PTFE in Pharmaceutical Manufacturing: Innovations Ahead
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PTFE in Pharma: Past and Future
Polytetrafluoroethylene (PTFE) has played a significant role in pharmaceutical manufacturing since its introduction in the 1940s. Initially developed as a byproduct of refrigerant research, PTFE quickly found applications in various industries due to its unique properties. In the pharmaceutical sector, PTFE's chemical inertness, low friction coefficient, and high temperature resistance made it an ideal material for equipment components and packaging.
The early adoption of PTFE in pharmaceutical manufacturing focused primarily on its use in gaskets, seals, and linings for processing equipment. These applications leveraged PTFE's ability to withstand harsh chemical environments and prevent contamination. As the industry evolved, so did the applications of PTFE, expanding to include filtration membranes, tubing, and specialized coatings for tablet manufacturing equipment.
Throughout the 1960s and 1970s, advancements in PTFE production techniques led to the development of modified PTFE materials with enhanced properties. These innovations allowed for broader applications in pharmaceutical manufacturing, including the creation of more efficient drug delivery systems and improved packaging solutions.
The 1980s and 1990s saw a surge in PTFE use for controlled release formulations and transdermal patches. PTFE's non-stick properties and biocompatibility made it an excellent choice for these applications, enabling more precise and consistent drug delivery. During this period, PTFE also became increasingly important in the production of high-purity active pharmaceutical ingredients (APIs), where its resistance to chemical reactions helped maintain product integrity.
In recent years, the focus has shifted towards developing PTFE nanocomposites and surface-modified PTFE materials. These innovations aim to enhance the material's performance in specific pharmaceutical applications, such as improving drug dissolution rates or creating more effective barrier properties for packaging.
Looking to the future, PTFE is poised to play an even more critical role in pharmaceutical manufacturing. Emerging trends include the development of PTFE-based 3D printing materials for personalized medicine applications and the integration of PTFE in smart packaging solutions. Additionally, research into PTFE's potential in advanced drug delivery systems, such as targeted nanocarriers, shows promise for revolutionizing treatment approaches.
As sustainability becomes increasingly important in the pharmaceutical industry, efforts are underway to develop more environmentally friendly PTFE production methods and recycling techniques. These advancements will likely shape the future use of PTFE in pharmaceutical manufacturing, ensuring its continued relevance while addressing environmental concerns.
The early adoption of PTFE in pharmaceutical manufacturing focused primarily on its use in gaskets, seals, and linings for processing equipment. These applications leveraged PTFE's ability to withstand harsh chemical environments and prevent contamination. As the industry evolved, so did the applications of PTFE, expanding to include filtration membranes, tubing, and specialized coatings for tablet manufacturing equipment.
Throughout the 1960s and 1970s, advancements in PTFE production techniques led to the development of modified PTFE materials with enhanced properties. These innovations allowed for broader applications in pharmaceutical manufacturing, including the creation of more efficient drug delivery systems and improved packaging solutions.
The 1980s and 1990s saw a surge in PTFE use for controlled release formulations and transdermal patches. PTFE's non-stick properties and biocompatibility made it an excellent choice for these applications, enabling more precise and consistent drug delivery. During this period, PTFE also became increasingly important in the production of high-purity active pharmaceutical ingredients (APIs), where its resistance to chemical reactions helped maintain product integrity.
In recent years, the focus has shifted towards developing PTFE nanocomposites and surface-modified PTFE materials. These innovations aim to enhance the material's performance in specific pharmaceutical applications, such as improving drug dissolution rates or creating more effective barrier properties for packaging.
Looking to the future, PTFE is poised to play an even more critical role in pharmaceutical manufacturing. Emerging trends include the development of PTFE-based 3D printing materials for personalized medicine applications and the integration of PTFE in smart packaging solutions. Additionally, research into PTFE's potential in advanced drug delivery systems, such as targeted nanocarriers, shows promise for revolutionizing treatment approaches.
As sustainability becomes increasingly important in the pharmaceutical industry, efforts are underway to develop more environmentally friendly PTFE production methods and recycling techniques. These advancements will likely shape the future use of PTFE in pharmaceutical manufacturing, ensuring its continued relevance while addressing environmental concerns.
Market Demand Analysis
The pharmaceutical industry's demand for PTFE (Polytetrafluoroethylene) has been steadily increasing due to its unique properties and versatile applications in drug manufacturing processes. PTFE's exceptional chemical resistance, low friction, and non-stick characteristics make it an invaluable material in various pharmaceutical equipment and components.
One of the primary drivers of market demand for PTFE in pharmaceutical manufacturing is the growing emphasis on contamination control and product purity. PTFE's inertness and resistance to chemical reactions make it ideal for use in critical processing equipment, such as reactors, filters, and transfer lines. This helps prevent unwanted interactions between the manufacturing equipment and pharmaceutical compounds, ensuring the integrity and quality of the final products.
The increasing adoption of single-use technologies in pharmaceutical manufacturing has also contributed to the rising demand for PTFE. Single-use systems often incorporate PTFE-based components, such as tubing, gaskets, and seals, due to their compatibility with a wide range of chemicals and their ability to maintain product purity. This trend is expected to continue as pharmaceutical companies seek to improve flexibility, reduce cross-contamination risks, and lower production costs.
Another factor driving market demand is the expansion of biopharmaceutical manufacturing. PTFE's excellent biocompatibility and resistance to protein adsorption make it particularly suitable for use in bioprocessing equipment. As the biopharmaceutical sector continues to grow, the demand for PTFE in applications such as cell culture systems, chromatography columns, and filtration membranes is expected to increase correspondingly.
The pharmaceutical industry's focus on process optimization and efficiency has also led to increased interest in PTFE-based solutions. PTFE coatings and components can improve equipment performance, reduce maintenance requirements, and extend the lifespan of manufacturing assets. This translates to lower operational costs and improved productivity for pharmaceutical manufacturers, further driving the adoption of PTFE-based technologies.
Regulatory requirements and quality standards in pharmaceutical manufacturing have become increasingly stringent, creating additional demand for materials that can meet these high standards. PTFE's compliance with FDA regulations and its ability to withstand sterilization processes make it a preferred choice for many pharmaceutical applications. As regulatory scrutiny continues to intensify, the demand for PTFE in pharmaceutical manufacturing is expected to grow further.
The global trend towards personalized medicine and small-batch production is also influencing the market demand for PTFE. Flexible manufacturing systems that can handle a variety of products and batch sizes often rely on PTFE components due to their versatility and ease of cleaning. This trend is likely to drive innovation in PTFE-based solutions tailored to the needs of adaptable pharmaceutical manufacturing processes.
One of the primary drivers of market demand for PTFE in pharmaceutical manufacturing is the growing emphasis on contamination control and product purity. PTFE's inertness and resistance to chemical reactions make it ideal for use in critical processing equipment, such as reactors, filters, and transfer lines. This helps prevent unwanted interactions between the manufacturing equipment and pharmaceutical compounds, ensuring the integrity and quality of the final products.
The increasing adoption of single-use technologies in pharmaceutical manufacturing has also contributed to the rising demand for PTFE. Single-use systems often incorporate PTFE-based components, such as tubing, gaskets, and seals, due to their compatibility with a wide range of chemicals and their ability to maintain product purity. This trend is expected to continue as pharmaceutical companies seek to improve flexibility, reduce cross-contamination risks, and lower production costs.
Another factor driving market demand is the expansion of biopharmaceutical manufacturing. PTFE's excellent biocompatibility and resistance to protein adsorption make it particularly suitable for use in bioprocessing equipment. As the biopharmaceutical sector continues to grow, the demand for PTFE in applications such as cell culture systems, chromatography columns, and filtration membranes is expected to increase correspondingly.
The pharmaceutical industry's focus on process optimization and efficiency has also led to increased interest in PTFE-based solutions. PTFE coatings and components can improve equipment performance, reduce maintenance requirements, and extend the lifespan of manufacturing assets. This translates to lower operational costs and improved productivity for pharmaceutical manufacturers, further driving the adoption of PTFE-based technologies.
Regulatory requirements and quality standards in pharmaceutical manufacturing have become increasingly stringent, creating additional demand for materials that can meet these high standards. PTFE's compliance with FDA regulations and its ability to withstand sterilization processes make it a preferred choice for many pharmaceutical applications. As regulatory scrutiny continues to intensify, the demand for PTFE in pharmaceutical manufacturing is expected to grow further.
The global trend towards personalized medicine and small-batch production is also influencing the market demand for PTFE. Flexible manufacturing systems that can handle a variety of products and batch sizes often rely on PTFE components due to their versatility and ease of cleaning. This trend is likely to drive innovation in PTFE-based solutions tailored to the needs of adaptable pharmaceutical manufacturing processes.
Technical Challenges
The use of PTFE (Polytetrafluoroethylene) in pharmaceutical manufacturing faces several technical challenges that hinder its widespread adoption and optimal performance. One of the primary issues is the material's inherent non-stick properties, which, while beneficial in many applications, can lead to difficulties in achieving proper adhesion and bonding with other materials or surfaces in pharmaceutical processes.
Another significant challenge lies in the processing and fabrication of PTFE components for pharmaceutical equipment. The high melting point and unique molecular structure of PTFE make it resistant to conventional molding and shaping techniques, requiring specialized and often costly manufacturing processes. This limitation can impact the design flexibility and production efficiency of PTFE-based pharmaceutical equipment.
The potential for particle shedding is a critical concern in pharmaceutical manufacturing. Despite PTFE's excellent chemical resistance, there is a risk of microscopic particles detaching from PTFE surfaces during high-stress operations or over extended periods of use. These particles can potentially contaminate pharmaceutical products, posing risks to product quality and patient safety.
PTFE's thermal properties present both advantages and challenges. While its high temperature resistance is beneficial, the material's poor heat conductivity can lead to inefficiencies in heat transfer processes, which are crucial in many pharmaceutical manufacturing operations. This characteristic may necessitate the development of innovative heat management solutions when incorporating PTFE components.
The chemical inertness of PTFE, while generally an asset, can sometimes be a drawback in pharmaceutical applications that require specific surface interactions or modifications. Developing methods to selectively modify PTFE surfaces without compromising its core properties remains a technical challenge, particularly for applications requiring controlled drug release or specific biomolecular interactions.
Regulatory compliance and validation pose additional challenges. The use of PTFE in pharmaceutical manufacturing must meet stringent regulatory standards, including those set by the FDA and other global health authorities. Demonstrating the long-term stability, biocompatibility, and absence of leachables from PTFE components in various pharmaceutical processes requires extensive testing and documentation.
Lastly, the integration of PTFE with emerging technologies in pharmaceutical manufacturing, such as continuous manufacturing processes and advanced process analytical technologies (PAT), presents new technical hurdles. Adapting PTFE components to these innovative manufacturing paradigms while maintaining their beneficial properties and meeting regulatory requirements is an ongoing challenge that requires continuous research and development efforts.
Another significant challenge lies in the processing and fabrication of PTFE components for pharmaceutical equipment. The high melting point and unique molecular structure of PTFE make it resistant to conventional molding and shaping techniques, requiring specialized and often costly manufacturing processes. This limitation can impact the design flexibility and production efficiency of PTFE-based pharmaceutical equipment.
The potential for particle shedding is a critical concern in pharmaceutical manufacturing. Despite PTFE's excellent chemical resistance, there is a risk of microscopic particles detaching from PTFE surfaces during high-stress operations or over extended periods of use. These particles can potentially contaminate pharmaceutical products, posing risks to product quality and patient safety.
PTFE's thermal properties present both advantages and challenges. While its high temperature resistance is beneficial, the material's poor heat conductivity can lead to inefficiencies in heat transfer processes, which are crucial in many pharmaceutical manufacturing operations. This characteristic may necessitate the development of innovative heat management solutions when incorporating PTFE components.
The chemical inertness of PTFE, while generally an asset, can sometimes be a drawback in pharmaceutical applications that require specific surface interactions or modifications. Developing methods to selectively modify PTFE surfaces without compromising its core properties remains a technical challenge, particularly for applications requiring controlled drug release or specific biomolecular interactions.
Regulatory compliance and validation pose additional challenges. The use of PTFE in pharmaceutical manufacturing must meet stringent regulatory standards, including those set by the FDA and other global health authorities. Demonstrating the long-term stability, biocompatibility, and absence of leachables from PTFE components in various pharmaceutical processes requires extensive testing and documentation.
Lastly, the integration of PTFE with emerging technologies in pharmaceutical manufacturing, such as continuous manufacturing processes and advanced process analytical technologies (PAT), presents new technical hurdles. Adapting PTFE components to these innovative manufacturing paradigms while maintaining their beneficial properties and meeting regulatory requirements is an ongoing challenge that requires continuous research and development efforts.
Current PTFE Solutions
01 PTFE manufacturing processes
Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding methods. These processes aim to improve the quality, efficiency, and properties of the resulting PTFE products.- PTFE manufacturing processes: Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding methods. These processes aim to improve the quality, consistency, and properties of PTFE products for different applications.
- PTFE composite materials: PTFE is combined with other materials to create composite structures with enhanced properties. These composites may include reinforcing fibers, nanoparticles, or other polymers to improve mechanical strength, thermal stability, or electrical properties.
- Surface modification of PTFE: Techniques for modifying the surface of PTFE to enhance its properties or compatibility with other materials are explored. These may include chemical treatments, plasma treatments, or the application of coatings to improve adhesion or other surface characteristics.
- PTFE in membrane technology: PTFE is utilized in the development of advanced membrane technologies for various applications, including filtration, separation, and gas diffusion. These membranes leverage PTFE's unique properties such as chemical resistance and hydrophobicity.
- PTFE in electronic and electrical applications: The use of PTFE in electronic and electrical components is explored, taking advantage of its excellent dielectric properties and thermal stability. Applications may include insulation materials, printed circuit boards, and high-frequency components.
02 PTFE composite materials
PTFE is combined with other materials to create composite structures with enhanced properties. These composites may include reinforcing fibers, nanoparticles, or other polymers to improve mechanical strength, thermal stability, or electrical properties.Expand Specific Solutions03 Surface modification of PTFE
Techniques for modifying the surface of PTFE to enhance its properties or compatibility with other materials. This may include chemical treatments, plasma treatments, or the application of coatings to improve adhesion, wettability, or other surface characteristics.Expand Specific Solutions04 PTFE in membrane technology
Applications of PTFE in membrane technology, including the development of porous PTFE membranes for filtration, separation, and other industrial processes. These membranes may be used in water treatment, gas separation, or medical applications.Expand Specific Solutions05 PTFE in electronic applications
The use of PTFE in electronic and electrical applications, such as insulation materials, printed circuit boards, and cable coatings. PTFE's excellent dielectric properties and thermal stability make it suitable for various electronic components and assemblies.Expand Specific Solutions
Key Industry Players
The PTFE market in pharmaceutical manufacturing is in a mature growth phase, with increasing demand driven by stringent regulatory requirements and the need for high-performance materials. The global market size is substantial, expected to reach several billion dollars by 2025. Technologically, PTFE applications are well-established, but innovations continue to emerge. Key players like DAIKIN INDUSTRIES, DuPont, and W. L. Gore & Associates are leading advancements in PTFE formulations and processing techniques. Emerging companies such as Guarniflon and Teknor Apex are also contributing to market dynamics with specialized PTFE products. Research institutions like the Swiss Federal Institute of Technology and Zhejiang University are pushing boundaries in PTFE applications, potentially opening new avenues for pharmaceutical manufacturing.
DAIKIN INDUSTRIES Ltd.
Technical Solution: Daikin has pioneered the development of PTFE materials tailored for pharmaceutical applications. Their POLYFLON™ PTFE series includes grades specifically designed for drug manufacturing equipment, offering superior chemical resistance and low friction properties[1]. Daikin has innovated in the area of PTFE micropowders, which are used as glidants and lubricants in tablet formulations, improving flow characteristics and reducing sticking during compression[2]. They have also developed PTFE-based membrane filters with precise pore sizes for sterile filtration in pharmaceutical processes, enhancing product quality and safety[3]. Daikin's research extends to PTFE-based coatings for drug-eluting stents, demonstrating the material's versatility in medical devices[4].
Strengths: Diverse PTFE product portfolio for pharmaceuticals, strong focus on innovation, global presence. Weaknesses: Potential regulatory challenges in some markets, competition from alternative materials in certain applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced PTFE formulations specifically for pharmaceutical manufacturing. Their Teflon™ PTFE fluoropolymer resins offer exceptional chemical resistance and non-stick properties, crucial for preventing drug adherence to processing equipment[1]. DuPont's innovations include PTFE-based coatings for tablet compression tools, reducing sticking issues and improving production efficiency[2]. They have also engineered PTFE-lined piping systems that maintain product purity throughout the manufacturing process, minimizing contamination risks[3]. DuPont's research has led to the development of PTFE grades with enhanced permeability control, vital for controlled-release drug delivery systems[4].
Strengths: Extensive experience in PTFE technology, wide range of pharmaceutical-grade products, strong R&D capabilities. Weaknesses: Higher cost compared to some alternatives, potential environmental concerns associated with fluoropolymer production.
Innovative PTFE Patents
Methods of making polytetrafluoroethylene/polymer composites and uses thereof
PatentActiveUS20210221961A1
Innovation
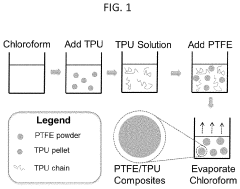
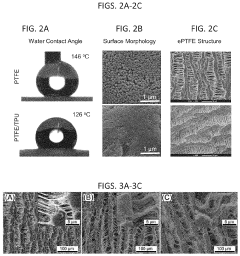
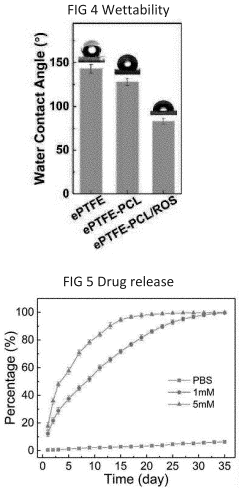
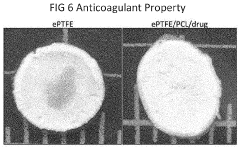
- A method involving dissolving a polymer with a solvent, immersing PTFE into the solution, and extruding the blend to form composite products, allowing for the addition of biodegradable polymers and bio-functional materials, which can be used to create PTFE/polymer composites with improved biocompatibility, bioactivity, and controlled drug release.
Medical devices utilizing melt-processible poly(tetrafluoroethylene)
PatentInactiveUS6939593B2
Innovation
- Development of melt-processible poly(tetrafluoroethylene) (MP-PTFE) with specific physical characteristics, allowing for extrusions and coextrusions to form medical device parts with low friction and good trackability, eliminating the need for additional coatings or layers.
Regulatory Compliance
Regulatory compliance is a critical aspect of PTFE use in pharmaceutical manufacturing. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established stringent guidelines for the use of PTFE in drug production processes. These regulations primarily focus on ensuring the safety and efficacy of pharmaceutical products that come into contact with PTFE materials.
The FDA's Code of Federal Regulations (CFR) Title 21, specifically parts 177 and 178, outlines the requirements for PTFE used in food contact applications, which also apply to pharmaceutical manufacturing. These regulations stipulate the acceptable grades of PTFE, maximum use levels, and specific conditions under which PTFE can be used in drug production equipment and packaging.
Similarly, the EMA has implemented guidelines through the European Pharmacopoeia, which sets standards for PTFE materials used in pharmaceutical applications. These guidelines address issues such as chemical composition, purity, and potential leachables from PTFE components.
Manufacturers must demonstrate compliance with these regulations through rigorous testing and documentation. This includes providing detailed information on the PTFE materials used, their chemical composition, and any potential interactions with pharmaceutical ingredients. Extensive stability studies and leachable/extractable testing are often required to ensure that PTFE components do not compromise drug safety or efficacy.
Recent innovations in PTFE technology have led to the development of new grades specifically designed for pharmaceutical applications. These advanced materials offer improved purity profiles and reduced potential for particle shedding, addressing key regulatory concerns. Manufacturers are increasingly focusing on developing PTFE products that not only meet current regulatory standards but also anticipate future regulatory changes.
Compliance with Good Manufacturing Practices (GMP) is another crucial aspect of PTFE use in pharmaceutical production. GMP guidelines require manufacturers to implement robust quality control systems, maintain detailed documentation, and ensure traceability of all materials used in drug production, including PTFE components.
As regulatory bodies continue to refine their requirements, the pharmaceutical industry is seeing a trend towards more stringent controls on PTFE materials. This includes increased scrutiny of supply chains, more comprehensive material characterization, and enhanced monitoring of PTFE performance throughout the drug manufacturing process.
The FDA's Code of Federal Regulations (CFR) Title 21, specifically parts 177 and 178, outlines the requirements for PTFE used in food contact applications, which also apply to pharmaceutical manufacturing. These regulations stipulate the acceptable grades of PTFE, maximum use levels, and specific conditions under which PTFE can be used in drug production equipment and packaging.
Similarly, the EMA has implemented guidelines through the European Pharmacopoeia, which sets standards for PTFE materials used in pharmaceutical applications. These guidelines address issues such as chemical composition, purity, and potential leachables from PTFE components.
Manufacturers must demonstrate compliance with these regulations through rigorous testing and documentation. This includes providing detailed information on the PTFE materials used, their chemical composition, and any potential interactions with pharmaceutical ingredients. Extensive stability studies and leachable/extractable testing are often required to ensure that PTFE components do not compromise drug safety or efficacy.
Recent innovations in PTFE technology have led to the development of new grades specifically designed for pharmaceutical applications. These advanced materials offer improved purity profiles and reduced potential for particle shedding, addressing key regulatory concerns. Manufacturers are increasingly focusing on developing PTFE products that not only meet current regulatory standards but also anticipate future regulatory changes.
Compliance with Good Manufacturing Practices (GMP) is another crucial aspect of PTFE use in pharmaceutical production. GMP guidelines require manufacturers to implement robust quality control systems, maintain detailed documentation, and ensure traceability of all materials used in drug production, including PTFE components.
As regulatory bodies continue to refine their requirements, the pharmaceutical industry is seeing a trend towards more stringent controls on PTFE materials. This includes increased scrutiny of supply chains, more comprehensive material characterization, and enhanced monitoring of PTFE performance throughout the drug manufacturing process.
Environmental Impact
The use of PTFE in pharmaceutical manufacturing has significant environmental implications that must be carefully considered. PTFE, while offering numerous benefits in terms of chemical resistance and non-stick properties, poses challenges in terms of its production, use, and disposal.
The manufacturing process of PTFE involves the use of fluoropolymers, which can have a substantial environmental footprint. The production of these materials often requires energy-intensive processes and can result in the emission of greenhouse gases. Additionally, the use of certain precursor chemicals in PTFE production, such as perfluorooctanoic acid (PFOA), has raised concerns due to their persistence in the environment and potential health risks.
In pharmaceutical manufacturing, the use of PTFE in equipment and components can contribute to reduced contamination and improved product quality. However, the disposal of PTFE-containing materials at the end of their lifecycle presents environmental challenges. PTFE is not biodegradable and can persist in the environment for extended periods. Incineration of PTFE waste can lead to the release of toxic substances, including hydrogen fluoride and other fluorinated compounds.
Recent innovations in PTFE technology are addressing some of these environmental concerns. Researchers are exploring more sustainable production methods that reduce the use of harmful precursors and minimize emissions. Some manufacturers are developing PTFE alternatives that maintain similar performance characteristics while offering improved environmental profiles.
Recycling of PTFE materials is another area of focus for reducing environmental impact. While challenging due to PTFE's chemical stability, advancements in recycling technologies are making it possible to recover and reprocess PTFE waste. This not only reduces the amount of material sent to landfills but also decreases the demand for virgin PTFE production.
The pharmaceutical industry is increasingly adopting green chemistry principles, which include the use of more environmentally friendly materials and processes. This trend is driving innovation in PTFE alternatives and more sustainable manufacturing practices. Companies are exploring bio-based polymers and other materials that can provide similar benefits to PTFE while reducing environmental impact.
As regulations around environmental protection become more stringent, pharmaceutical manufacturers are likely to face increased pressure to address the environmental impact of PTFE use. This may lead to further innovations in material science and manufacturing processes, aimed at developing more sustainable solutions for the industry.
The manufacturing process of PTFE involves the use of fluoropolymers, which can have a substantial environmental footprint. The production of these materials often requires energy-intensive processes and can result in the emission of greenhouse gases. Additionally, the use of certain precursor chemicals in PTFE production, such as perfluorooctanoic acid (PFOA), has raised concerns due to their persistence in the environment and potential health risks.
In pharmaceutical manufacturing, the use of PTFE in equipment and components can contribute to reduced contamination and improved product quality. However, the disposal of PTFE-containing materials at the end of their lifecycle presents environmental challenges. PTFE is not biodegradable and can persist in the environment for extended periods. Incineration of PTFE waste can lead to the release of toxic substances, including hydrogen fluoride and other fluorinated compounds.
Recent innovations in PTFE technology are addressing some of these environmental concerns. Researchers are exploring more sustainable production methods that reduce the use of harmful precursors and minimize emissions. Some manufacturers are developing PTFE alternatives that maintain similar performance characteristics while offering improved environmental profiles.
Recycling of PTFE materials is another area of focus for reducing environmental impact. While challenging due to PTFE's chemical stability, advancements in recycling technologies are making it possible to recover and reprocess PTFE waste. This not only reduces the amount of material sent to landfills but also decreases the demand for virgin PTFE production.
The pharmaceutical industry is increasingly adopting green chemistry principles, which include the use of more environmentally friendly materials and processes. This trend is driving innovation in PTFE alternatives and more sustainable manufacturing practices. Companies are exploring bio-based polymers and other materials that can provide similar benefits to PTFE while reducing environmental impact.
As regulations around environmental protection become more stringent, pharmaceutical manufacturers are likely to face increased pressure to address the environmental impact of PTFE use. This may lead to further innovations in material science and manufacturing processes, aimed at developing more sustainable solutions for the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!