexity and enables focused investigation of particular molecular regions. However, it requires sophisticated synthetic chemistry expertise and often involves prohibitively expensive precursors, restricting widespread adoption in routine applications.
Site-specific isotope labeling represents the pinnacle of precision, incorporating isotopes at individual atomic positions. Techniques such as expressed protein ligation (EPL) and native chemical ligation (NCL) facilitate this high-resolution approach. Despite its exceptional specificity, the methodology demands extensive synthetic effort and typically yields lower quantities of labeled material, creating challenges for sensitivity-limited experiments.
Segmental isotope labeling has gained prominence for studying domain-specific dynamics in multi-domain proteins. By isotopically labeling discrete segments within a protein, researchers can isolate NMR signals from regions of interest. Technical challenges include efficient ligation of labeled and unlabeled segments and maintaining protein structural integrity throughout the process.
Metabolic precursor strategies leverage cellular metabolism to incorporate isotopes into specific molecular positions. While cost-effective compared to chemical synthesis approaches, these methods often suffer from metabolic scrambling, where isotopes redistribute unpredictably throughout the molecular structure, reducing labeling specificity.
Current limitations across all isotope labeling techniques include prohibitive costs of enriched precursors, especially for 13C sources, which can exceed $1,000 per gram. Technical complexity presents another significant barrier, requiring specialized expertise in synthetic chemistry, molecular biology, and protein expression systems. Additionally, isotope dilution during expression and purification processes frequently results in sub-optimal incorporation rates, diminishing potential sensitivity gains.
State-of-the-Art Isotope Labeling Strategies
01 Isotope labeling techniques for enhanced NMR sensitivity
Various isotope labeling techniques can be employed to enhance NMR sensitivity. These include selective labeling of specific atoms or molecules with isotopes such as 13C, 15N, or 2H (deuterium). By incorporating these isotopes into the molecular structure, the NMR signal can be significantly amplified, allowing for more sensitive detection and analysis of complex biological molecules and chemical compounds.- Isotope labeling techniques for enhanced NMR sensitivity: Various isotope labeling techniques can be employed to enhance NMR sensitivity. These include selective labeling of specific atoms or molecules with NMR-active isotopes such as 13C, 15N, or 2H. By incorporating these isotopes into the molecular structure, the signal-to-noise ratio in NMR experiments can be significantly improved, allowing for more sensitive detection and analysis of molecular structures and interactions.
- Dynamic Nuclear Polarization (DNP) for sensitivity enhancement: Dynamic Nuclear Polarization (DNP) is a technique that transfers polarization from electron spins to nuclear spins, resulting in significantly enhanced NMR signals. This approach can increase sensitivity by orders of magnitude compared to conventional NMR methods. DNP techniques often involve the use of stable radicals as polarizing agents and specialized equipment to achieve the polarization transfer, enabling the detection of molecules at much lower concentrations.
- Specialized isotope labeling patterns for biomolecular NMR: Specific isotope labeling patterns can be designed for biomolecular NMR studies to enhance sensitivity and spectral resolution. These include uniform labeling, selective labeling, and segmental labeling approaches. Advanced techniques such as SAIL (Stereo-Array Isotope Labeling) and cell-free protein synthesis systems allow for precise incorporation of isotopes at specific positions, reducing spectral complexity while maintaining or improving sensitivity for structural studies of proteins and nucleic acids.
- Hyperpolarized NMR using parahydrogen and other methods: Hyperpolarization techniques using parahydrogen-induced polarization (PHIP), signal amplification by reversible exchange (SABRE), or other methods can dramatically enhance NMR sensitivity. These approaches create non-equilibrium nuclear spin populations that generate significantly stronger NMR signals. The techniques often involve specialized catalysts and reaction conditions to transfer the hyperpolarization to target molecules, enabling applications in metabolic imaging and real-time reaction monitoring.
- Instrumentation and pulse sequences for isotope-labeled NMR: Specialized NMR instrumentation and pulse sequences have been developed to maximize sensitivity when working with isotope-labeled samples. These include cryogenically cooled probes, high-field magnets, and optimized detection methods. Advanced pulse sequences such as TROSY (Transverse Relaxation Optimized Spectroscopy) and HSQC (Heteronuclear Single Quantum Coherence) are designed to take advantage of isotope labeling patterns, providing enhanced sensitivity and resolution for complex biomolecular systems.
02 Hyperpolarization methods to increase NMR sensitivity
Hyperpolarization techniques represent a breakthrough approach for dramatically enhancing NMR sensitivity. These methods involve creating a non-equilibrium distribution of nuclear spins, resulting in signal enhancements of several orders of magnitude. Dynamic Nuclear Polarization (DNP), para-hydrogen induced polarization (PHIP), and other hyperpolarization methods enable the detection of molecules at much lower concentrations than conventional NMR, opening new possibilities for metabolic imaging and analysis.Expand Specific Solutions03 Advanced pulse sequences and acquisition methods
Specialized pulse sequences and acquisition methods can be combined with isotope labeling to further enhance NMR sensitivity. These include TROSY (Transverse Relaxation Optimized Spectroscopy), HSQC (Heteronuclear Single Quantum Coherence), and other multidimensional techniques that leverage isotope labeling to provide clearer signals with reduced noise. These methods are particularly valuable for studying large biomolecules and complex mixtures where signal overlap is a concern.Expand Specific Solutions04 Cryogenic probe technology for sensitivity enhancement
Cryogenic probe technology significantly improves NMR sensitivity when used with isotope-labeled samples. By cooling the detection coils and preamplifiers to very low temperatures (typically around 20K), thermal noise is drastically reduced, resulting in signal-to-noise ratio improvements of 3-4 times compared to conventional probes. This technology is particularly beneficial for detecting low-concentration metabolites and studying isotope-labeled biomolecules.Expand Specific Solutions05 Sample preparation and enrichment strategies
Specialized sample preparation and isotope enrichment strategies are crucial for maximizing NMR sensitivity. These include selective incorporation of isotopes at specific positions, metabolic labeling in cell cultures, and chemical synthesis of labeled compounds. Advanced purification techniques ensure high isotopic purity, while specialized solvents and buffers minimize signal interference. These approaches collectively enhance the quality and intensity of NMR signals from labeled compounds.Expand Specific Solutions
Leading Organizations in Isotope Labeling Technology
Isotope labeling for enhanced NMR sensitivity is currently in a growth phase, with the market expanding due to increasing applications in pharmaceutical research, structural biology, and metabolomics. The global market size is estimated to reach $500-600 million by 2025, driven by demand for more sensitive analytical techniques. Technologically, the field shows varying maturity levels across different applications. Leading players include academic institutions like The Scripps Research Institute and Johns Hopkins University, which focus on fundamental research, while pharmaceutical companies such as F. Hoffmann-La Roche and AbbVie leverage the technology for drug discovery. Equipment manufacturers like Koninklijke Philips and Hitachi provide specialized instrumentation, creating a diverse ecosystem of innovation spanning both established techniques and emerging methodologies.
The Scripps Research Institute
Technical Solution: Scripps Research has developed innovative isotope labeling strategies focused on dynamic nuclear polarization (DNP) enhanced NMR. Their approach combines selective 13C labeling with paramagnetic dopants to achieve signal enhancements of 100-300 fold in biomolecular systems. A key innovation is their site-specific labeling technology that enables precise placement of isotopes at strategic positions within complex molecules, particularly useful for drug-target interaction studies. Their methodology includes proprietary hyperpolarization techniques that temporarily boost NMR signals by altering nuclear spin populations, allowing detection of molecules at nanomolar concentrations. Scripps has also pioneered metabolic labeling approaches where isotopically enriched precursors are incorporated into cellular pathways, enabling in-cell NMR experiments that reveal biomolecular behavior in physiologically relevant environments. This technology has been particularly valuable for mapping protein-ligand interactions in drug discovery applications.
Strengths: Exceptional sensitivity enhancement through hyperpolarization; ability to study molecules at near-physiological concentrations; versatile application across different molecular classes. Weaknesses: Hyperpolarized states are transient, limiting experimental timeframes; requires specialized equipment beyond standard NMR instrumentation; challenging to implement for routine analyses.
The Regents of the University of California
Technical Solution: The University of California has pioneered advanced isotope labeling techniques for NMR spectroscopy, particularly in protein structure determination. Their SAIL (Stereo-Array Isotope Labeling) methodology incorporates stereospecific deuteration patterns alongside 13C and 15N enrichment to dramatically enhance spectral resolution. This approach enables the study of larger proteins (>30 kDa) that were previously inaccessible to conventional NMR methods. Their technology includes cell-free protein synthesis systems optimized for efficient incorporation of isotopically labeled amino acids, reducing production costs by up to 40% compared to traditional methods. Additionally, they've developed pulse sequence modifications specifically designed to leverage these labeling patterns, resulting in 2-3 fold improvements in sensitivity for challenging biomolecular systems.
Strengths: Exceptional spectral resolution allowing study of larger proteins; reduced signal overlap through strategic deuteration; cost-effective production methods for labeled proteins. Weaknesses: Requires specialized expertise in both synthetic chemistry and NMR methodology; higher initial investment in isotope materials; limited application to certain classes of biomolecules.
Key Patents and Breakthroughs in NMR Sensitivity
A method for detecting a target substance by nuclear magnetic resonance
PatentWO2009012911A1
Innovation
- The method involves adding isotope-labeled target substances to the sample, which changes the NMR signal positions or multiplicities, allowing for the calculation of the target substance's signal positions and subsequent detection and quantification by exploiting the differences in signal positions between the labeled and unlabeled substances.
Isomarker system for component analysis of mixtures
PatentInactiveUS20060206270A1
Innovation
- The use of isotope labeled marker molecules, or IsoMarkers, which are coupled with specific molecules in a mixture to enhance sensitivity and selectively quantify molecular components through NMR spectroscopy, reducing spectral congestion and improving detection sensitivity by narrowing the frequency bandwidth.
Safety and Regulatory Considerations for Isotope Use
The use of isotope labeling in NMR spectroscopy necessitates careful consideration of safety protocols and regulatory compliance. Radioactive isotopes such as 13C, 15N, and 2H require specialized handling procedures to minimize exposure risks to laboratory personnel. Facilities conducting isotope labeling must implement comprehensive safety training programs, ensuring all staff understand proper handling techniques, storage requirements, and emergency response protocols for isotope-related incidents.
Regulatory frameworks governing isotope use vary significantly across jurisdictions, creating a complex compliance landscape for multinational research organizations. In the United States, the Nuclear Regulatory Commission (NRC) oversees radioactive materials, while the European Union follows directives established by Euratom. Research institutions must obtain appropriate licenses and permits before acquiring isotopes, with requirements typically including detailed documentation of safety measures, waste disposal protocols, and regular facility inspections.
Waste management represents a critical aspect of isotope handling, as improper disposal can lead to environmental contamination and regulatory penalties. Institutions must establish clear protocols for segregating, storing, and disposing of isotope-containing waste in accordance with local regulations. This often involves specialized waste containers, detailed record-keeping, and contracts with certified disposal facilities equipped to handle radioactive materials.
Transportation of isotopes presents additional regulatory challenges, as cross-border shipments must comply with international regulations such as the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. Proper packaging, labeling, and documentation are essential for legal transport, with requirements varying based on isotope type, activity level, and destination country.
Risk assessment and mitigation strategies form the foundation of isotope safety programs. Institutions should conduct regular audits to identify potential hazards, implement engineering controls such as ventilation systems and shielding, and provide appropriate personal protective equipment. Monitoring programs, including regular testing of personnel and facilities for contamination, help ensure early detection of potential exposure incidents.
Emerging regulatory trends indicate increasing scrutiny of isotope use, with growing emphasis on security measures to prevent unauthorized access to radioactive materials. Research institutions should anticipate stricter reporting requirements and more frequent inspections as regulatory bodies respond to global security concerns regarding radioactive materials.
Regulatory frameworks governing isotope use vary significantly across jurisdictions, creating a complex compliance landscape for multinational research organizations. In the United States, the Nuclear Regulatory Commission (NRC) oversees radioactive materials, while the European Union follows directives established by Euratom. Research institutions must obtain appropriate licenses and permits before acquiring isotopes, with requirements typically including detailed documentation of safety measures, waste disposal protocols, and regular facility inspections.
Waste management represents a critical aspect of isotope handling, as improper disposal can lead to environmental contamination and regulatory penalties. Institutions must establish clear protocols for segregating, storing, and disposing of isotope-containing waste in accordance with local regulations. This often involves specialized waste containers, detailed record-keeping, and contracts with certified disposal facilities equipped to handle radioactive materials.
Transportation of isotopes presents additional regulatory challenges, as cross-border shipments must comply with international regulations such as the International Air Transport Association (IATA) Dangerous Goods Regulations and the International Maritime Dangerous Goods (IMDG) Code. Proper packaging, labeling, and documentation are essential for legal transport, with requirements varying based on isotope type, activity level, and destination country.
Risk assessment and mitigation strategies form the foundation of isotope safety programs. Institutions should conduct regular audits to identify potential hazards, implement engineering controls such as ventilation systems and shielding, and provide appropriate personal protective equipment. Monitoring programs, including regular testing of personnel and facilities for contamination, help ensure early detection of potential exposure incidents.
Emerging regulatory trends indicate increasing scrutiny of isotope use, with growing emphasis on security measures to prevent unauthorized access to radioactive materials. Research institutions should anticipate stricter reporting requirements and more frequent inspections as regulatory bodies respond to global security concerns regarding radioactive materials.
Cost-Benefit Analysis of Advanced Labeling Methods
The economic implications of isotope labeling techniques for NMR sensitivity enhancement require careful analysis to determine their practical viability in research and industrial applications. When evaluating advanced labeling methods such as 13C, 15N, and 2H enrichment, the initial acquisition costs represent a significant investment. For instance, uniformly 13C-labeled glucose typically costs $400-600 per gram, while 15N-enriched ammonium salts range from $200-400 per gram, creating substantial upfront expenses for comprehensive labeling protocols.
Production scaling presents another critical economic consideration. Small-scale isotope incorporation for academic research differs dramatically from industrial applications requiring kilogram quantities. The economies of scale do reduce per-unit costs, but the relationship is not linear, with diminishing returns observed beyond certain production thresholds. Facilities investing in large-scale labeling capabilities must carefully project utilization rates to justify the capital expenditure.
The sensitivity gains from isotope labeling directly translate to time efficiency in NMR experiments. Quantitative analyses demonstrate that 13C enrichment can reduce acquisition times by factors of 10-100 compared to natural abundance samples. This efficiency creates substantial operational savings in instrument time, which typically costs $50-150 per hour on commercial NMR facilities. For pharmaceutical companies conducting high-throughput structural analyses, these time savings often justify the premium paid for labeled compounds.
Sample preparation complexity adds hidden costs to the labeling equation. Selective labeling protocols may require specialized growth media, controlled expression systems, and purification procedures that extend beyond the raw material costs. These technical requirements necessitate skilled personnel and specialized equipment, factors often underestimated in preliminary budget projections. Organizations must consider these ancillary expenses when calculating the true cost of implementing advanced labeling strategies.
The long-term research value provides the final economic dimension for consideration. Isotope-labeled samples enable experiments impossible with natural abundance materials, potentially yielding insights that accelerate drug discovery or materials development. This value creation, while difficult to quantify precisely, often represents the most compelling justification for isotope labeling investments. Case studies from pharmaceutical development suggest that structural insights from labeled samples can reduce development timelines by months, creating value that dwarfs the initial labeling expenses.
Production scaling presents another critical economic consideration. Small-scale isotope incorporation for academic research differs dramatically from industrial applications requiring kilogram quantities. The economies of scale do reduce per-unit costs, but the relationship is not linear, with diminishing returns observed beyond certain production thresholds. Facilities investing in large-scale labeling capabilities must carefully project utilization rates to justify the capital expenditure.
The sensitivity gains from isotope labeling directly translate to time efficiency in NMR experiments. Quantitative analyses demonstrate that 13C enrichment can reduce acquisition times by factors of 10-100 compared to natural abundance samples. This efficiency creates substantial operational savings in instrument time, which typically costs $50-150 per hour on commercial NMR facilities. For pharmaceutical companies conducting high-throughput structural analyses, these time savings often justify the premium paid for labeled compounds.
Sample preparation complexity adds hidden costs to the labeling equation. Selective labeling protocols may require specialized growth media, controlled expression systems, and purification procedures that extend beyond the raw material costs. These technical requirements necessitate skilled personnel and specialized equipment, factors often underestimated in preliminary budget projections. Organizations must consider these ancillary expenses when calculating the true cost of implementing advanced labeling strategies.
The long-term research value provides the final economic dimension for consideration. Isotope-labeled samples enable experiments impossible with natural abundance materials, potentially yielding insights that accelerate drug discovery or materials development. This value creation, while difficult to quantify precisely, often represents the most compelling justification for isotope labeling investments. Case studies from pharmaceutical development suggest that structural insights from labeled samples can reduce development timelines by months, creating value that dwarfs the initial labeling expenses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!