PTFE: Leading the Way in Advanced Healthcare Solutions
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PTFE in Healthcare: Evolution and Objectives
Polytetrafluoroethylene (PTFE), commonly known as Teflon, has emerged as a revolutionary material in the healthcare industry since its accidental discovery in 1938. Initially developed for industrial applications, PTFE's unique properties quickly caught the attention of medical researchers and practitioners, leading to its widespread adoption in various healthcare solutions.
The evolution of PTFE in healthcare can be traced through several key milestones. In the 1960s, PTFE was first used in vascular grafts, marking its entry into the medical field. This breakthrough paved the way for its application in other medical devices, such as catheters and implants. The 1970s and 1980s saw further expansion of PTFE's use in healthcare, with the development of PTFE-coated surgical instruments and dental implants.
As research progressed, the biocompatibility and non-stick properties of PTFE became increasingly valuable in medical applications. The 1990s witnessed a surge in PTFE-based medical innovations, including its use in drug-eluting stents and advanced wound dressings. The turn of the millennium brought about nanotechnology applications of PTFE, opening up new possibilities in targeted drug delivery and tissue engineering.
The primary objective of PTFE in healthcare has been to enhance patient outcomes and improve medical procedures. Its non-reactive nature and low friction coefficient make it ideal for reducing infections, minimizing tissue trauma, and extending the lifespan of medical devices. PTFE's versatility allows for its integration into a wide range of medical products, from simple disposables to complex implantable devices.
Current research aims to further exploit PTFE's potential in healthcare. Objectives include developing PTFE-based scaffolds for tissue regeneration, improving the material's antimicrobial properties, and exploring its use in 3D-printed medical devices. Additionally, researchers are investigating ways to enhance PTFE's bioactivity while maintaining its inert characteristics, potentially leading to more effective drug delivery systems and improved implant integration.
The future of PTFE in healthcare looks promising, with ongoing efforts to combine it with other advanced materials and technologies. This includes the development of smart PTFE-based materials that can respond to biological stimuli, as well as the integration of PTFE into wearable medical devices. As healthcare continues to evolve towards more personalized and minimally invasive treatments, PTFE is expected to play a crucial role in enabling these advancements.
The evolution of PTFE in healthcare can be traced through several key milestones. In the 1960s, PTFE was first used in vascular grafts, marking its entry into the medical field. This breakthrough paved the way for its application in other medical devices, such as catheters and implants. The 1970s and 1980s saw further expansion of PTFE's use in healthcare, with the development of PTFE-coated surgical instruments and dental implants.
As research progressed, the biocompatibility and non-stick properties of PTFE became increasingly valuable in medical applications. The 1990s witnessed a surge in PTFE-based medical innovations, including its use in drug-eluting stents and advanced wound dressings. The turn of the millennium brought about nanotechnology applications of PTFE, opening up new possibilities in targeted drug delivery and tissue engineering.
The primary objective of PTFE in healthcare has been to enhance patient outcomes and improve medical procedures. Its non-reactive nature and low friction coefficient make it ideal for reducing infections, minimizing tissue trauma, and extending the lifespan of medical devices. PTFE's versatility allows for its integration into a wide range of medical products, from simple disposables to complex implantable devices.
Current research aims to further exploit PTFE's potential in healthcare. Objectives include developing PTFE-based scaffolds for tissue regeneration, improving the material's antimicrobial properties, and exploring its use in 3D-printed medical devices. Additionally, researchers are investigating ways to enhance PTFE's bioactivity while maintaining its inert characteristics, potentially leading to more effective drug delivery systems and improved implant integration.
The future of PTFE in healthcare looks promising, with ongoing efforts to combine it with other advanced materials and technologies. This includes the development of smart PTFE-based materials that can respond to biological stimuli, as well as the integration of PTFE into wearable medical devices. As healthcare continues to evolve towards more personalized and minimally invasive treatments, PTFE is expected to play a crucial role in enabling these advancements.
Market Demand Analysis for PTFE in Medical Applications
The global market for PTFE in medical applications has been experiencing significant growth, driven by the material's unique properties and the increasing demand for advanced healthcare solutions. PTFE's exceptional chemical resistance, biocompatibility, and low friction characteristics make it an ideal choice for various medical devices and implants.
In recent years, the healthcare industry has witnessed a surge in demand for minimally invasive surgical procedures, which has directly impacted the market for PTFE-based medical devices. Catheters, guidewires, and stent grafts utilizing PTFE coatings have become increasingly popular due to their ability to reduce patient trauma and improve surgical outcomes. This trend is expected to continue, further boosting the demand for PTFE in the medical sector.
The aging population in developed countries has also contributed to the growing market for PTFE-based medical implants. As the prevalence of chronic diseases and orthopedic conditions increases, there is a rising need for long-lasting, biocompatible implants. PTFE's non-reactive nature and ability to integrate with human tissue make it an excellent choice for joint replacements, vascular grafts, and other long-term implantable devices.
Another significant factor driving the demand for PTFE in medical applications is the increasing focus on infection control and prevention in healthcare settings. PTFE's non-stick properties and resistance to bacterial adhesion make it an attractive option for medical equipment and surfaces that require frequent cleaning and sterilization. This has led to a growing adoption of PTFE-coated instruments and components in hospitals and clinics worldwide.
The pharmaceutical industry has also contributed to the expanding market for PTFE in medical applications. PTFE's chemical inertness and thermal stability make it an ideal material for drug delivery systems and pharmaceutical processing equipment. As the pharmaceutical sector continues to innovate and develop new drug formulations, the demand for PTFE-based components is expected to rise.
Emerging markets, particularly in Asia-Pacific and Latin America, present significant growth opportunities for PTFE in medical applications. As healthcare infrastructure improves and access to advanced medical technologies increases in these regions, the demand for PTFE-based medical devices and implants is projected to grow substantially.
However, the market for PTFE in medical applications also faces challenges, including stringent regulatory requirements and the emergence of alternative materials. Manufacturers must navigate complex approval processes and demonstrate the long-term safety and efficacy of PTFE-based medical products. Additionally, ongoing research into new biomaterials may lead to the development of alternatives that could compete with PTFE in certain applications.
In recent years, the healthcare industry has witnessed a surge in demand for minimally invasive surgical procedures, which has directly impacted the market for PTFE-based medical devices. Catheters, guidewires, and stent grafts utilizing PTFE coatings have become increasingly popular due to their ability to reduce patient trauma and improve surgical outcomes. This trend is expected to continue, further boosting the demand for PTFE in the medical sector.
The aging population in developed countries has also contributed to the growing market for PTFE-based medical implants. As the prevalence of chronic diseases and orthopedic conditions increases, there is a rising need for long-lasting, biocompatible implants. PTFE's non-reactive nature and ability to integrate with human tissue make it an excellent choice for joint replacements, vascular grafts, and other long-term implantable devices.
Another significant factor driving the demand for PTFE in medical applications is the increasing focus on infection control and prevention in healthcare settings. PTFE's non-stick properties and resistance to bacterial adhesion make it an attractive option for medical equipment and surfaces that require frequent cleaning and sterilization. This has led to a growing adoption of PTFE-coated instruments and components in hospitals and clinics worldwide.
The pharmaceutical industry has also contributed to the expanding market for PTFE in medical applications. PTFE's chemical inertness and thermal stability make it an ideal material for drug delivery systems and pharmaceutical processing equipment. As the pharmaceutical sector continues to innovate and develop new drug formulations, the demand for PTFE-based components is expected to rise.
Emerging markets, particularly in Asia-Pacific and Latin America, present significant growth opportunities for PTFE in medical applications. As healthcare infrastructure improves and access to advanced medical technologies increases in these regions, the demand for PTFE-based medical devices and implants is projected to grow substantially.
However, the market for PTFE in medical applications also faces challenges, including stringent regulatory requirements and the emergence of alternative materials. Manufacturers must navigate complex approval processes and demonstrate the long-term safety and efficacy of PTFE-based medical products. Additionally, ongoing research into new biomaterials may lead to the development of alternatives that could compete with PTFE in certain applications.
Current PTFE Technology and Challenges in Healthcare
Polytetrafluoroethylene (PTFE) has emerged as a revolutionary material in the healthcare industry, offering unique properties that address various challenges in medical applications. The current state of PTFE technology in healthcare is characterized by its widespread use in medical devices, implants, and surgical equipment.
One of the primary advantages of PTFE in healthcare is its exceptional biocompatibility. The material's inert nature and low friction coefficient make it ideal for use in implantable devices, such as vascular grafts and heart valves. PTFE's non-stick properties also contribute to its effectiveness in preventing bacterial adhesion, reducing the risk of infections in medical settings.
In the field of cardiovascular medicine, PTFE-based vascular grafts have become a standard solution for bypass surgeries and other vascular reconstructions. The material's ability to integrate with surrounding tissues while maintaining patency has significantly improved patient outcomes in these procedures.
Despite its numerous advantages, PTFE technology in healthcare faces several challenges. One of the primary concerns is the potential for wear and degradation over time, particularly in high-stress applications such as joint replacements. Researchers are actively working on enhancing the material's durability and longevity through surface modifications and composite formulations.
Another challenge lies in the manufacturing process of PTFE-based medical devices. The material's high melting point and viscosity make it difficult to process using conventional techniques, necessitating specialized manufacturing methods. This complexity can lead to increased production costs and limited scalability for certain applications.
The biofilm formation on PTFE surfaces remains a persistent issue in some medical applications. While PTFE's non-stick properties offer some resistance to bacterial adhesion, long-term exposure in biological environments can still lead to biofilm development. Ongoing research focuses on developing surface treatments and coatings to further enhance PTFE's antimicrobial properties.
In the realm of drug delivery, PTFE's hydrophobic nature presents both opportunities and challenges. While this property can be advantageous for controlled release systems, it also limits the material's ability to incorporate water-soluble drugs effectively. Scientists are exploring various surface modification techniques and composite materials to overcome this limitation and expand PTFE's applications in drug delivery.
The environmental impact of PTFE production and disposal is an emerging concern in the healthcare sector. As sustainability becomes increasingly important, there is a growing need for eco-friendly alternatives or improved recycling methods for PTFE-based medical products.
One of the primary advantages of PTFE in healthcare is its exceptional biocompatibility. The material's inert nature and low friction coefficient make it ideal for use in implantable devices, such as vascular grafts and heart valves. PTFE's non-stick properties also contribute to its effectiveness in preventing bacterial adhesion, reducing the risk of infections in medical settings.
In the field of cardiovascular medicine, PTFE-based vascular grafts have become a standard solution for bypass surgeries and other vascular reconstructions. The material's ability to integrate with surrounding tissues while maintaining patency has significantly improved patient outcomes in these procedures.
Despite its numerous advantages, PTFE technology in healthcare faces several challenges. One of the primary concerns is the potential for wear and degradation over time, particularly in high-stress applications such as joint replacements. Researchers are actively working on enhancing the material's durability and longevity through surface modifications and composite formulations.
Another challenge lies in the manufacturing process of PTFE-based medical devices. The material's high melting point and viscosity make it difficult to process using conventional techniques, necessitating specialized manufacturing methods. This complexity can lead to increased production costs and limited scalability for certain applications.
The biofilm formation on PTFE surfaces remains a persistent issue in some medical applications. While PTFE's non-stick properties offer some resistance to bacterial adhesion, long-term exposure in biological environments can still lead to biofilm development. Ongoing research focuses on developing surface treatments and coatings to further enhance PTFE's antimicrobial properties.
In the realm of drug delivery, PTFE's hydrophobic nature presents both opportunities and challenges. While this property can be advantageous for controlled release systems, it also limits the material's ability to incorporate water-soluble drugs effectively. Scientists are exploring various surface modification techniques and composite materials to overcome this limitation and expand PTFE's applications in drug delivery.
The environmental impact of PTFE production and disposal is an emerging concern in the healthcare sector. As sustainability becomes increasingly important, there is a growing need for eco-friendly alternatives or improved recycling methods for PTFE-based medical products.
Existing PTFE Applications in Healthcare
01 PTFE manufacturing processes
Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding methods. These processes aim to improve the quality, efficiency, and properties of the resulting PTFE materials.- PTFE manufacturing processes: Various methods for producing PTFE are described, including polymerization techniques, extrusion processes, and molding methods. These processes aim to improve the quality, efficiency, and properties of the resulting PTFE materials.
- PTFE composite materials: PTFE is combined with other materials to create composite structures with enhanced properties. These composites may include reinforcing fibers, nanoparticles, or other polymers to improve mechanical strength, thermal stability, or specific functional characteristics.
- Surface modification of PTFE: Techniques for modifying the surface properties of PTFE are explored, including chemical treatments, plasma treatments, and coating methods. These modifications aim to improve adhesion, wettability, or introduce specific functionalities to PTFE surfaces.
- PTFE in membrane applications: PTFE is utilized in various membrane applications, including filtration, separation, and gas diffusion. The development of porous PTFE membranes with controlled pore sizes and structures is described for use in diverse industries such as water treatment and fuel cells.
- PTFE in coating formulations: PTFE is incorporated into coating formulations to impart non-stick, low friction, and chemical resistance properties. These coatings find applications in cookware, industrial equipment, and automotive components. Methods for improving the adhesion and durability of PTFE coatings are also discussed.
02 PTFE composite materials
The development of PTFE-based composite materials involves combining PTFE with other substances to enhance its properties or create new functionalities. These composites can include reinforced PTFE, PTFE-metal combinations, or PTFE-polymer blends.Expand Specific Solutions03 Surface modification of PTFE
Techniques for modifying the surface properties of PTFE are explored to improve its adhesion, wettability, or compatibility with other materials. These modifications can involve chemical treatments, plasma processing, or the application of coatings.Expand Specific Solutions04 PTFE applications in various industries
PTFE finds applications in diverse industries due to its unique properties. These applications include use in cookware, automotive parts, electronics, medical devices, and industrial equipment. The material's non-stick, heat-resistant, and chemically inert properties make it valuable in many fields.Expand Specific Solutions05 Recycling and environmental considerations of PTFE
Methods for recycling PTFE and addressing environmental concerns related to its production and disposal are discussed. This includes techniques for reprocessing PTFE waste, reducing environmental impact during manufacturing, and developing more sustainable PTFE alternatives.Expand Specific Solutions
Key Players in PTFE Medical Solutions
The PTFE healthcare solutions market is in a growth phase, driven by increasing demand for advanced medical devices and implants. The market size is expanding rapidly, with major players like Medtronic, Boston Scientific, and W. L. Gore & Associates leading the way. These companies are investing heavily in R&D to develop innovative PTFE-based products for cardiovascular, orthopedic, and general surgery applications. The technology is relatively mature, with established manufacturing processes, but there is ongoing research to enhance PTFE's properties for specific medical uses. Emerging players like Zeus Co. and Nanjing Comptech Composites are also contributing to market growth by offering specialized PTFE solutions, indicating a competitive and dynamic landscape.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced PTFE-based solutions for healthcare applications, particularly in cardiovascular devices. Their ePTFE (expanded PTFE) technology is used in vascular grafts and stent-grafts, offering improved biocompatibility and reduced thrombogenicity[1]. The company has also incorporated PTFE into their catheter systems, enhancing lubricity and reducing friction during minimally invasive procedures[2]. Medtronic's research has focused on optimizing PTFE surface modifications to improve cell adhesion and tissue integration, potentially leading to better long-term outcomes for implanted devices[3].
Strengths: Extensive experience in medical device manufacturing, strong R&D capabilities, and a wide range of PTFE applications. Weaknesses: High development costs and potential regulatory challenges for novel PTFE-based technologies.
Boston Scientific Ltd.
Technical Solution: Boston Scientific has pioneered the use of PTFE in various medical devices, particularly in the field of interventional cardiology and peripheral interventions. Their PTFE-coated guidewires and catheters offer exceptional lubricity and navigability through complex vascular anatomies[4]. The company has also developed PTFE-based stent grafts for treating aortic aneurysms, leveraging the material's durability and biocompatibility[5]. Boston Scientific's research efforts have focused on creating hybrid PTFE materials that combine the benefits of PTFE with other polymers to enhance overall device performance and longevity[6].
Strengths: Strong market presence in interventional devices, innovative PTFE coating technologies, and a diverse product portfolio. Weaknesses: Intense competition in the medical device market and potential for patent disputes over PTFE applications.
Innovative PTFE Technologies for Medical Use
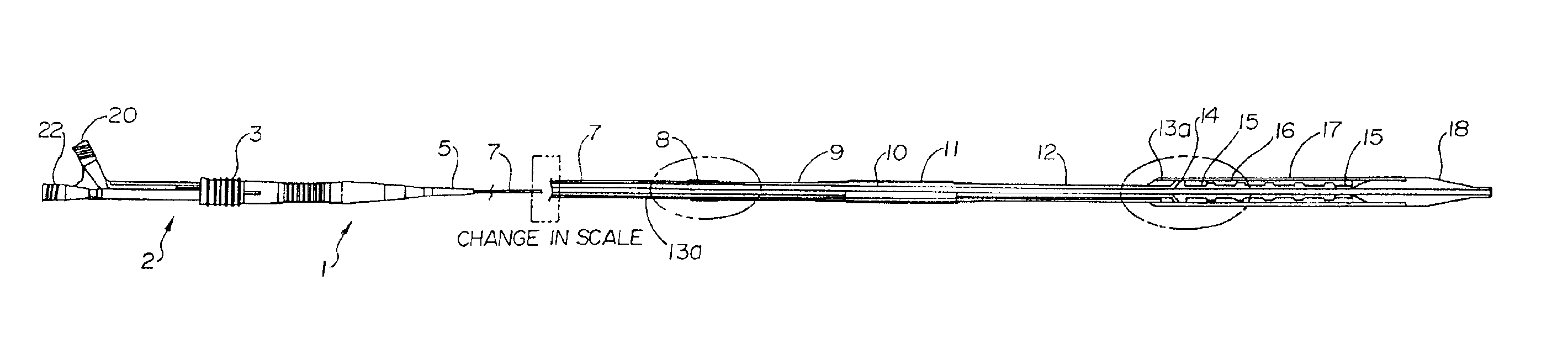
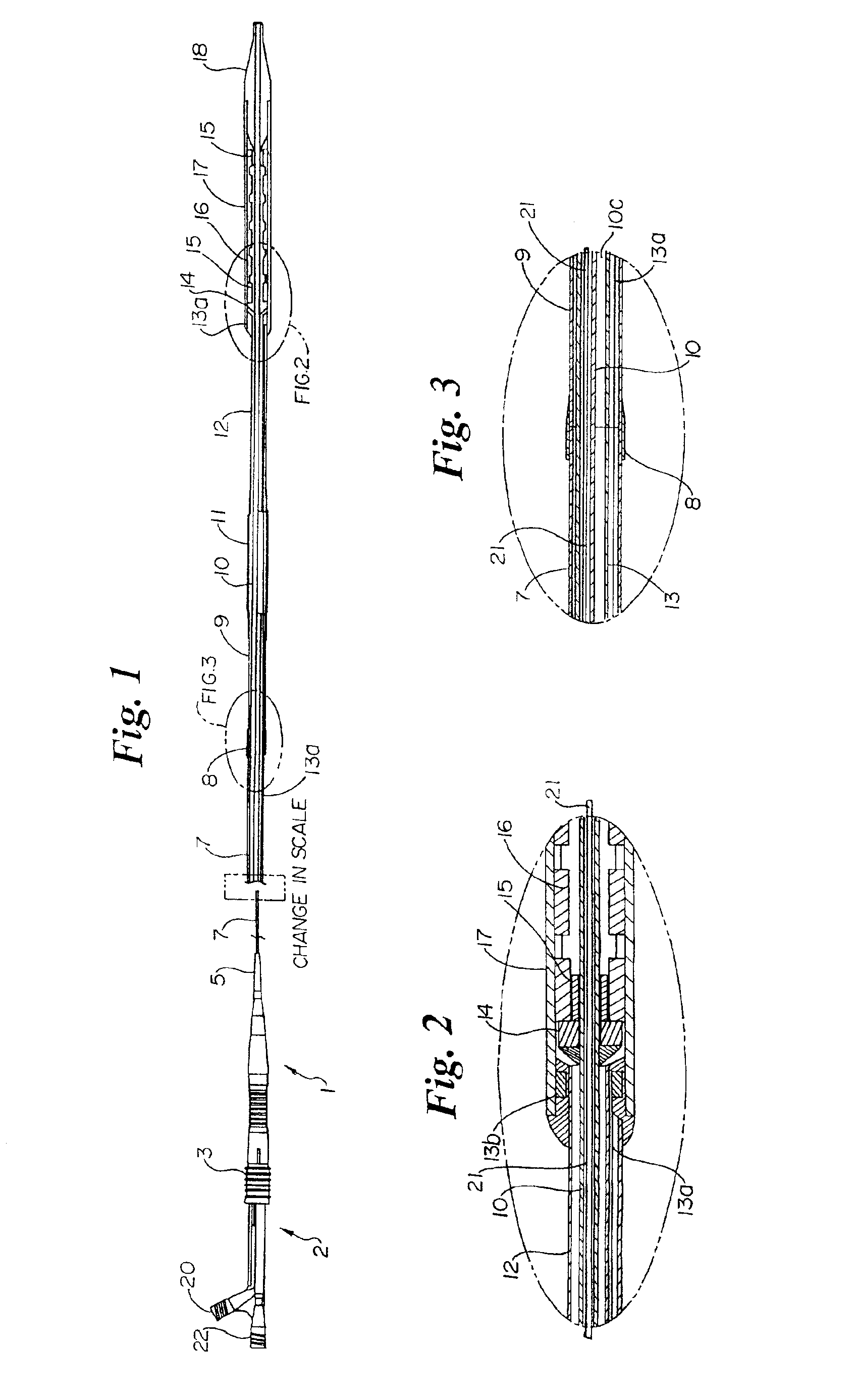
Medical devices utilizing melt-processible poly(tetrafluoroethylene)
PatentInactiveUS6939593B2
Innovation
- Development of melt-processible poly(tetrafluoroethylene) (MP-PTFE) with specific physical characteristics, allowing for extrusions and coextrusions to form medical device parts with low friction and good trackability, eliminating the need for additional coatings or layers.
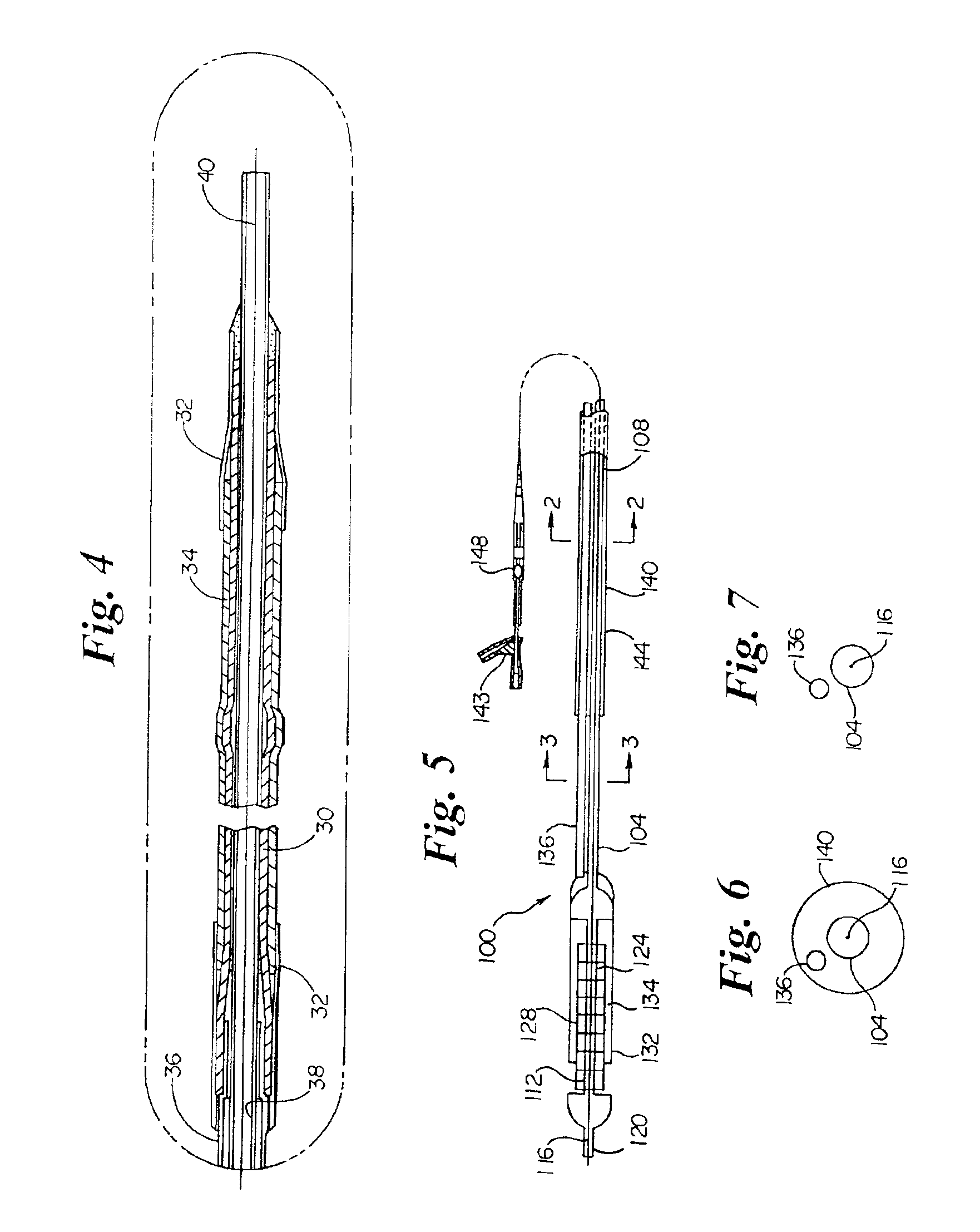
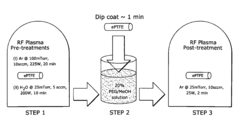
Wettable ePTFE medical devices
PatentActiveUS8029562B2
Innovation
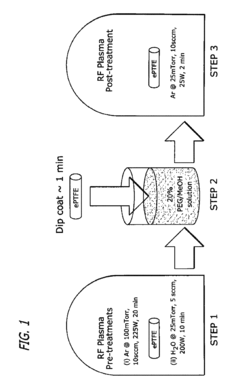
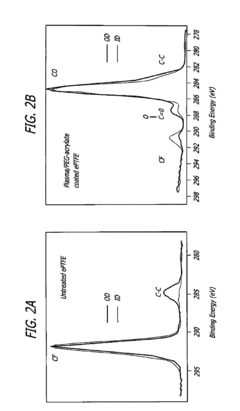
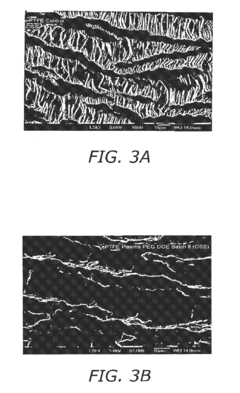
- Surface modification of ePTFE using a multi-step process involving radio frequency (RF)-generated plasmas to pre-treat, coat with a hydrophilic agent, and crosslink the coating, specifically using argon and H2O plasmas with a PEG-acrylate coating to enhance wettability.
Regulatory Framework for PTFE in Healthcare
The regulatory framework for PTFE in healthcare is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of medical devices and applications utilizing this versatile material. At the forefront of this framework is the U.S. Food and Drug Administration (FDA), which has established stringent guidelines for the use of PTFE in medical devices and implants.
The FDA classifies PTFE-based medical devices into different categories based on their intended use and potential risk. Class I devices, such as surgical instruments coated with PTFE, are subject to general controls. Class II devices, including certain vascular grafts and stents, require special controls and premarket notification. Class III devices, which may include more complex implants, undergo the most rigorous premarket approval process.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of PTFE in healthcare applications. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust quality management systems and conduct post-market surveillance.
The International Organization for Standardization (ISO) has developed several standards relevant to PTFE in healthcare, including ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems in medical device manufacturing. These standards are often incorporated into regulatory requirements worldwide, ensuring a harmonized approach to PTFE safety and quality.
Regulatory bodies also focus on the manufacturing processes of PTFE for healthcare applications. Good Manufacturing Practices (GMP) are enforced to ensure consistent quality and purity of PTFE materials used in medical devices. This includes strict controls on raw materials, processing conditions, and contamination prevention.
Environmental regulations play an increasingly important role in the PTFE regulatory framework. The production and disposal of PTFE have come under scrutiny due to concerns about perfluorooctanoic acid (PFOA), a chemical historically used in PTFE manufacturing. Many jurisdictions now require PFOA-free production methods and have implemented restrictions on PFOA content in products.
As nanotechnology advances, regulatory agencies are developing guidelines for the use of PTFE nanoparticles in healthcare applications. These emerging regulations aim to address potential risks associated with the unique properties of nanoscale materials while fostering innovation in areas such as drug delivery and tissue engineering.
The global nature of the medical device industry necessitates international regulatory cooperation. Initiatives like the Medical Device Single Audit Program (MDSAP) aim to streamline auditing processes across multiple jurisdictions, reducing the regulatory burden on manufacturers while maintaining high standards of safety and efficacy for PTFE-based healthcare products.
The FDA classifies PTFE-based medical devices into different categories based on their intended use and potential risk. Class I devices, such as surgical instruments coated with PTFE, are subject to general controls. Class II devices, including certain vascular grafts and stents, require special controls and premarket notification. Class III devices, which may include more complex implants, undergo the most rigorous premarket approval process.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of PTFE in healthcare applications. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust quality management systems and conduct post-market surveillance.
The International Organization for Standardization (ISO) has developed several standards relevant to PTFE in healthcare, including ISO 10993 for biocompatibility testing and ISO 13485 for quality management systems in medical device manufacturing. These standards are often incorporated into regulatory requirements worldwide, ensuring a harmonized approach to PTFE safety and quality.
Regulatory bodies also focus on the manufacturing processes of PTFE for healthcare applications. Good Manufacturing Practices (GMP) are enforced to ensure consistent quality and purity of PTFE materials used in medical devices. This includes strict controls on raw materials, processing conditions, and contamination prevention.
Environmental regulations play an increasingly important role in the PTFE regulatory framework. The production and disposal of PTFE have come under scrutiny due to concerns about perfluorooctanoic acid (PFOA), a chemical historically used in PTFE manufacturing. Many jurisdictions now require PFOA-free production methods and have implemented restrictions on PFOA content in products.
As nanotechnology advances, regulatory agencies are developing guidelines for the use of PTFE nanoparticles in healthcare applications. These emerging regulations aim to address potential risks associated with the unique properties of nanoscale materials while fostering innovation in areas such as drug delivery and tissue engineering.
The global nature of the medical device industry necessitates international regulatory cooperation. Initiatives like the Medical Device Single Audit Program (MDSAP) aim to streamline auditing processes across multiple jurisdictions, reducing the regulatory burden on manufacturers while maintaining high standards of safety and efficacy for PTFE-based healthcare products.
Biocompatibility and Safety Considerations of PTFE
Polytetrafluoroethylene (PTFE) has emerged as a leading material in advanced healthcare solutions, largely due to its exceptional biocompatibility and safety profile. The biocompatibility of PTFE is attributed to its chemical inertness, which minimizes adverse reactions when in contact with biological tissues. This property makes PTFE an ideal candidate for various medical applications, including implants, surgical materials, and drug delivery systems.
One of the primary safety considerations of PTFE in healthcare is its resistance to degradation within the human body. Unlike some other polymers, PTFE does not break down or leach harmful substances over time, ensuring long-term stability and reducing the risk of complications. This characteristic is particularly crucial for implantable devices and long-term medical applications where material integrity is paramount.
The non-stick nature of PTFE also contributes to its safety profile by reducing the risk of bacterial adhesion and biofilm formation. This property is especially valuable in preventing infections associated with medical devices and implants. Furthermore, PTFE's low coefficient of friction helps minimize tissue irritation and inflammation, enhancing patient comfort and reducing the likelihood of adverse reactions.
In terms of regulatory compliance, PTFE has undergone extensive testing and evaluation to meet stringent safety standards set by regulatory bodies such as the FDA and EMA. These assessments include cytotoxicity studies, sensitization tests, and long-term implantation trials to ensure the material's safety for various medical applications.
Despite its numerous advantages, there are some safety considerations that researchers and manufacturers must address when working with PTFE in healthcare settings. One concern is the potential release of ultrafine particles during manufacturing or wear processes, which may have implications for respiratory health. To mitigate this risk, strict manufacturing protocols and quality control measures are implemented to minimize particle generation and ensure product safety.
Another aspect of PTFE safety in healthcare applications is its interaction with other materials and substances. While generally inert, PTFE can potentially interact with certain chemicals or undergo changes under extreme conditions. Researchers continue to investigate these interactions to optimize PTFE formulations and expand its range of applications while maintaining its excellent safety profile.
As the healthcare industry continues to advance, ongoing research focuses on further enhancing the biocompatibility and safety of PTFE-based materials. This includes developing surface modification techniques to improve cell adhesion for tissue engineering applications and exploring new PTFE composites that combine the material's inherent safety with additional functionalities.
One of the primary safety considerations of PTFE in healthcare is its resistance to degradation within the human body. Unlike some other polymers, PTFE does not break down or leach harmful substances over time, ensuring long-term stability and reducing the risk of complications. This characteristic is particularly crucial for implantable devices and long-term medical applications where material integrity is paramount.
The non-stick nature of PTFE also contributes to its safety profile by reducing the risk of bacterial adhesion and biofilm formation. This property is especially valuable in preventing infections associated with medical devices and implants. Furthermore, PTFE's low coefficient of friction helps minimize tissue irritation and inflammation, enhancing patient comfort and reducing the likelihood of adverse reactions.
In terms of regulatory compliance, PTFE has undergone extensive testing and evaluation to meet stringent safety standards set by regulatory bodies such as the FDA and EMA. These assessments include cytotoxicity studies, sensitization tests, and long-term implantation trials to ensure the material's safety for various medical applications.
Despite its numerous advantages, there are some safety considerations that researchers and manufacturers must address when working with PTFE in healthcare settings. One concern is the potential release of ultrafine particles during manufacturing or wear processes, which may have implications for respiratory health. To mitigate this risk, strict manufacturing protocols and quality control measures are implemented to minimize particle generation and ensure product safety.
Another aspect of PTFE safety in healthcare applications is its interaction with other materials and substances. While generally inert, PTFE can potentially interact with certain chemicals or undergo changes under extreme conditions. Researchers continue to investigate these interactions to optimize PTFE formulations and expand its range of applications while maintaining its excellent safety profile.
As the healthcare industry continues to advance, ongoing research focuses on further enhancing the biocompatibility and safety of PTFE-based materials. This includes developing surface modification techniques to improve cell adhesion for tissue engineering applications and exploring new PTFE composites that combine the material's inherent safety with additional functionalities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!