PTFE in Medical Devices: Innovations and Benefits
PTFE Medical Evolution
Polytetrafluoroethylene (PTFE) has undergone a remarkable evolution in its medical applications since its accidental discovery in 1938. Initially developed for industrial use, PTFE's unique properties quickly caught the attention of the medical community. The material's journey in medical devices began in the 1950s when it was first used in artificial heart valves and vascular grafts.
The 1960s and 1970s saw a rapid expansion of PTFE's medical applications. Surgeons began using PTFE-coated instruments due to its non-stick properties, significantly improving surgical precision and reducing tissue damage. During this period, PTFE also found its way into dental implants and orthopedic devices, leveraging its biocompatibility and low friction characteristics.
The 1980s marked a significant milestone with the development of expanded PTFE (ePTFE). This innovation allowed for the creation of porous structures, opening up new possibilities in tissue engineering and regenerative medicine. ePTFE became a crucial component in hernia repair meshes and vascular grafts, offering improved integration with the body's tissues.
In the 1990s and early 2000s, PTFE's role in minimally invasive procedures grew substantially. Its use in catheters and guidewires revolutionized interventional cardiology and radiology, enabling more precise and less traumatic procedures. Concurrently, PTFE-based membranes found applications in regenerative dentistry and ophthalmology.
The past two decades have seen PTFE evolve further with nanotechnology advancements. Nano-modified PTFE surfaces have been developed to enhance antimicrobial properties and improve cell adhesion in specific applications. This has led to innovations in wound dressings and implantable devices with reduced infection risks.
Recent years have witnessed the integration of PTFE with other advanced materials and technologies. Hybrid materials combining PTFE with bioactive compounds have shown promise in drug delivery systems and tissue scaffolds. Additionally, 3D printing technologies have enabled the creation of custom PTFE implants with complex geometries, tailored to individual patient needs.
Looking ahead, the evolution of PTFE in medical devices continues to focus on enhancing biocompatibility, reducing inflammatory responses, and improving long-term performance. Research is ongoing into self-healing PTFE composites and smart PTFE materials that can respond to physiological changes, potentially revolutionizing implantable medical devices and tissue engineering scaffolds.
Market Demand Analysis
The market demand for PTFE in medical devices has been steadily increasing due to its unique properties and versatile applications. PTFE, known for its exceptional chemical resistance, low friction coefficient, and biocompatibility, has become a crucial material in various medical applications, driving significant growth in the healthcare sector.
In the cardiovascular field, PTFE is widely used in vascular grafts and heart valves. The aging population and rising incidence of cardiovascular diseases have led to a surge in demand for these devices. PTFE-based vascular grafts offer superior patency rates and reduced risk of infection, making them preferred choices for bypass surgeries and hemodialysis access.
The orthopedic market has also witnessed increased adoption of PTFE-coated implants and joint replacements. These devices provide enhanced wear resistance and reduced friction, leading to improved longevity and patient outcomes. As the global population ages and the prevalence of osteoarthritis rises, the demand for such implants is expected to grow significantly.
In the field of minimally invasive surgery, PTFE-coated guidewires and catheters have gained popularity due to their low friction properties, allowing for smoother navigation through blood vessels and reducing the risk of tissue damage. The trend towards less invasive procedures has further boosted the demand for these PTFE-enhanced devices.
The dental industry has embraced PTFE membranes for guided tissue regeneration procedures. These membranes promote bone and soft tissue growth while preventing unwanted cell migration, making them invaluable in periodontal and implant surgeries. The growing awareness of dental health and increasing demand for cosmetic dentistry have contributed to the expansion of this market segment.
PTFE's non-stick properties have made it an ideal material for medical packaging and drug delivery systems. Its use in pharmaceutical packaging helps prevent drug adherence and ensures accurate dosing. The increasing focus on patient compliance and drug efficacy has driven the demand for PTFE-based packaging solutions.
The global pandemic has highlighted the importance of personal protective equipment (PPE), with PTFE finding applications in high-performance face masks and protective clothing. Its ability to repel liquids while allowing breathability has made it a valuable material in creating effective barriers against pathogens.
As healthcare systems worldwide strive for improved patient outcomes and cost-effectiveness, the demand for PTFE in medical devices is expected to continue its upward trajectory. The material's ability to enhance device performance, reduce complications, and extend product lifespan aligns well with these objectives, positioning PTFE as a critical component in the future of medical technology.
PTFE Tech Challenges
Despite its widespread use in medical devices, PTFE (Polytetrafluoroethylene) faces several technical challenges that researchers and manufacturers are actively addressing. One of the primary concerns is the potential for PTFE particle shedding, which can occur during the manufacturing process or over time as the material wears. These particles may lead to inflammation or other adverse reactions in patients, particularly in long-term implantable devices.
Another significant challenge lies in the surface properties of PTFE. While its non-stick nature is beneficial in many applications, it can also hinder cell adhesion and tissue integration in certain medical contexts. This limitation becomes particularly problematic in implants where tissue attachment is crucial for proper functioning and long-term stability.
The sterilization of PTFE-based medical devices presents another hurdle. Traditional sterilization methods, such as high-temperature autoclaving, can potentially alter the material's properties or structure. This necessitates the development of alternative sterilization techniques that maintain the integrity of PTFE while ensuring complete microbial elimination.
PTFE's inherent chemical inertness, while advantageous in many scenarios, poses challenges in bonding and coating processes. Achieving strong, durable bonds between PTFE and other materials used in medical devices often requires specialized surface treatment techniques or the development of novel adhesive systems.
The manufacturing of complex PTFE structures, especially those with intricate geometries or micro-features, remains a technical challenge. Traditional machining methods can be difficult to apply to PTFE due to its softness and tendency to deform under pressure. This limitation has spurred research into advanced manufacturing techniques, including 3D printing and micro-molding of PTFE.
Environmental concerns also present a growing challenge for PTFE in medical applications. The material's non-biodegradability and the environmental impact of its production process have led to increased scrutiny and a push for more sustainable alternatives or improved recycling methods for PTFE-based medical devices.
Lastly, the biocompatibility of PTFE, while generally good, still faces challenges in certain specialized applications. For instance, in blood-contacting devices, there is an ongoing need to improve PTFE's hemocompatibility to reduce the risk of thrombosis and enhance long-term performance. This has led to research into surface modifications and the development of composite materials that combine PTFE with other biocompatible substances.
Current PTFE Solutions
01 PTFE manufacturing processes
Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding procedures. These processes aim to improve the quality, consistency, and properties of PTFE products for different applications.- PTFE manufacturing processes: Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding methods. These processes aim to improve the quality, efficiency, and properties of the resulting PTFE materials.
- PTFE composite materials: PTFE is often combined with other materials to create composite structures with enhanced properties. These composites may include reinforcing fibers, nanoparticles, or other polymers to improve strength, conductivity, or other specific characteristics.
- Surface modification of PTFE: Techniques for modifying the surface of PTFE materials are explored to enhance properties such as adhesion, wettability, or biocompatibility. These modifications may involve chemical treatments, plasma processing, or the application of coatings.
- PTFE in membrane technology: PTFE is widely used in membrane applications due to its chemical resistance and non-stick properties. Innovations in this area focus on creating porous PTFE membranes for filtration, separation, and other specialized applications.
- PTFE recycling and environmental considerations: Research into recycling methods for PTFE waste and developing more environmentally friendly production processes. This includes techniques for breaking down PTFE for reuse and exploring bio-based alternatives or additives to reduce environmental impact.
02 PTFE composites and blends
The development of PTFE composites and blends with other materials to enhance specific properties such as wear resistance, thermal conductivity, or mechanical strength. These combinations create new materials with improved performance for specialized applications.Expand Specific Solutions03 Surface modification of PTFE
Techniques for modifying the surface of PTFE to improve its adhesion, wettability, or compatibility with other materials. These modifications can include chemical treatments, plasma treatments, or the application of coatings to enhance PTFE's functionality in various applications.Expand Specific Solutions04 PTFE in membrane and filtration applications
The use of PTFE in the development of membranes and filtration systems, leveraging its non-stick and chemical-resistant properties. These applications include water treatment, gas separation, and industrial filtration processes.Expand Specific Solutions05 PTFE in electronic and electrical applications
The incorporation of PTFE in electronic and electrical components due to its excellent dielectric properties and thermal stability. Applications include insulation materials, printed circuit boards, and high-frequency communication devices.Expand Specific Solutions
Key Industry Players
The PTFE in medical devices market is in a mature growth phase, characterized by steady innovation and expanding applications. The global market size for PTFE in medical devices is substantial, driven by increasing demand for minimally invasive procedures and advanced medical technologies. Technologically, PTFE applications are well-established, with ongoing refinements focused on enhancing biocompatibility and performance. Key players like Medtronic, Boston Scientific, and W. L. Gore & Associates are at the forefront, continuously developing new PTFE-based solutions for cardiovascular, orthopedic, and general surgery applications. These companies leverage their extensive R&D capabilities and market presence to maintain competitive advantages in this specialized field.
Boston Scientific Ltd.
Medtronic, Inc.
PTFE Patent Landscape
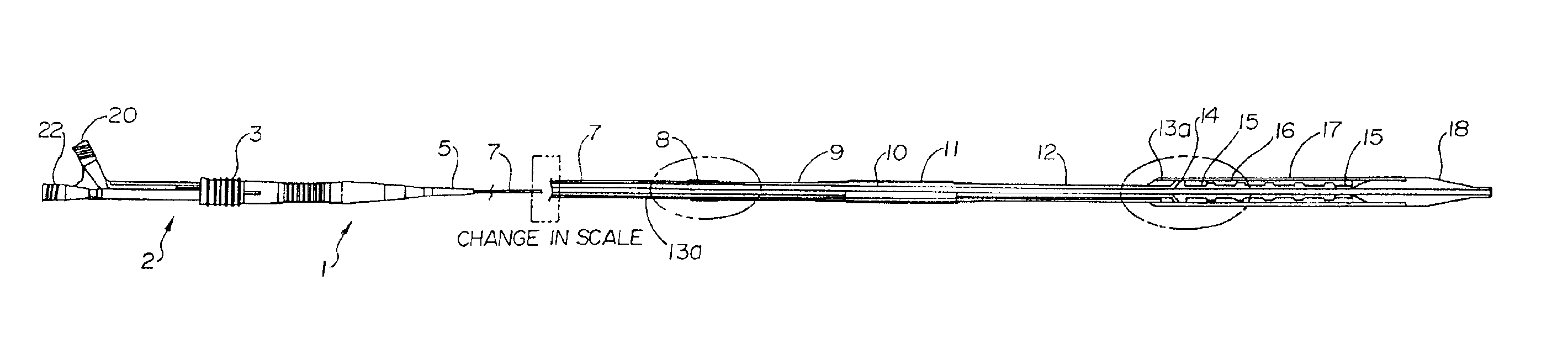
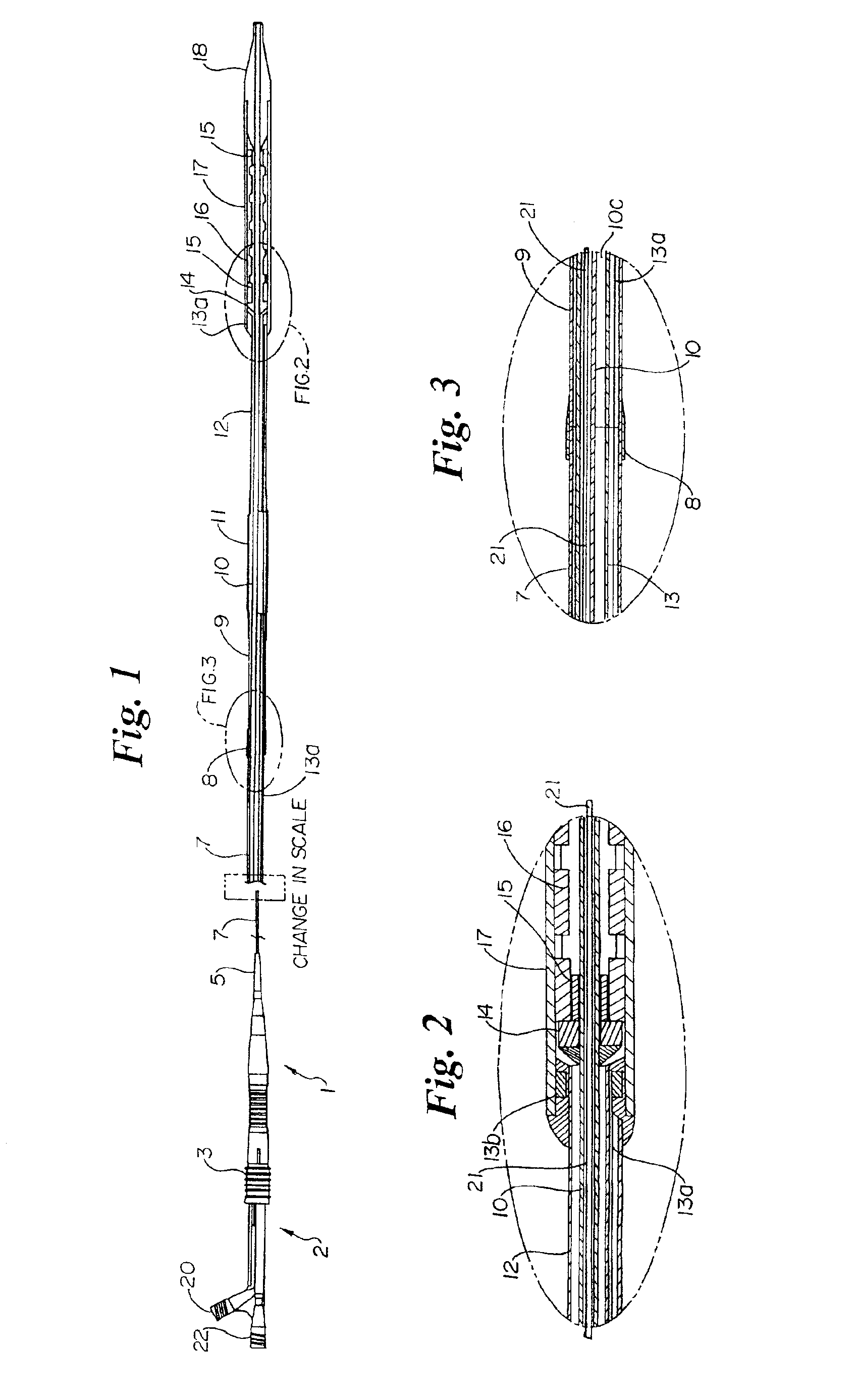
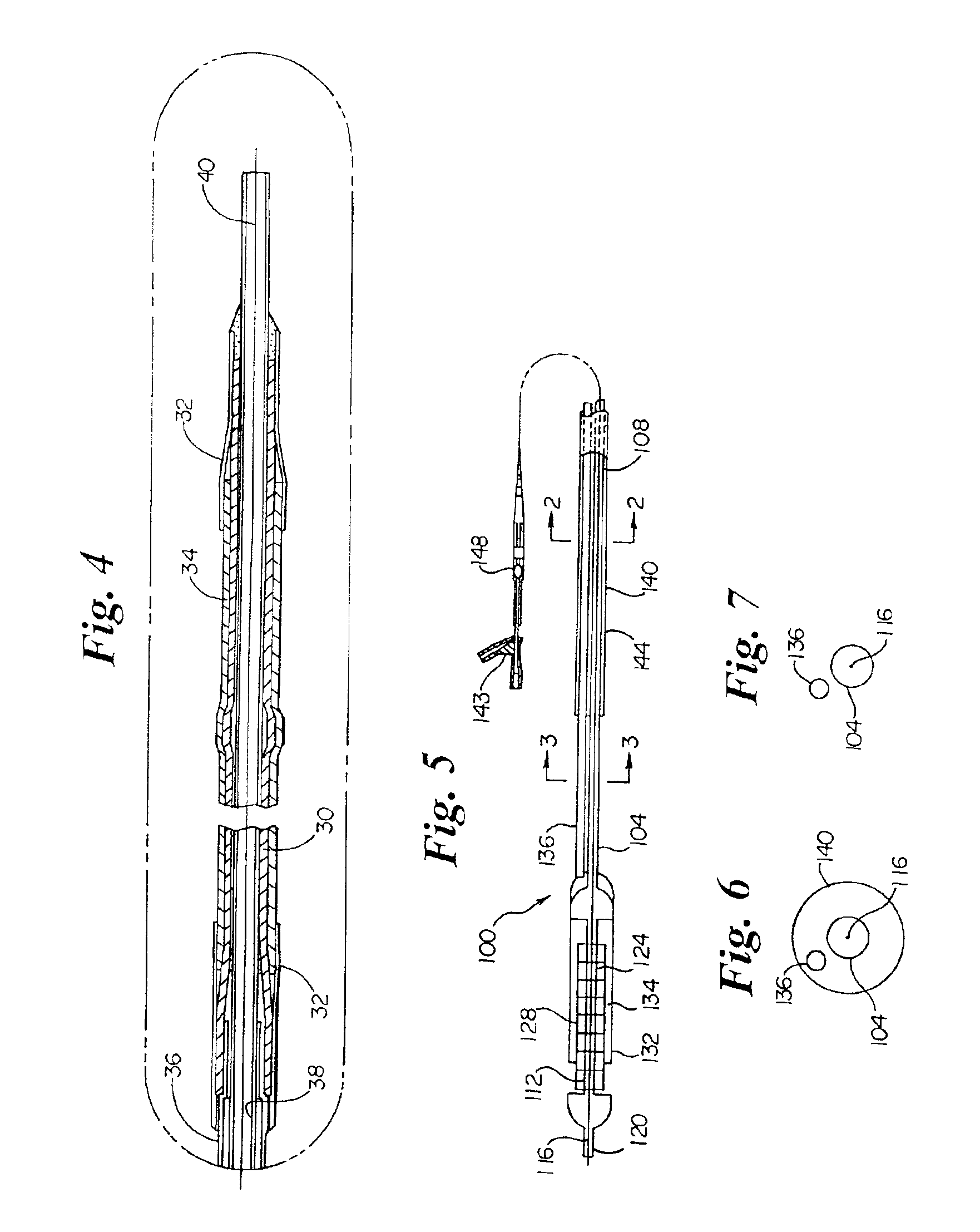
- Development of melt-processible poly(tetrafluoroethylene) (MP-PTFE) with specific physical characteristics, allowing for extrusions and coextrusions to form medical device parts with low friction and good trackability, eliminating the need for additional coatings or layers.
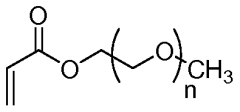
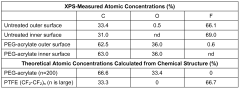
- Surface modification of ePTFE using a multi-step process involving radio frequency (RF)-generated plasmas to pre-treat, coat with a hydrophilic polymer, and crosslink the coating, specifically using argon and H2O plasmas with a PEG-acrylate coating to enhance wettability.
Biocompatibility Aspect
Biocompatibility is a critical aspect of PTFE (Polytetrafluoroethylene) in medical devices, playing a pivotal role in its widespread adoption and success. PTFE's exceptional biocompatibility stems from its unique chemical structure, which renders it inert and non-reactive within the human body. This characteristic makes PTFE an ideal material for various medical applications, ranging from implants to surgical instruments.
One of the key factors contributing to PTFE's biocompatibility is its low surface energy, which minimizes protein adsorption and cellular adhesion. This property significantly reduces the risk of thrombosis and inflammatory responses when PTFE is used in blood-contacting devices such as vascular grafts and heart valves. The material's resistance to bacterial colonization further enhances its suitability for long-term implantation, reducing the risk of device-associated infections.
PTFE's chemical stability also contributes to its biocompatibility. The strong carbon-fluorine bonds in its molecular structure make it highly resistant to degradation by bodily fluids and enzymes. This stability ensures that PTFE maintains its structural integrity and functional properties over extended periods, minimizing the release of potentially harmful degradation products into the surrounding tissues.
Recent innovations in PTFE technology have focused on enhancing its biocompatibility even further. Surface modification techniques, such as plasma treatment and grafting of bioactive molecules, have been developed to improve cell adhesion and tissue integration in specific applications. These advancements have expanded the range of medical devices that can benefit from PTFE's properties, including orthopedic implants and tissue engineering scaffolds.
The biocompatibility of PTFE has been extensively studied and validated through numerous in vitro and in vivo studies. These investigations have consistently demonstrated low cytotoxicity, minimal inflammatory responses, and excellent tissue compatibility. Furthermore, long-term clinical studies of PTFE-based medical devices have shown favorable outcomes, reinforcing its status as a safe and reliable biomaterial.
Despite its excellent biocompatibility profile, ongoing research continues to explore potential improvements and address specific challenges. For instance, efforts are being made to develop PTFE composites that combine its biocompatibility with enhanced mechanical properties or drug-eluting capabilities. These innovations aim to expand the applications of PTFE in medical devices while maintaining its core biocompatibility benefits.
Regulatory Compliance
The regulatory landscape for PTFE in medical devices is complex and constantly evolving. Manufacturers must navigate a web of regulations and standards to ensure compliance and market access. In the United States, the Food and Drug Administration (FDA) oversees the approval process for medical devices containing PTFE. The FDA classifies these devices based on their intended use and risk level, with Class III devices requiring the most stringent premarket approval.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of PTFE in medical devices. These regulations emphasize the importance of clinical evidence, post-market surveillance, and traceability throughout the device lifecycle. Manufacturers must obtain CE marking to demonstrate compliance with these regulations before placing their products on the EU market.
International standards, such as ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems, play a crucial role in ensuring the safety and efficacy of PTFE-containing medical devices. Adherence to these standards is often necessary to meet regulatory requirements in various jurisdictions.
The regulatory framework also addresses the potential risks associated with PTFE, such as the release of perfluorooctanoic acid (PFOA) during manufacturing. Many regulatory bodies have implemented restrictions on PFOA content in PTFE materials used in medical devices. Manufacturers must demonstrate compliance with these limits through rigorous testing and documentation.
Post-market surveillance is an essential aspect of regulatory compliance for PTFE-containing medical devices. Manufacturers are required to monitor the performance and safety of their products in real-world settings, reporting any adverse events or product defects to the relevant authorities. This ongoing vigilance helps identify potential issues and ensures the continued safety and effectiveness of PTFE-based medical devices.
As environmental concerns grow, regulatory bodies are increasingly focusing on the sustainability and end-of-life management of medical devices containing PTFE. Manufacturers may need to consider recyclability, disposal methods, and potential environmental impacts when designing and producing these devices to meet evolving regulatory expectations.
Regulatory compliance for PTFE in medical devices requires a comprehensive approach, encompassing product development, manufacturing processes, clinical evaluation, and post-market activities. Staying abreast of regulatory changes and proactively addressing compliance requirements is crucial for manufacturers to maintain market access and ensure patient safety in this dynamic field.