Quantitative NMR Methods in Pharmaceuticals: Purity Assurance
SEP 22, 20256 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
qNMR Evolution and Objectives in Pharmaceutical Analysis
Quantitative Nuclear Magnetic Resonance (qNMR) spectroscopy has evolved significantly since its inception in the 1960s, transforming from a purely qualitative analytical technique to a powerful quantitative tool in pharmaceutical analysis. The evolution of qNMR has been driven by advancements in instrumentation, methodology, and computational capabilities, enabling increasingly precise and reliable measurements critical for pharmaceutical quality control.
In the early stages of NMR development, the technique was primarily used for structural elucidation of organic compounds. The transition to quantitative applications began when researchers recognized that NMR signal intensity is directly proportional to the number of nuclei generating the signal. This fundamental principle established the foundation for qNMR as a primary ratio method, requiring no compound-specific calibration standards.
The 1980s and 1990s witnessed significant improvements in NMR hardware, including higher magnetic field strengths, more stable electronics, and advanced probe designs. These technological advancements substantially enhanced sensitivity and resolution, making qNMR increasingly viable for pharmaceutical applications. The introduction of digital signal processing and Fourier transform techniques further improved spectral quality and data handling capabilities.
By the early 2000s, qNMR had gained recognition from regulatory bodies and pharmacopeias as a reliable method for purity assessment. The European Pharmacopoeia was among the first to include qNMR methods, followed by the United States Pharmacopeia and other international standards organizations. This regulatory acceptance marked a crucial milestone in the technique's evolution and adoption in pharmaceutical quality control.
Recent developments have focused on addressing the remaining limitations of qNMR, including enhanced automation, reduced sample requirements, and improved data processing algorithms. The integration of artificial intelligence and machine learning approaches has enabled more sophisticated spectral analysis and interpretation, reducing human error and increasing throughput.
The primary objectives of qNMR in pharmaceutical analysis center on ensuring product quality, safety, and efficacy through accurate purity determination. Unlike chromatographic methods that require compound-specific reference standards, qNMR offers the advantage of using a single, well-characterized reference standard for multiple analytes, significantly streamlining the analytical process.
Additional objectives include the development of robust, validated methods suitable for routine quality control, the establishment of standardized protocols for method transfer between laboratories, and the integration of qNMR into comprehensive analytical workflows. The pharmaceutical industry increasingly aims to implement qNMR as a complementary technique to traditional methods, leveraging its unique strengths in direct quantification without separation.
Looking forward, the evolution of qNMR continues toward greater sensitivity, accessibility, and integration with other analytical techniques. The ultimate goal remains the establishment of qNMR as a primary method for pharmaceutical purity assessment, providing accurate, reliable, and efficient analysis throughout the drug development and manufacturing lifecycle.
In the early stages of NMR development, the technique was primarily used for structural elucidation of organic compounds. The transition to quantitative applications began when researchers recognized that NMR signal intensity is directly proportional to the number of nuclei generating the signal. This fundamental principle established the foundation for qNMR as a primary ratio method, requiring no compound-specific calibration standards.
The 1980s and 1990s witnessed significant improvements in NMR hardware, including higher magnetic field strengths, more stable electronics, and advanced probe designs. These technological advancements substantially enhanced sensitivity and resolution, making qNMR increasingly viable for pharmaceutical applications. The introduction of digital signal processing and Fourier transform techniques further improved spectral quality and data handling capabilities.
By the early 2000s, qNMR had gained recognition from regulatory bodies and pharmacopeias as a reliable method for purity assessment. The European Pharmacopoeia was among the first to include qNMR methods, followed by the United States Pharmacopeia and other international standards organizations. This regulatory acceptance marked a crucial milestone in the technique's evolution and adoption in pharmaceutical quality control.
Recent developments have focused on addressing the remaining limitations of qNMR, including enhanced automation, reduced sample requirements, and improved data processing algorithms. The integration of artificial intelligence and machine learning approaches has enabled more sophisticated spectral analysis and interpretation, reducing human error and increasing throughput.
The primary objectives of qNMR in pharmaceutical analysis center on ensuring product quality, safety, and efficacy through accurate purity determination. Unlike chromatographic methods that require compound-specific reference standards, qNMR offers the advantage of using a single, well-characterized reference standard for multiple analytes, significantly streamlining the analytical process.
Additional objectives include the development of robust, validated methods suitable for routine quality control, the establishment of standardized protocols for method transfer between laboratories, and the integration of qNMR into comprehensive analytical workflows. The pharmaceutical industry increasingly aims to implement qNMR as a complementary technique to traditional methods, leveraging its unique strengths in direct quantification without separation.
Looking forward, the evolution of qNMR continues toward greater sensitivity, accessibility, and integration with other analytical techniques. The ultimate goal remains the establishment of qNMR as a primary method for pharmaceutical purity assessment, providing accurate, reliable, and efficient analysis throughout the drug development and manufacturing lifecycle.
Market Demand for Precise Pharmaceutical Purity Testing
The pharmaceutical industry's demand for precise purity testing methods has experienced significant growth over the past decade, driven by increasingly stringent regulatory requirements and the rising complexity of drug formulations. The global pharmaceutical analytical testing market, which includes purity testing services, was valued at approximately $5.6 billion in 2022 and is projected to reach $11.4 billion by 2028, growing at a CAGR of 12.5% during the forecast period.
Quantitative Nuclear Magnetic Resonance (qNMR) methods have emerged as a critical solution within this expanding market. Unlike traditional chromatographic techniques, qNMR offers direct proportionality between signal intensity and the number of nuclei responsible for the signal, enabling absolute quantification without the need for identical reference standards. This capability addresses a fundamental market need in pharmaceutical quality control.
Regulatory bodies worldwide, including the FDA, EMA, and ICH, have intensified their focus on pharmaceutical purity standards, creating substantial market pull for advanced analytical methods. The FDA's increasing emphasis on Quality by Design (QbD) principles has further accelerated demand for precise, reproducible purity testing methodologies like qNMR. Additionally, the implementation of ICH Q3D guidelines for elemental impurities has created new testing requirements that traditional methods struggle to meet efficiently.
The biosimilars and generic pharmaceuticals sectors represent particularly high-growth segments for qNMR adoption. With the patent cliff resulting in numerous blockbuster drugs losing exclusivity, manufacturers require cost-effective yet highly accurate methods to demonstrate bioequivalence and purity. The global generics market, valued at $411.8 billion in 2022, is expected to reach $571.3 billion by 2027, creating substantial demand for efficient purity testing solutions.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) have become significant consumers of advanced analytical services, including qNMR testing. The pharmaceutical outsourcing market has grown at 7.9% annually, with analytical testing representing one of the most frequently outsourced services. This trend has created a specialized market segment focused exclusively on providing sophisticated purity testing services.
Emerging markets, particularly in Asia-Pacific regions, are experiencing the fastest growth in demand for pharmaceutical purity testing. China and India, as major pharmaceutical manufacturing hubs, have implemented more rigorous quality standards to align with international requirements, driving adoption of advanced analytical methods including qNMR. Market research indicates that analytical testing services in these regions are growing at approximately 15% annually, outpacing global averages.
Quantitative Nuclear Magnetic Resonance (qNMR) methods have emerged as a critical solution within this expanding market. Unlike traditional chromatographic techniques, qNMR offers direct proportionality between signal intensity and the number of nuclei responsible for the signal, enabling absolute quantification without the need for identical reference standards. This capability addresses a fundamental market need in pharmaceutical quality control.
Regulatory bodies worldwide, including the FDA, EMA, and ICH, have intensified their focus on pharmaceutical purity standards, creating substantial market pull for advanced analytical methods. The FDA's increasing emphasis on Quality by Design (QbD) principles has further accelerated demand for precise, reproducible purity testing methodologies like qNMR. Additionally, the implementation of ICH Q3D guidelines for elemental impurities has created new testing requirements that traditional methods struggle to meet efficiently.
The biosimilars and generic pharmaceuticals sectors represent particularly high-growth segments for qNMR adoption. With the patent cliff resulting in numerous blockbuster drugs losing exclusivity, manufacturers require cost-effective yet highly accurate methods to demonstrate bioequivalence and purity. The global generics market, valued at $411.8 billion in 2022, is expected to reach $571.3 billion by 2027, creating substantial demand for efficient purity testing solutions.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) have become significant consumers of advanced analytical services, including qNMR testing. The pharmaceutical outsourcing market has grown at 7.9% annually, with analytical testing representing one of the most frequently outsourced services. This trend has created a specialized market segment focused exclusively on providing sophisticated purity testing services.
Emerging markets, particularly in Asia-Pacific regions, are experiencing the fastest growth in demand for pharmaceutical purity testing. China and India, as major pharmaceutical manufacturing hubs, have implemented more rigorous quality standards to align with international requirements, driving adoption of advanced analytical methods including qNMR. Market research indicates that analytical testing services in these regions are growing at approximately 15% annually, outpacing global averages.
Current qNMR Capabilities and Technical Limitations
Quantitative NMR (qNMR) has established itself as a powerful analytical technique in pharmaceutical quality control, offering unique capabilities for purity determination without the need for reference standards of identical chemical structure. Current qNMR methodologies primarily utilize 1H NMR due to its high sensitivity, natural abundance, and relatively short relaxation times, enabling efficient and accurate quantification.
The precision of modern qNMR methods has reached impressive levels, with relative standard deviations typically below 1% when performed under optimal conditions. High-field NMR spectrometers (400-600 MHz) equipped with cryoprobes have significantly enhanced sensitivity, allowing for detection limits in the microgram range. This sensitivity improvement has expanded qNMR applications to low-concentration analytes and complex matrices common in pharmaceutical development.
Digital signal processing and advanced integration algorithms have substantially improved the accuracy of peak area measurements, particularly for overlapping signals. Modern qNMR software packages now incorporate sophisticated baseline correction, phase adjustment, and deconvolution tools that minimize human intervention and reduce operator-dependent variations in results.
Despite these advancements, qNMR faces several technical limitations. Signal overlap remains a significant challenge, particularly in complex pharmaceutical formulations containing multiple active ingredients and excipients. While 2D NMR techniques offer potential solutions, they often compromise quantitative accuracy due to variable magnetization transfer efficiencies.
Sample preparation inconsistencies continue to introduce variability in qNMR results. Factors such as solvent effects, concentration dependencies, and pH variations can significantly impact chemical shifts and integration accuracy. Standardization of sample preparation protocols across the industry remains incomplete.
The requirement for relatively large sample amounts (typically 1-10 mg) compared to chromatographic methods presents another limitation, particularly for early-stage drug development where material availability is often restricted. This constraint becomes especially problematic for high-potency active pharmaceutical ingredients.
Relaxation time considerations impose practical limitations on experiment duration and accuracy. Incomplete relaxation between pulses can lead to systematic quantification errors, while ensuring complete relaxation often results in prohibitively long acquisition times for routine quality control applications.
Matrix effects from pharmaceutical excipients frequently complicate analysis by introducing additional signals and altering the magnetic environment of analyte nuclei. Current deconvolution algorithms struggle with highly complex spectral regions, limiting the application of qNMR in finished pharmaceutical products without prior separation steps.
The precision of modern qNMR methods has reached impressive levels, with relative standard deviations typically below 1% when performed under optimal conditions. High-field NMR spectrometers (400-600 MHz) equipped with cryoprobes have significantly enhanced sensitivity, allowing for detection limits in the microgram range. This sensitivity improvement has expanded qNMR applications to low-concentration analytes and complex matrices common in pharmaceutical development.
Digital signal processing and advanced integration algorithms have substantially improved the accuracy of peak area measurements, particularly for overlapping signals. Modern qNMR software packages now incorporate sophisticated baseline correction, phase adjustment, and deconvolution tools that minimize human intervention and reduce operator-dependent variations in results.
Despite these advancements, qNMR faces several technical limitations. Signal overlap remains a significant challenge, particularly in complex pharmaceutical formulations containing multiple active ingredients and excipients. While 2D NMR techniques offer potential solutions, they often compromise quantitative accuracy due to variable magnetization transfer efficiencies.
Sample preparation inconsistencies continue to introduce variability in qNMR results. Factors such as solvent effects, concentration dependencies, and pH variations can significantly impact chemical shifts and integration accuracy. Standardization of sample preparation protocols across the industry remains incomplete.
The requirement for relatively large sample amounts (typically 1-10 mg) compared to chromatographic methods presents another limitation, particularly for early-stage drug development where material availability is often restricted. This constraint becomes especially problematic for high-potency active pharmaceutical ingredients.
Relaxation time considerations impose practical limitations on experiment duration and accuracy. Incomplete relaxation between pulses can lead to systematic quantification errors, while ensuring complete relaxation often results in prohibitively long acquisition times for routine quality control applications.
Matrix effects from pharmaceutical excipients frequently complicate analysis by introducing additional signals and altering the magnetic environment of analyte nuclei. Current deconvolution algorithms struggle with highly complex spectral regions, limiting the application of qNMR in finished pharmaceutical products without prior separation steps.
Established qNMR Protocols for Pharmaceutical Purity Assessment
01 Quantitative NMR methods for purity determination
Quantitative Nuclear Magnetic Resonance (qNMR) spectroscopy provides a direct method for determining the purity of compounds by comparing signal intensities of the analyte with those of a reference standard. This technique offers high accuracy and precision without requiring reference standards of the same substance, making it particularly valuable for pharmaceutical quality control and assurance. The method relies on the principle that NMR signal intensity is directly proportional to the number of nuclei responsible for the signal.- Internal standard methods for quantitative NMR analysis: Internal standard methods involve adding a known amount of a reference compound to the sample for quantitative NMR analysis. This approach allows for direct comparison between the signal intensities of the analyte and the reference compound, enabling accurate determination of purity. The method compensates for variations in instrument parameters and sample conditions, providing reliable quantitative results without the need for calibration curves.
- Advanced pulse sequences for improved quantitative NMR accuracy: Specialized pulse sequences have been developed to enhance the accuracy of quantitative NMR measurements for purity assurance. These techniques include ERETIC (Electronic REference To access In vivo Concentrations), PULCON (PULse length based CONcentration determination), and various relaxation-edited sequences. These methods improve signal-to-noise ratio, reduce interference from overlapping signals, and compensate for relaxation effects, resulting in more precise quantification of analytes.
- Automated data processing algorithms for qNMR purity assessment: Automated data processing algorithms have been developed to streamline quantitative NMR analysis for purity assessment. These algorithms include automated phase correction, baseline correction, peak integration, and statistical analysis tools. Machine learning approaches are also being implemented to improve the accuracy and efficiency of data interpretation. These computational methods reduce human error and increase throughput in quality control applications.
- Multi-nuclear and multi-dimensional NMR techniques for purity verification: Multi-nuclear and multi-dimensional NMR techniques provide enhanced capabilities for purity verification. These approaches utilize various nuclei (such as 1H, 13C, 19F, and 31P) and correlation experiments (like HSQC, HMBC, and DOSY) to obtain comprehensive structural information and detect impurities that might be missed by conventional methods. These techniques are particularly valuable for complex samples with multiple components or when dealing with overlapping signals.
- Reference standards and validation protocols for qNMR in quality control: Standardized reference materials and validation protocols have been established to ensure the reliability of quantitative NMR for purity assurance in quality control settings. These include certified reference materials with known purity, method validation guidelines, and system suitability tests. Interlaboratory comparison studies and proficiency testing schemes have also been developed to demonstrate the reproducibility and robustness of qNMR methods across different laboratories and instrument platforms.
02 Internal standard selection and optimization for qNMR
The selection of appropriate internal standards is crucial for accurate quantitative NMR analysis. Ideal internal standards should have well-resolved signals that don't overlap with the analyte, exhibit good solubility in the chosen solvent, remain chemically inert with the sample, and possess similar relaxation properties. Common internal standards include maleic acid, benzoic acid, and dimethyl sulfone. Optimization of experimental parameters such as pulse sequences, relaxation delays, and acquisition times is essential to ensure accurate quantification.Expand Specific Solutions03 Advanced pulse sequences and data processing for qNMR
Advanced pulse sequences have been developed to enhance the sensitivity and selectivity of quantitative NMR measurements. These include ERETIC (Electronic REference To access In vivo Concentrations), PULCON (PULse length based CONcentration determination), and various 2D qNMR methods. Sophisticated data processing algorithms are employed to improve spectral resolution, phase correction, baseline correction, and peak integration, which are critical for accurate quantification. Machine learning approaches are increasingly being applied to automate and optimize these processes.Expand Specific Solutions04 Validation and standardization of qNMR methods
Validation protocols for quantitative NMR methods include assessment of linearity, accuracy, precision, specificity, limit of detection, and limit of quantification. Standardization efforts aim to establish consistent methodologies across laboratories, including the use of certified reference materials and round-robin testing. Regulatory bodies increasingly recognize qNMR as a primary method for purity determination, with specific guidelines being developed for pharmaceutical and food safety applications.Expand Specific Solutions05 Specialized qNMR applications for complex matrices
Specialized quantitative NMR techniques have been developed for analyzing complex matrices such as biological samples, natural products, and formulated products. These include solvent suppression methods, diffusion-ordered spectroscopy (DOSY), and selective excitation techniques to isolate signals of interest. Hyphenated techniques combining NMR with other analytical methods such as LC-NMR and SPE-NMR provide enhanced capabilities for purity assessment in complex mixtures. These approaches are particularly valuable for impurity profiling and structural elucidation of unknown compounds.Expand Specific Solutions
Leading Pharmaceutical Companies and Instrument Manufacturers
The pharmaceutical NMR quantitative analysis market is in a growth phase, with increasing adoption driven by regulatory demands for higher purity standards. The global market size is expanding steadily as pharmaceutical companies seek more precise analytical methods for quality control. Technologically, quantitative NMR has reached moderate maturity with established protocols, though innovation continues. Leading players include Bruker BioSpin, a dominant instrumentation provider, alongside pharmaceutical giants like Sanofi-Aventis, Teva, and Eli Lilly who have integrated advanced NMR methods into their quality assurance workflows. Academic institutions such as University of Strasbourg and New York University contribute significant research advancements, while specialized companies like Valisure LLC are pioneering chemical validation services using NMR technology for medication quality verification.
Bruker Switzerland AG
Technical Solution: Bruker has developed advanced quantitative NMR (qNMR) solutions specifically designed for pharmaceutical purity analysis. Their AVANCE NEO and AVANCE III HD NMR spectrometers incorporate proprietary PotencyMR™ software that enables high-precision quantification of active pharmaceutical ingredients (APIs) without the need for reference standards of identical chemical structure. The system utilizes PULCON (Pulse Length based Concentration determination) methodology that allows for absolute quantification with accuracy better than 1.5% in many applications. Bruker's qNMR technology implements ERETIC2 (Electronic REference To access In vivo Concentrations) digital signal processing for internal reference calibration, eliminating the need for physical reference standards that might contaminate samples. Their systems are compliant with regulatory requirements including ICH, USP, EP, and JP guidelines for pharmaceutical analysis, with validation protocols that ensure traceability to SI units through NIST-traceable standards[1][3].
Strengths: Industry-leading precision (>99% accuracy in optimal conditions); comprehensive software integration for automated workflow; regulatory compliance built into systems; extensive validation protocols. Weaknesses: High initial investment cost; requires specialized training for optimal operation; limited to laboratories with appropriate NMR infrastructure; maintenance requirements can be significant.
Teva Pharmaceutical Industries Ltd.
Technical Solution: Teva Pharmaceutical Industries has implemented comprehensive qNMR strategies for generic pharmaceutical development and quality control. Their approach focuses on high-throughput qNMR methodologies suitable for diverse pharmaceutical products across their extensive portfolio. Teva's qNMR platform incorporates automated sample preparation systems with robotic handling that enables processing of up to 100 samples per day with minimal operator intervention. Their methodology employs standardized acquisition parameters and internal reference compounds validated across multiple NMR field strengths (300-600 MHz) to ensure consistent results across different laboratory sites. Teva has developed specialized qNMR approaches for challenging pharmaceutical matrices including sustained-release formulations, combination products, and complex generics. Their systems implement automated structural verification alongside quantification, providing simultaneous identity and purity assessment in a single analytical run. Teva's qNMR platform features integration with their laboratory information management system (LIMS) for complete data traceability and compliance with 21 CFR Part 11 requirements[9][10].
Strengths: High-throughput capabilities for diverse product portfolio; standardized methods across multiple manufacturing sites; extensive experience with regulatory submissions using qNMR data; comprehensive validation across different pharmaceutical formulations. Weaknesses: Methods optimized for generic products may require adaptation for novel compounds; focus on routine analysis rather than cutting-edge methodology development; requires significant infrastructure investment across multiple sites.
Key Innovations in Reference Standards and Pulse Sequences
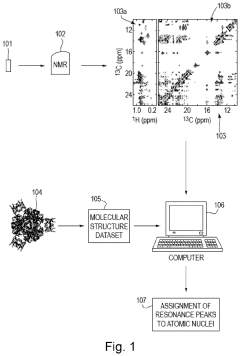
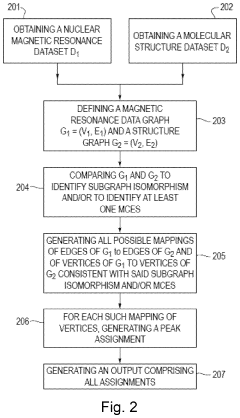
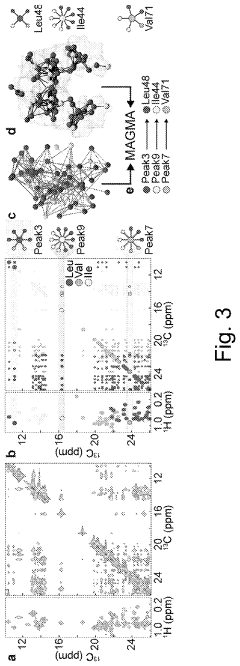
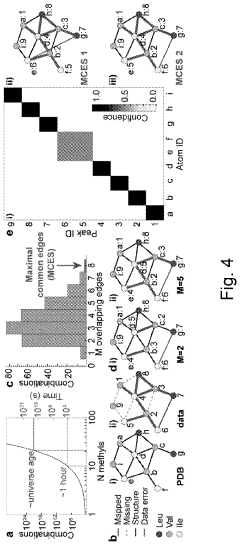
Method for processing nuclear magnetic resonance (NMR) spectroscopic data
PatentInactiveUS10866295B2
Innovation
- A graph-matching algorithm that combines structural models with experimental multidimensional magnetic resonance data to accurately identify confident and ambiguous peak assignments by comparing experimental distance restraints with structural models, reducing the need for laborious experiments and providing exact sets of plausible assignments.
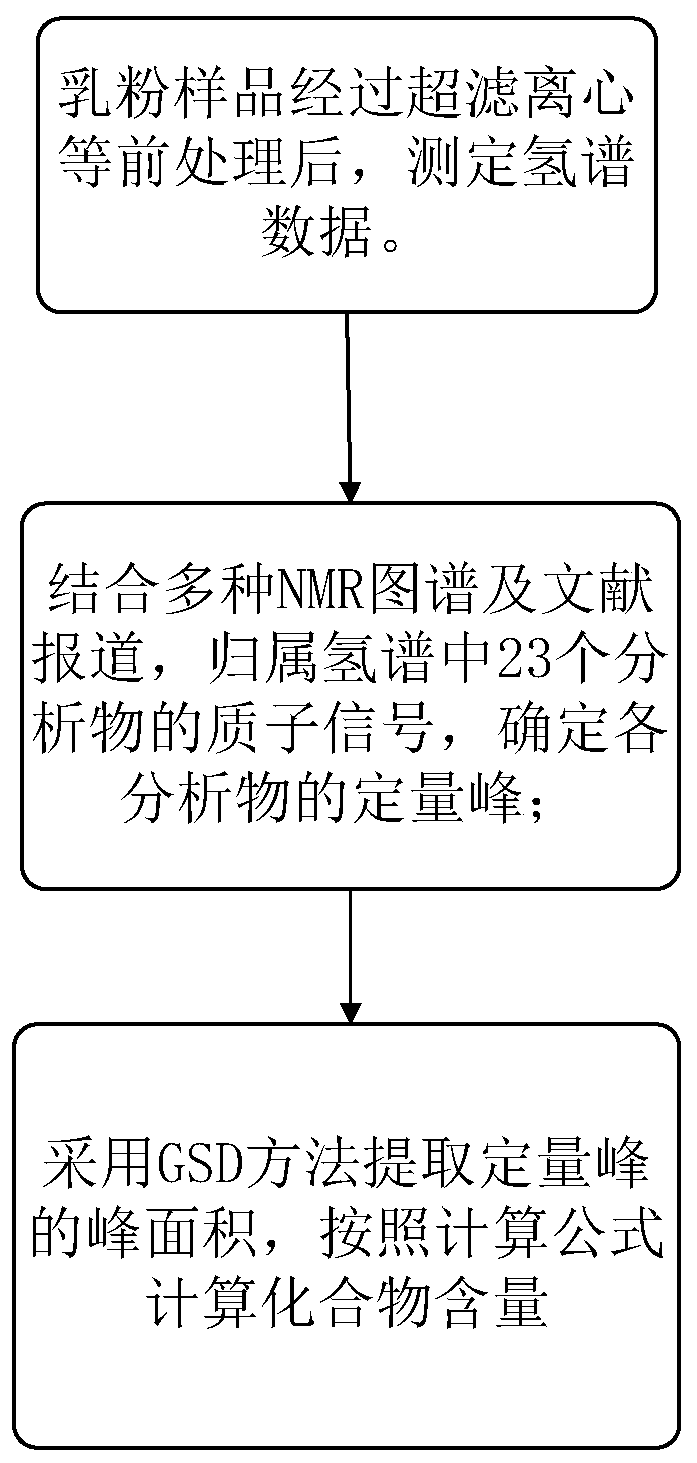
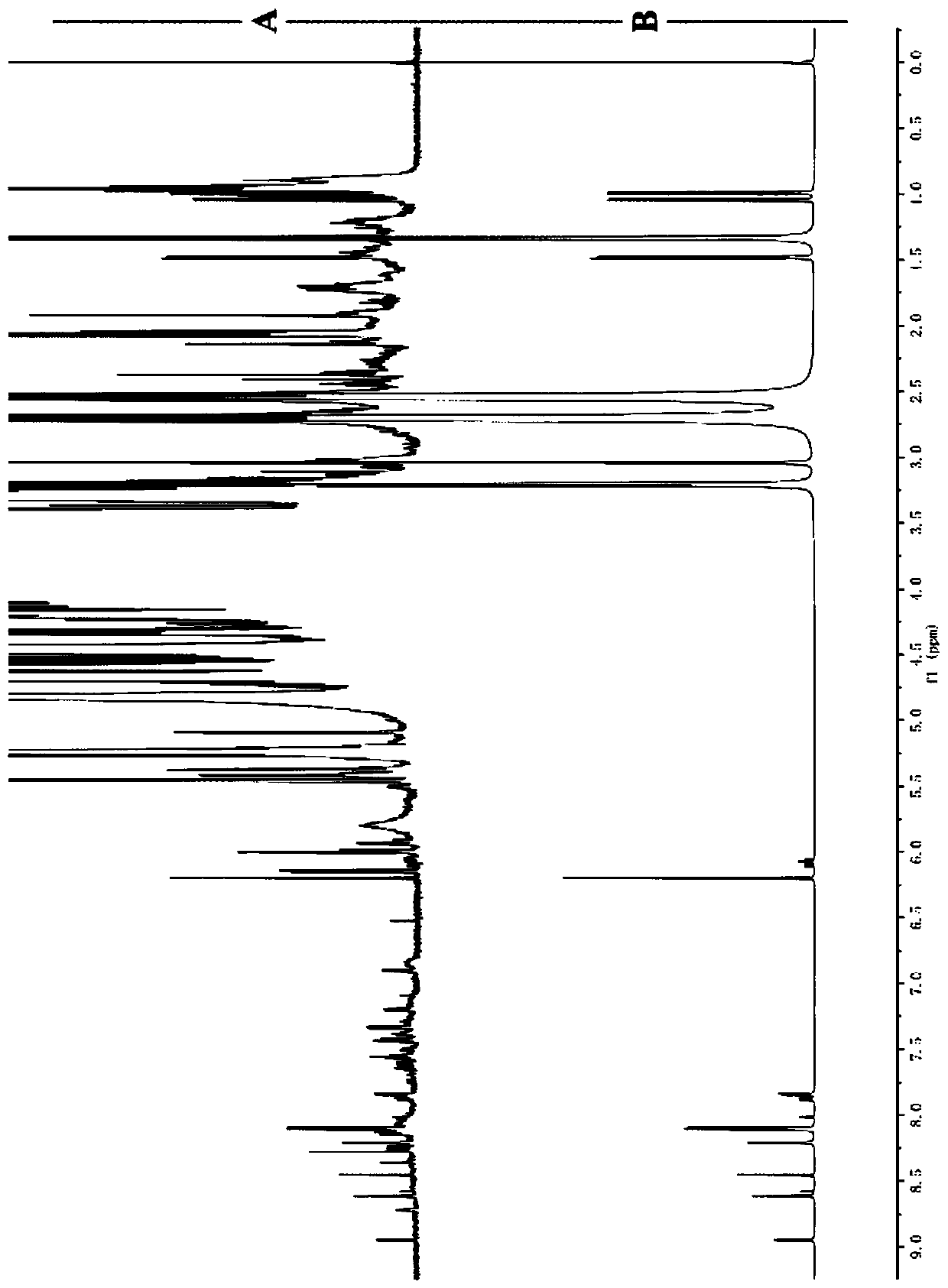
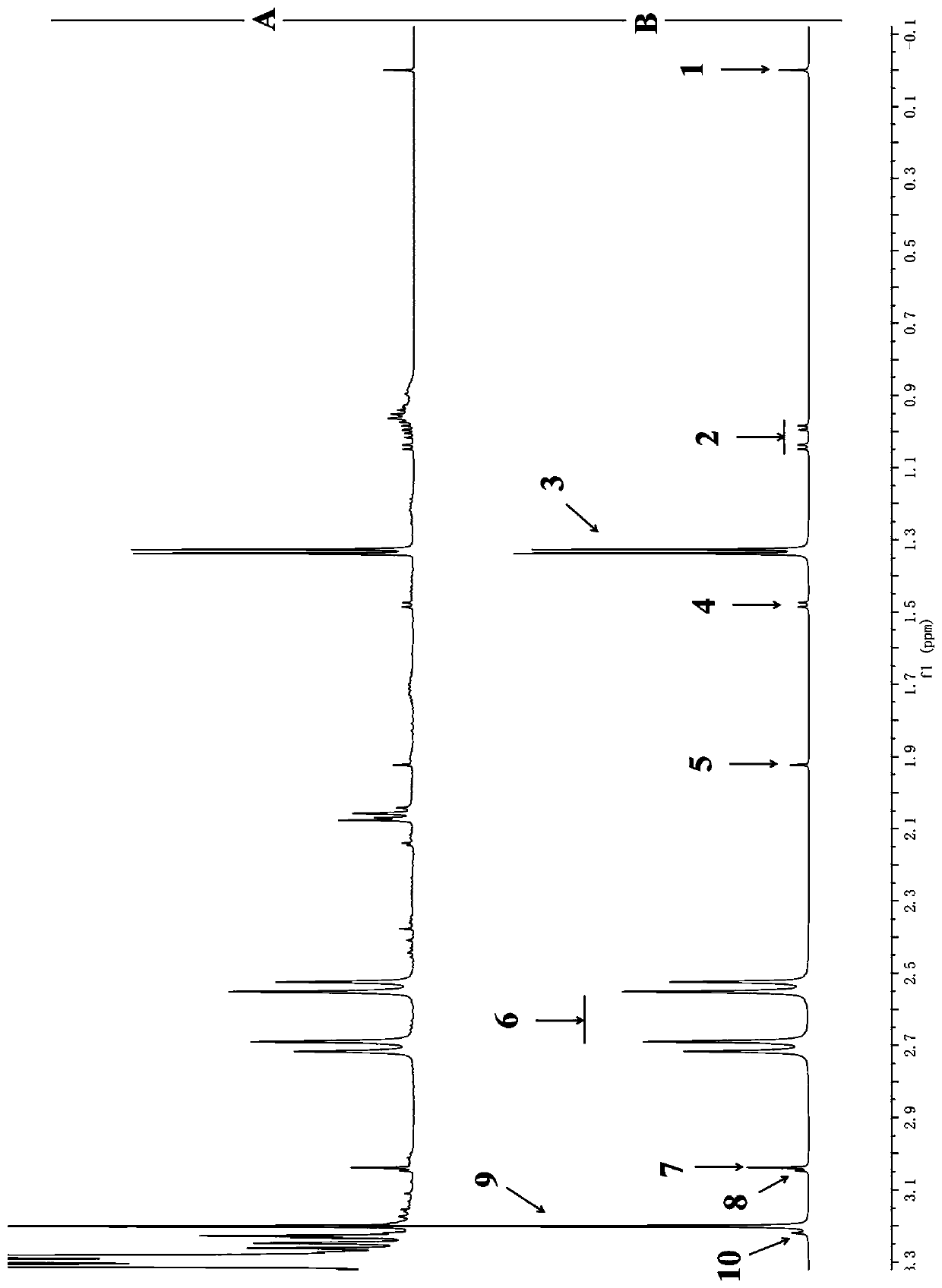
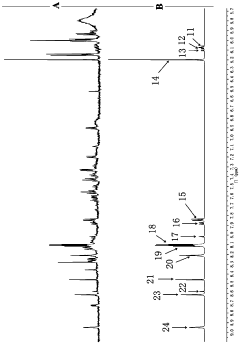
Method for detecting multiple micro-molecule water-soluble matter in milk powder product
PatentPendingCN109991259A
Innovation
- Quantitative nuclear magnetic resonance (qNMR) combined with full spectrum deconvolution (GSD) signal processing method was used to stabilize the sample pH value through ultrafiltration centrifugation and buffer solution, using 3-(trimethylsilyl)deuterated sodium propionate (TMSP). ) is used as an internal standard to perform deconvolution processing on 1H-NMR spectra to quantitatively analyze a variety of small molecule water-soluble components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!