PTFE Innovations in Medical Biocompatibility
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PTFE Medical Evolution
Polytetrafluoroethylene (PTFE) has undergone a remarkable evolution in medical applications since its accidental discovery in 1938. Initially developed as an industrial material, PTFE's unique properties quickly caught the attention of the medical community. The 1960s marked a significant milestone when PTFE was first used in vascular grafts, revolutionizing cardiovascular surgery.
Throughout the 1970s and 1980s, researchers focused on enhancing PTFE's biocompatibility, leading to the development of expanded PTFE (ePTFE). This innovation greatly improved the material's porosity and tissue integration capabilities, expanding its use in various medical implants and devices. The 1990s saw a surge in PTFE applications, including its use in dental implants, orthopedic devices, and as a coating for medical instruments.
The turn of the millennium brought about a new era of PTFE innovation, with a focus on nanotechnology. Researchers began exploring ways to manipulate PTFE at the molecular level, creating nanostructured surfaces that could better mimic natural tissue environments. This led to improved cell adhesion and reduced foreign body responses, further enhancing PTFE's biocompatibility.
In the 2010s, the medical community witnessed the emergence of PTFE composites and hybrid materials. By combining PTFE with other biocompatible substances, such as hydrogels or carbon nanotubes, researchers developed materials with enhanced mechanical properties and biological performance. These advancements opened up new possibilities in tissue engineering and regenerative medicine.
Recent years have seen a shift towards "smart" PTFE materials. Researchers are now exploring ways to incorporate drug-eluting capabilities and responsive elements into PTFE-based medical devices. This allows for controlled release of therapeutic agents and adaptive responses to physiological changes, potentially revolutionizing long-term implantable devices and drug delivery systems.
The ongoing evolution of PTFE in medical applications continues to focus on improving its biocompatibility, reducing inflammation, and enhancing integration with host tissues. Current research is exploring surface modification techniques, such as plasma treatment and biofunctionalization, to further optimize PTFE's interaction with biological systems. As we look to the future, the development of PTFE-based materials that can actively promote tissue regeneration and seamlessly integrate with the body's natural processes represents the next frontier in medical biocompatibility.
Throughout the 1970s and 1980s, researchers focused on enhancing PTFE's biocompatibility, leading to the development of expanded PTFE (ePTFE). This innovation greatly improved the material's porosity and tissue integration capabilities, expanding its use in various medical implants and devices. The 1990s saw a surge in PTFE applications, including its use in dental implants, orthopedic devices, and as a coating for medical instruments.
The turn of the millennium brought about a new era of PTFE innovation, with a focus on nanotechnology. Researchers began exploring ways to manipulate PTFE at the molecular level, creating nanostructured surfaces that could better mimic natural tissue environments. This led to improved cell adhesion and reduced foreign body responses, further enhancing PTFE's biocompatibility.
In the 2010s, the medical community witnessed the emergence of PTFE composites and hybrid materials. By combining PTFE with other biocompatible substances, such as hydrogels or carbon nanotubes, researchers developed materials with enhanced mechanical properties and biological performance. These advancements opened up new possibilities in tissue engineering and regenerative medicine.
Recent years have seen a shift towards "smart" PTFE materials. Researchers are now exploring ways to incorporate drug-eluting capabilities and responsive elements into PTFE-based medical devices. This allows for controlled release of therapeutic agents and adaptive responses to physiological changes, potentially revolutionizing long-term implantable devices and drug delivery systems.
The ongoing evolution of PTFE in medical applications continues to focus on improving its biocompatibility, reducing inflammation, and enhancing integration with host tissues. Current research is exploring surface modification techniques, such as plasma treatment and biofunctionalization, to further optimize PTFE's interaction with biological systems. As we look to the future, the development of PTFE-based materials that can actively promote tissue regeneration and seamlessly integrate with the body's natural processes represents the next frontier in medical biocompatibility.
Biocompatibility Demand
The demand for biocompatible materials in medical applications has been steadily increasing, driven by advancements in medical technology and the growing need for long-term implantable devices. PTFE (Polytetrafluoroethylene) has emerged as a crucial material in this field due to its exceptional biocompatibility properties. The medical industry's focus on patient safety and improved treatment outcomes has led to a surge in demand for PTFE-based products.
In recent years, the global market for biocompatible materials has experienced significant growth, with PTFE playing a pivotal role. The cardiovascular sector, in particular, has shown a strong demand for PTFE-based vascular grafts and heart valves. This is primarily due to PTFE's ability to resist thrombosis and its long-term stability in the human body. Additionally, the orthopedic field has seen an increased adoption of PTFE-coated implants, which reduce friction and wear in joint replacements.
The dental industry has also contributed to the rising demand for PTFE innovations. With the growing popularity of dental implants and the need for materials that promote osseointegration, PTFE membranes have become essential in guided tissue regeneration procedures. This application has opened up new avenues for PTFE usage in regenerative medicine and tissue engineering.
Another factor driving the demand for PTFE in medical biocompatibility is the aging population in many developed countries. As the elderly population grows, there is an increased need for long-term implantable devices that can withstand the physiological environment without causing adverse reactions. PTFE's chemical inertness and resistance to degradation make it an ideal candidate for such applications.
The pharmaceutical industry has also recognized the potential of PTFE in drug delivery systems. The material's non-stick properties and chemical resistance make it suitable for controlled release mechanisms and drug-eluting implants. This has led to collaborations between medical device manufacturers and pharmaceutical companies, further fueling the demand for innovative PTFE-based solutions.
Regulatory bodies and healthcare providers are increasingly emphasizing the importance of biocompatibility in medical devices. This has resulted in stricter guidelines and standards for materials used in medical applications. PTFE's proven track record in biocompatibility has positioned it as a preferred material for manufacturers looking to meet these stringent requirements.
As the field of personalized medicine continues to evolve, there is a growing demand for customizable PTFE-based products. This includes patient-specific implants and tailored drug delivery systems, which require advanced manufacturing techniques such as 3D printing. The ability to create complex geometries and precise structures using PTFE has opened up new possibilities in personalized healthcare, driving further innovation in the field.
In recent years, the global market for biocompatible materials has experienced significant growth, with PTFE playing a pivotal role. The cardiovascular sector, in particular, has shown a strong demand for PTFE-based vascular grafts and heart valves. This is primarily due to PTFE's ability to resist thrombosis and its long-term stability in the human body. Additionally, the orthopedic field has seen an increased adoption of PTFE-coated implants, which reduce friction and wear in joint replacements.
The dental industry has also contributed to the rising demand for PTFE innovations. With the growing popularity of dental implants and the need for materials that promote osseointegration, PTFE membranes have become essential in guided tissue regeneration procedures. This application has opened up new avenues for PTFE usage in regenerative medicine and tissue engineering.
Another factor driving the demand for PTFE in medical biocompatibility is the aging population in many developed countries. As the elderly population grows, there is an increased need for long-term implantable devices that can withstand the physiological environment without causing adverse reactions. PTFE's chemical inertness and resistance to degradation make it an ideal candidate for such applications.
The pharmaceutical industry has also recognized the potential of PTFE in drug delivery systems. The material's non-stick properties and chemical resistance make it suitable for controlled release mechanisms and drug-eluting implants. This has led to collaborations between medical device manufacturers and pharmaceutical companies, further fueling the demand for innovative PTFE-based solutions.
Regulatory bodies and healthcare providers are increasingly emphasizing the importance of biocompatibility in medical devices. This has resulted in stricter guidelines and standards for materials used in medical applications. PTFE's proven track record in biocompatibility has positioned it as a preferred material for manufacturers looking to meet these stringent requirements.
As the field of personalized medicine continues to evolve, there is a growing demand for customizable PTFE-based products. This includes patient-specific implants and tailored drug delivery systems, which require advanced manufacturing techniques such as 3D printing. The ability to create complex geometries and precise structures using PTFE has opened up new possibilities in personalized healthcare, driving further innovation in the field.
PTFE Challenges
Despite its widespread use in medical applications, Polytetrafluoroethylene (PTFE) faces several challenges in the context of medical biocompatibility. One of the primary concerns is the potential for PTFE to release fluoride ions under certain conditions, which can lead to local tissue inflammation and potential systemic effects. This issue is particularly critical in long-term implantable devices where prolonged exposure to PTFE may occur.
Another significant challenge is the hydrophobic nature of PTFE, which can limit its interaction with biological tissues and fluids. While this property is beneficial in some applications, it can hinder cell adhesion and tissue integration in others, potentially compromising the effectiveness of certain medical devices or implants. Researchers are actively exploring surface modification techniques to address this limitation without compromising PTFE's other desirable properties.
The manufacturing process of medical-grade PTFE also presents challenges. Ensuring consistent purity and eliminating potential contaminants during production is crucial for maintaining biocompatibility. The high temperatures required for PTFE processing can introduce thermal degradation products, which must be carefully controlled and removed to meet stringent medical standards.
PTFE's mechanical properties, while generally favorable, can be a double-edged sword in some medical applications. Its low coefficient of friction, while beneficial in many scenarios, can lead to issues with device fixation or stability in certain anatomical locations. Additionally, the material's creep behavior under long-term stress can result in dimensional changes over time, potentially affecting the performance and safety of implanted devices.
The sterilization of PTFE medical devices poses another challenge. Common sterilization methods like ethylene oxide treatment or gamma irradiation can potentially alter PTFE's surface properties or induce chemical changes, affecting its biocompatibility. Developing sterilization protocols that maintain PTFE's integrity while ensuring complete microbial elimination is an ongoing area of research.
Lastly, the environmental persistence of PTFE raises concerns about its long-term impact. While generally inert in the body, the disposal or degradation of PTFE-containing medical devices at the end of their lifecycle presents environmental challenges. This has prompted research into more biodegradable alternatives or recycling methods for PTFE medical waste.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians. Innovations in surface modification, composite materials, and manufacturing processes are being pursued to overcome these limitations and enhance PTFE's biocompatibility profile in medical applications.
Another significant challenge is the hydrophobic nature of PTFE, which can limit its interaction with biological tissues and fluids. While this property is beneficial in some applications, it can hinder cell adhesion and tissue integration in others, potentially compromising the effectiveness of certain medical devices or implants. Researchers are actively exploring surface modification techniques to address this limitation without compromising PTFE's other desirable properties.
The manufacturing process of medical-grade PTFE also presents challenges. Ensuring consistent purity and eliminating potential contaminants during production is crucial for maintaining biocompatibility. The high temperatures required for PTFE processing can introduce thermal degradation products, which must be carefully controlled and removed to meet stringent medical standards.
PTFE's mechanical properties, while generally favorable, can be a double-edged sword in some medical applications. Its low coefficient of friction, while beneficial in many scenarios, can lead to issues with device fixation or stability in certain anatomical locations. Additionally, the material's creep behavior under long-term stress can result in dimensional changes over time, potentially affecting the performance and safety of implanted devices.
The sterilization of PTFE medical devices poses another challenge. Common sterilization methods like ethylene oxide treatment or gamma irradiation can potentially alter PTFE's surface properties or induce chemical changes, affecting its biocompatibility. Developing sterilization protocols that maintain PTFE's integrity while ensuring complete microbial elimination is an ongoing area of research.
Lastly, the environmental persistence of PTFE raises concerns about its long-term impact. While generally inert in the body, the disposal or degradation of PTFE-containing medical devices at the end of their lifecycle presents environmental challenges. This has prompted research into more biodegradable alternatives or recycling methods for PTFE medical waste.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, biomedical engineers, and clinicians. Innovations in surface modification, composite materials, and manufacturing processes are being pursued to overcome these limitations and enhance PTFE's biocompatibility profile in medical applications.
Current PTFE Solutions
01 Surface modification of PTFE for improved biocompatibility
Various surface modification techniques are employed to enhance the biocompatibility of PTFE. These methods include plasma treatment, chemical etching, and coating with biocompatible materials. Such modifications can improve cell adhesion, reduce protein adsorption, and enhance overall tissue integration, making PTFE more suitable for medical applications.- Surface modification of PTFE for improved biocompatibility: Various surface modification techniques are employed to enhance the biocompatibility of PTFE. These methods include plasma treatment, chemical etching, and coating with biocompatible materials. Such modifications can improve cell adhesion, reduce protein adsorption, and enhance overall tissue integration, making PTFE more suitable for medical applications.
- PTFE composites for biomedical applications: PTFE is often combined with other materials to create composites with enhanced biocompatibility. These composites may incorporate bioactive agents, nanoparticles, or other polymers to improve properties such as antimicrobial activity, cell growth promotion, or mechanical strength while maintaining PTFE's inherent chemical inertness.
- Biocompatible PTFE coatings for medical devices: PTFE coatings are developed to improve the biocompatibility of medical devices such as implants, catheters, and stents. These coatings can reduce friction, prevent bacterial adhesion, and minimize inflammatory responses, thereby enhancing the overall performance and safety of medical devices in contact with biological tissues.
- Porous PTFE structures for tissue engineering: Porous PTFE scaffolds are designed for tissue engineering applications. These structures provide a biocompatible framework for cell growth and tissue regeneration. The porosity and surface properties of PTFE can be tailored to optimize cell attachment, proliferation, and differentiation for various tissue types.
- Biocompatibility testing and evaluation of PTFE materials: Various methods and protocols are developed to assess the biocompatibility of PTFE materials. These include in vitro cell culture studies, in vivo animal implantation tests, and long-term compatibility evaluations. Such testing is crucial for ensuring the safety and efficacy of PTFE-based medical devices and implants.
02 PTFE composites for biomedical applications
PTFE is often combined with other materials to create composites with enhanced biocompatibility. These composites may incorporate bioactive agents, nanoparticles, or other polymers to improve properties such as antimicrobial activity, cell growth promotion, or mechanical strength while maintaining PTFE's inherent biocompatibility.Expand Specific Solutions03 Biocompatible PTFE coatings for medical devices
PTFE coatings are applied to various medical devices to improve their biocompatibility. These coatings can reduce friction, prevent bacterial adhesion, and enhance the overall performance of implants, catheters, and other medical instruments. The development of specialized coating techniques ensures strong adhesion and durability of the PTFE layer.Expand Specific Solutions04 Porous PTFE structures for tissue engineering
Porous PTFE scaffolds are developed for tissue engineering applications. These structures provide a biocompatible framework for cell growth and tissue regeneration. The porosity and surface properties of PTFE can be tailored to optimize cell attachment, proliferation, and differentiation for specific tissue types.Expand Specific Solutions05 Biocompatibility testing and evaluation of PTFE materials
Various methods and protocols are developed to assess the biocompatibility of PTFE materials. These include in vitro cell culture studies, in vivo animal implantation tests, and long-term biocompatibility evaluations. Such testing is crucial for ensuring the safety and efficacy of PTFE-based medical devices and implants.Expand Specific Solutions
PTFE Industry Leaders
The PTFE innovations in medical biocompatibility market is in a growth phase, driven by increasing demand for advanced medical devices and implants. The global market size is estimated to be in the billions, with steady annual growth. Technologically, PTFE biocompatibility is advancing rapidly, with key players like Boston Scientific, Medtronic, and W. L. Gore & Associates leading innovation. These companies are developing novel PTFE formulations and surface modifications to enhance biocompatibility and reduce complications in medical applications. Research institutions like Zhejiang University and Northwestern University are also contributing to technological advancements, focusing on improving PTFE's interaction with biological systems and expanding its potential applications in the medical field.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has made significant strides in PTFE innovations for medical biocompatibility, particularly in the field of vascular implants. The company has developed a novel PTFE-based material with a microporous structure that mimics natural blood vessels, promoting better integration with the patient's tissue[4]. This material, used in their vascular grafts, has shown improved patency rates and reduced risk of infection compared to traditional PTFE grafts. Medtronic has also explored the use of drug-eluting PTFE materials, where the polymer is impregnated with anticoagulants or growth factors to enhance biocompatibility and reduce complications such as restenosis[5]. Their research has extended to the development of composite materials, combining PTFE with other biocompatible polymers to achieve optimal mechanical and biological properties for specific medical applications[6].
Strengths: Strong focus on vascular applications, innovative microporous PTFE structures, and integration of drug-eluting technologies. Weaknesses: Potential regulatory challenges with novel materials and limited application outside of vascular devices.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has developed advanced PTFE-based materials for medical applications, focusing on improving biocompatibility. Their ePTFE (expanded PTFE) technology, known as GORE-TEX, has been widely used in various medical devices. The company has innovated in surface modifications of PTFE to enhance cell adhesion and reduce thrombogenicity. They have developed a proprietary surface modification technique that alters the PTFE surface at the molecular level, improving its interaction with biological tissues without compromising the material's mechanical properties[1]. This modified PTFE has shown improved endothelialization in vascular grafts, potentially reducing the risk of thrombosis and improving long-term patency rates[2]. Gore has also explored the incorporation of bioactive molecules into PTFE structures to further enhance biocompatibility and tissue integration[3].
Strengths: Extensive experience in PTFE technology, proven track record in medical applications, and advanced surface modification techniques. Weaknesses: High cost of proprietary technologies and potential limitations in certain biological environments.
PTFE Biocompatibility
Wettable ePTFE Medical Devices
PatentActiveUS20110060402A1
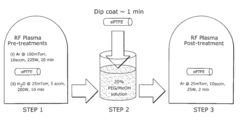
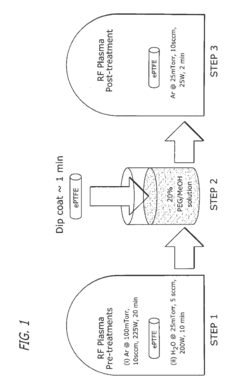
Innovation
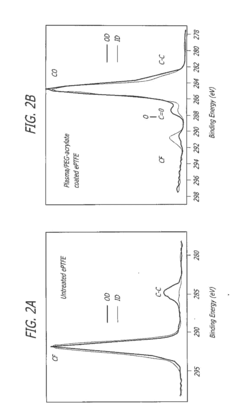
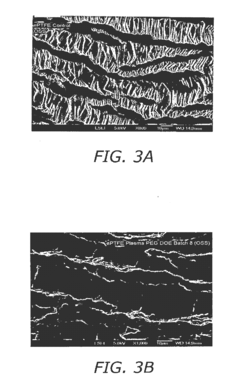
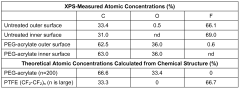
- Surface modification of ePTFE using a multi-step process involving radio frequency (RF)-generated plasmas to pre-treat, coat with a hydrophilic agent, and crosslink the coating, specifically using argon and H2O plasmas with a PEG-acrylate coating to enhance wettability.
Wettable eptfe medical devices
PatentWO2007127989A2
Innovation
- Surface modification of ePTFE using a multi-step process involving radio frequency (RF)-generated plasmas to pre-treat, coat with a hydrophilic polymer, and crosslink the coating, specifically using argon and H2O plasmas with a PEG-acrylate coating to enhance wettability.
Regulatory Compliance
Regulatory compliance is a critical aspect of PTFE innovations in medical biocompatibility. The use of PTFE in medical devices and implants is subject to stringent regulations and standards set by various regulatory bodies worldwide. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the safety and efficacy of medical devices containing PTFE.
The FDA classifies medical devices into three categories based on their risk level and intended use. PTFE-containing devices may fall into different classes depending on their specific applications. Class I devices, such as PTFE-coated surgical instruments, are subject to general controls. Class II devices, which include many PTFE-based implants, require special controls and often necessitate 510(k) premarket notification. Class III devices, such as certain PTFE-based vascular grafts, undergo the most rigorous scrutiny and typically require premarket approval (PMA).
Manufacturers must adhere to Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) to ensure consistent production of safe and effective PTFE-based medical devices. These regulations cover various aspects of the manufacturing process, including design controls, process validation, and risk management.
In the European Union, PTFE-based medical devices must comply with the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR). These regulations require manufacturers to obtain CE marking, demonstrating conformity with essential requirements for safety and performance. The MDR and IVDR emphasize the importance of clinical evidence and post-market surveillance for PTFE-containing devices.
Biocompatibility testing is a crucial component of regulatory compliance for PTFE innovations. ISO 10993 provides a framework for evaluating the biological safety of medical devices, including those containing PTFE. This standard outlines various tests, such as cytotoxicity, sensitization, and implantation studies, which are essential for demonstrating the biocompatibility of PTFE-based materials.
As PTFE innovations continue to advance, regulatory bodies are adapting their guidelines to address emerging technologies and applications. For instance, the use of PTFE in combination products or as part of novel drug-eluting devices may require additional regulatory considerations. Manufacturers must stay abreast of evolving regulatory landscapes and engage in early dialogue with regulatory agencies to ensure compliance throughout the product development lifecycle.
The FDA classifies medical devices into three categories based on their risk level and intended use. PTFE-containing devices may fall into different classes depending on their specific applications. Class I devices, such as PTFE-coated surgical instruments, are subject to general controls. Class II devices, which include many PTFE-based implants, require special controls and often necessitate 510(k) premarket notification. Class III devices, such as certain PTFE-based vascular grafts, undergo the most rigorous scrutiny and typically require premarket approval (PMA).
Manufacturers must adhere to Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) to ensure consistent production of safe and effective PTFE-based medical devices. These regulations cover various aspects of the manufacturing process, including design controls, process validation, and risk management.
In the European Union, PTFE-based medical devices must comply with the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR). These regulations require manufacturers to obtain CE marking, demonstrating conformity with essential requirements for safety and performance. The MDR and IVDR emphasize the importance of clinical evidence and post-market surveillance for PTFE-containing devices.
Biocompatibility testing is a crucial component of regulatory compliance for PTFE innovations. ISO 10993 provides a framework for evaluating the biological safety of medical devices, including those containing PTFE. This standard outlines various tests, such as cytotoxicity, sensitization, and implantation studies, which are essential for demonstrating the biocompatibility of PTFE-based materials.
As PTFE innovations continue to advance, regulatory bodies are adapting their guidelines to address emerging technologies and applications. For instance, the use of PTFE in combination products or as part of novel drug-eluting devices may require additional regulatory considerations. Manufacturers must stay abreast of evolving regulatory landscapes and engage in early dialogue with regulatory agencies to ensure compliance throughout the product development lifecycle.
Safety and Longevity
PTFE (polytetrafluoroethylene) has long been recognized for its exceptional biocompatibility in medical applications. Its safety profile and longevity have made it a preferred material for various implantable devices and medical equipment. The inert nature of PTFE contributes significantly to its safety, as it does not react with bodily tissues or fluids, minimizing the risk of adverse reactions or inflammatory responses.
One of the key factors contributing to PTFE's longevity in medical applications is its resistance to degradation. Unlike many other polymers, PTFE maintains its structural integrity and chemical properties even when exposed to harsh biological environments for extended periods. This stability ensures that PTFE-based medical devices and implants can function effectively for years, reducing the need for frequent replacements and minimizing patient discomfort.
The non-stick properties of PTFE also play a crucial role in its safety and longevity. By preventing the adhesion of proteins, cells, and other biological materials, PTFE-coated surfaces reduce the risk of thrombosis and infection. This characteristic is particularly valuable in cardiovascular applications, where blood clot formation can have severe consequences.
Recent innovations in PTFE technology have focused on enhancing its already impressive safety and longevity profiles. Surface modification techniques, such as plasma treatment and chemical grafting, have been developed to further improve PTFE's biocompatibility. These modifications can promote better integration with surrounding tissues, reduce the risk of implant rejection, and extend the functional lifespan of PTFE-based medical devices.
Nanotechnology has also been employed to create PTFE composites with enhanced properties. By incorporating nanoparticles or nanostructures into the PTFE matrix, researchers have developed materials with improved wear resistance, antimicrobial properties, and even drug-eluting capabilities. These advancements not only extend the lifespan of PTFE-based implants but also expand their potential applications in the medical field.
The long-term safety of PTFE in medical applications has been extensively studied and documented. Numerous clinical trials and post-market surveillance studies have consistently demonstrated the material's excellent biocompatibility and low incidence of adverse events. This wealth of data provides confidence to both healthcare professionals and patients regarding the use of PTFE in critical medical applications.
As medical technology continues to advance, the demand for materials with exceptional safety and longevity profiles is expected to grow. PTFE, with its proven track record and ongoing innovations, is well-positioned to meet these evolving needs. Future research is likely to focus on further optimizing PTFE's properties for specific medical applications, potentially leading to even safer and more durable medical devices and implants.
One of the key factors contributing to PTFE's longevity in medical applications is its resistance to degradation. Unlike many other polymers, PTFE maintains its structural integrity and chemical properties even when exposed to harsh biological environments for extended periods. This stability ensures that PTFE-based medical devices and implants can function effectively for years, reducing the need for frequent replacements and minimizing patient discomfort.
The non-stick properties of PTFE also play a crucial role in its safety and longevity. By preventing the adhesion of proteins, cells, and other biological materials, PTFE-coated surfaces reduce the risk of thrombosis and infection. This characteristic is particularly valuable in cardiovascular applications, where blood clot formation can have severe consequences.
Recent innovations in PTFE technology have focused on enhancing its already impressive safety and longevity profiles. Surface modification techniques, such as plasma treatment and chemical grafting, have been developed to further improve PTFE's biocompatibility. These modifications can promote better integration with surrounding tissues, reduce the risk of implant rejection, and extend the functional lifespan of PTFE-based medical devices.
Nanotechnology has also been employed to create PTFE composites with enhanced properties. By incorporating nanoparticles or nanostructures into the PTFE matrix, researchers have developed materials with improved wear resistance, antimicrobial properties, and even drug-eluting capabilities. These advancements not only extend the lifespan of PTFE-based implants but also expand their potential applications in the medical field.
The long-term safety of PTFE in medical applications has been extensively studied and documented. Numerous clinical trials and post-market surveillance studies have consistently demonstrated the material's excellent biocompatibility and low incidence of adverse events. This wealth of data provides confidence to both healthcare professionals and patients regarding the use of PTFE in critical medical applications.
As medical technology continues to advance, the demand for materials with exceptional safety and longevity profiles is expected to grow. PTFE, with its proven track record and ongoing innovations, is well-positioned to meet these evolving needs. Future research is likely to focus on further optimizing PTFE's properties for specific medical applications, potentially leading to even safer and more durable medical devices and implants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!