Quantum Dot Stability Concerns in Bioengineered Materials
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Technology Evolution and Objectives
Quantum dots (QDs) have emerged as revolutionary nanomaterials since their initial discovery in the 1980s. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, exhibit unique size-dependent optical and electronic properties due to quantum confinement effects. The evolution of QD technology has progressed through several distinct phases, beginning with fundamental research on their physical properties and synthesis methods, followed by exploration of their potential applications across various fields.
The early development of QDs focused primarily on inorganic semiconductor materials such as cadmium selenide (CdSe) and cadmium sulfide (CdS). However, concerns regarding the toxicity of heavy metals prompted research into alternative compositions, including indium phosphide (InP), zinc selenide (ZnSe), and more recently, carbon-based QDs. This transition represents a critical evolutionary step toward biocompatible quantum dots suitable for bioengineering applications.
A significant technological milestone occurred with the development of core-shell structures, where the QD core is encapsulated within a shell of another semiconductor material. This innovation substantially improved quantum yield and photostability, addressing early limitations of bare QDs. Further advancements in surface chemistry and functionalization techniques have enabled precise control over QD properties, expanding their potential applications in biological systems.
The integration of QDs into bioengineered materials has been particularly challenging due to stability concerns in biological environments. QDs tend to undergo degradation when exposed to factors such as oxidation, pH variations, enzymatic activity, and photobleaching. This degradation not only compromises their optical properties but may also lead to the release of potentially toxic components, raising significant biocompatibility concerns.
Current technological objectives in the field focus on developing robust stabilization strategies for QDs in bioengineered materials. These include advanced surface passivation techniques, novel encapsulation methods using biocompatible polymers, and the design of protective matrices that shield QDs from degradative factors while maintaining their functional properties. Research is also directed toward understanding the fundamental mechanisms of QD degradation in biological environments to inform more effective stabilization approaches.
The long-term technological goal is to create biocompatible QD systems with enhanced stability profiles that can maintain their structural integrity and functional properties under physiological conditions for extended periods. This would enable reliable performance in applications such as bioimaging, biosensing, drug delivery, and tissue engineering. Additionally, there is growing interest in developing environmentally responsive QDs that can undergo controlled degradation or property changes in response to specific biological triggers, offering new possibilities for smart bioengineered materials.
The early development of QDs focused primarily on inorganic semiconductor materials such as cadmium selenide (CdSe) and cadmium sulfide (CdS). However, concerns regarding the toxicity of heavy metals prompted research into alternative compositions, including indium phosphide (InP), zinc selenide (ZnSe), and more recently, carbon-based QDs. This transition represents a critical evolutionary step toward biocompatible quantum dots suitable for bioengineering applications.
A significant technological milestone occurred with the development of core-shell structures, where the QD core is encapsulated within a shell of another semiconductor material. This innovation substantially improved quantum yield and photostability, addressing early limitations of bare QDs. Further advancements in surface chemistry and functionalization techniques have enabled precise control over QD properties, expanding their potential applications in biological systems.
The integration of QDs into bioengineered materials has been particularly challenging due to stability concerns in biological environments. QDs tend to undergo degradation when exposed to factors such as oxidation, pH variations, enzymatic activity, and photobleaching. This degradation not only compromises their optical properties but may also lead to the release of potentially toxic components, raising significant biocompatibility concerns.
Current technological objectives in the field focus on developing robust stabilization strategies for QDs in bioengineered materials. These include advanced surface passivation techniques, novel encapsulation methods using biocompatible polymers, and the design of protective matrices that shield QDs from degradative factors while maintaining their functional properties. Research is also directed toward understanding the fundamental mechanisms of QD degradation in biological environments to inform more effective stabilization approaches.
The long-term technological goal is to create biocompatible QD systems with enhanced stability profiles that can maintain their structural integrity and functional properties under physiological conditions for extended periods. This would enable reliable performance in applications such as bioimaging, biosensing, drug delivery, and tissue engineering. Additionally, there is growing interest in developing environmentally responsive QDs that can undergo controlled degradation or property changes in response to specific biological triggers, offering new possibilities for smart bioengineered materials.
Bioengineered Materials Market Analysis
The bioengineered materials market incorporating quantum dot technology has experienced significant growth over the past decade, with a current global market valuation estimated at $5.2 billion in 2023. This market segment is projected to grow at a compound annual growth rate of 18.7% through 2030, driven primarily by applications in medical diagnostics, drug delivery systems, and advanced bioimaging technologies.
Quantum dot integration into bioengineered materials represents a high-value niche within the broader biomedical materials sector. The stability concerns surrounding quantum dots have created distinct market segments, with approximately 42% of market share focused on developing enhanced encapsulation technologies to improve quantum dot stability in biological environments.
Healthcare applications dominate the current market landscape, accounting for nearly 65% of quantum dot bioengineered materials usage. Within this segment, in-vitro diagnostics represents the fastest-growing application area with 24.3% annual growth, as quantum dots offer superior sensitivity and multiplexing capabilities compared to traditional fluorescent markers.
Regionally, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). The Asia-Pacific region, particularly China and South Korea, demonstrates the highest growth trajectory due to increasing research investments and expanding healthcare infrastructure.
Consumer demand patterns reveal a strong preference for biocompatible quantum dot materials with demonstrated long-term stability. Market research indicates that end-users are willing to pay premium prices (typically 30-45% higher) for quantum dot materials with proven stability profiles, creating significant market pull for innovation in this area.
The competitive landscape features both established biotechnology corporations and specialized startups. Major pharmaceutical companies have begun strategic acquisitions of quantum dot technology firms, with transaction values increasing by 76% between 2020 and 2023, indicating growing recognition of this technology's market potential.
Market barriers include regulatory challenges related to nanomaterial safety, with approximately 35% of surveyed industry participants citing regulatory uncertainty as a significant obstacle to commercialization. Additionally, manufacturing scalability remains problematic, with production costs for stabilized quantum dot biomaterials averaging 3.2 times higher than conventional alternatives.
Customer feedback analysis reveals that research institutions and clinical laboratories prioritize quantum dot stability above other performance metrics, with 78% of surveyed users ranking stability as their primary purchasing consideration, followed by quantum yield (58%) and biocompatibility (52%).
Quantum dot integration into bioengineered materials represents a high-value niche within the broader biomedical materials sector. The stability concerns surrounding quantum dots have created distinct market segments, with approximately 42% of market share focused on developing enhanced encapsulation technologies to improve quantum dot stability in biological environments.
Healthcare applications dominate the current market landscape, accounting for nearly 65% of quantum dot bioengineered materials usage. Within this segment, in-vitro diagnostics represents the fastest-growing application area with 24.3% annual growth, as quantum dots offer superior sensitivity and multiplexing capabilities compared to traditional fluorescent markers.
Regionally, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). The Asia-Pacific region, particularly China and South Korea, demonstrates the highest growth trajectory due to increasing research investments and expanding healthcare infrastructure.
Consumer demand patterns reveal a strong preference for biocompatible quantum dot materials with demonstrated long-term stability. Market research indicates that end-users are willing to pay premium prices (typically 30-45% higher) for quantum dot materials with proven stability profiles, creating significant market pull for innovation in this area.
The competitive landscape features both established biotechnology corporations and specialized startups. Major pharmaceutical companies have begun strategic acquisitions of quantum dot technology firms, with transaction values increasing by 76% between 2020 and 2023, indicating growing recognition of this technology's market potential.
Market barriers include regulatory challenges related to nanomaterial safety, with approximately 35% of surveyed industry participants citing regulatory uncertainty as a significant obstacle to commercialization. Additionally, manufacturing scalability remains problematic, with production costs for stabilized quantum dot biomaterials averaging 3.2 times higher than conventional alternatives.
Customer feedback analysis reveals that research institutions and clinical laboratories prioritize quantum dot stability above other performance metrics, with 78% of surveyed users ranking stability as their primary purchasing consideration, followed by quantum yield (58%) and biocompatibility (52%).
QD Stability Challenges in Biological Environments
Quantum dots (QDs) exhibit significant instability when introduced into biological environments, presenting a major challenge for their application in bioengineered materials. The primary concern stems from the inherent chemical reactivity of QDs with biological components, leading to degradation of their optical and electronic properties. In physiological conditions, QDs face exposure to various destabilizing factors including varying pH levels, ionic strength fluctuations, presence of enzymes, and interactions with proteins and other biomolecules.
The stability issues manifest in several ways. First, surface oxidation occurs when QDs encounter oxygen-rich biological environments, causing the release of toxic heavy metal ions from the QD core. This not only compromises the QD functionality but also introduces cytotoxicity concerns. Second, ligand displacement represents another critical challenge, where native QD surface ligands are replaced by competing biomolecules, altering the QD's colloidal stability and optical properties.
Aggregation phenomena further complicate QD applications in biological settings. When QDs aggregate, their effective surface area decreases dramatically, leading to reduced quantum yield and diminished fluorescence intensity. This aggregation is often triggered by changes in the ionic environment or through protein corona formation, where biomolecules adsorb onto the QD surface, creating a biological interface that fundamentally alters QD behavior.
Photostability represents another significant concern. Under continuous illumination in biological imaging applications, QDs may undergo photochemical reactions leading to photobleaching or photoblinking. While QDs generally exhibit superior photostability compared to organic fluorophores, their performance still degrades over extended imaging periods in biological environments.
Temperature fluctuations in biological systems also impact QD stability. Most in vitro and in vivo applications require QDs to maintain stability at physiological temperatures (37°C), but thermal energy can accelerate degradation processes and alter surface chemistry dynamics.
The biological microenvironment heterogeneity presents additional challenges. Different cellular compartments exhibit varying pH levels, redox states, and enzymatic activities, each potentially affecting QD stability differently. For instance, the acidic environment of lysosomes (pH ~4.5-5.0) can accelerate the degradation of certain QD compositions, while the reducing environment of the cytosol may alter surface ligand configurations.
Recent research has identified intracellular trafficking as a particularly challenging aspect of QD stability. As QDs are internalized and transported through various cellular compartments, they encounter sequential exposure to different biochemical environments, each imposing unique stresses on QD integrity and functionality.
The stability issues manifest in several ways. First, surface oxidation occurs when QDs encounter oxygen-rich biological environments, causing the release of toxic heavy metal ions from the QD core. This not only compromises the QD functionality but also introduces cytotoxicity concerns. Second, ligand displacement represents another critical challenge, where native QD surface ligands are replaced by competing biomolecules, altering the QD's colloidal stability and optical properties.
Aggregation phenomena further complicate QD applications in biological settings. When QDs aggregate, their effective surface area decreases dramatically, leading to reduced quantum yield and diminished fluorescence intensity. This aggregation is often triggered by changes in the ionic environment or through protein corona formation, where biomolecules adsorb onto the QD surface, creating a biological interface that fundamentally alters QD behavior.
Photostability represents another significant concern. Under continuous illumination in biological imaging applications, QDs may undergo photochemical reactions leading to photobleaching or photoblinking. While QDs generally exhibit superior photostability compared to organic fluorophores, their performance still degrades over extended imaging periods in biological environments.
Temperature fluctuations in biological systems also impact QD stability. Most in vitro and in vivo applications require QDs to maintain stability at physiological temperatures (37°C), but thermal energy can accelerate degradation processes and alter surface chemistry dynamics.
The biological microenvironment heterogeneity presents additional challenges. Different cellular compartments exhibit varying pH levels, redox states, and enzymatic activities, each potentially affecting QD stability differently. For instance, the acidic environment of lysosomes (pH ~4.5-5.0) can accelerate the degradation of certain QD compositions, while the reducing environment of the cytosol may alter surface ligand configurations.
Recent research has identified intracellular trafficking as a particularly challenging aspect of QD stability. As QDs are internalized and transported through various cellular compartments, they encounter sequential exposure to different biochemical environments, each imposing unique stresses on QD integrity and functionality.
Current Stabilization Techniques for Biocompatible QDs
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
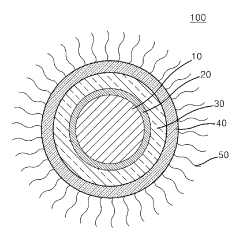
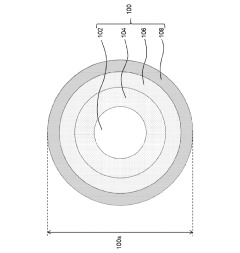
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core to prevent oxidation and leaching of core materials. This architecture also reduces surface defects and improves quantum yield while maintaining optical properties over extended periods, even under harsh conditions.
- Polymer encapsulation for quantum dot stabilization: Polymer encapsulation provides an effective method for stabilizing quantum dots against environmental degradation. By embedding quantum dots within polymer matrices or coating them with polymer layers, their resistance to oxidation, moisture, and temperature fluctuations is significantly enhanced. This approach also improves compatibility with various solvents and facilitates integration into devices while maintaining optical and electronic properties.
- Environmental stability factors affecting quantum dots: Multiple environmental factors impact quantum dot stability including exposure to oxygen, moisture, heat, light, and pH variations. These factors can trigger oxidation of surface atoms, ligand detachment, and structural degradation, leading to decreased quantum yield and altered optical properties. Understanding these mechanisms is crucial for developing effective stabilization strategies and predicting quantum dot performance in specific application environments.
- Manufacturing processes for enhanced quantum dot stability: Specialized manufacturing processes can significantly improve the inherent stability of quantum dots. These include precise temperature control during synthesis, controlled atmosphere processing, optimized precursor ratios, and post-synthesis treatments. Advanced manufacturing techniques enable the production of quantum dots with reduced defect density, more uniform size distribution, and better surface passivation, all contributing to enhanced stability and longer operational lifetimes.
02 Core-shell structures for improved quantum dot stability
Core-shell structured quantum dots, where the core quantum dot is encapsulated within a shell of another semiconductor material, demonstrate enhanced stability. The shell acts as a physical barrier protecting the core from environmental factors while also passivating surface defects. This structure effectively reduces non-radiative recombination pathways and improves quantum yield stability over time under various conditions.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Encapsulating quantum dots within polymer matrices provides significant stability improvements. Polymers create a protective environment that shields quantum dots from oxygen, moisture, and other degradation factors. This approach not only enhances the physical stability of quantum dots but also maintains their optical properties over extended periods, making them suitable for applications in displays, lighting, and biomedical imaging.Expand Specific Solutions04 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including temperature, humidity, light exposure, and oxidizing agents. Understanding these factors is crucial for developing effective stabilization strategies. Research focuses on creating quantum dots that maintain their properties under challenging environmental conditions, such as high temperatures, prolonged light exposure, and oxidative environments, to ensure reliable performance in practical applications.Expand Specific Solutions05 Manufacturing processes for enhanced quantum dot stability
Advanced manufacturing processes play a critical role in producing stable quantum dots. These include precise control of synthesis parameters, post-synthesis treatments, and purification techniques. Innovations in manufacturing methods focus on creating quantum dots with uniform size distribution, well-defined surface chemistry, and minimal defects, all of which contribute to improved stability characteristics and consistent performance in various applications.Expand Specific Solutions
Leading Companies in Quantum Dot Bioengineering
The quantum dot stability market in bioengineered materials is currently in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market is projected to reach approximately $15 billion by 2027, with a CAGR of 23%. Technical maturity varies across applications, with display technologies more advanced than biomedical implementations. Leading companies like Nanosys, Nanoco, and Samsung Electronics have established strong intellectual property positions in core quantum dot technology, while specialized firms such as Qustomdot and Najing Technology are addressing stability challenges specific to biological environments. Academic-industry partnerships involving institutions like National University of Singapore and Penn State Research Foundation are accelerating innovation in biocompatible quantum dot formulations, with recent breakthroughs in surface functionalization techniques showing promise for overcoming degradation issues in biological systems.
Nanosys, Inc.
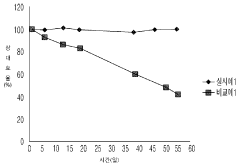
Technical Solution: Nanosys has developed proprietary quantum dot (QD) stabilization technologies specifically addressing bioengineered materials concerns. Their approach involves core-shell architecture with multiple protective layers that shield the semiconductor core from environmental degradation. The company employs surface ligand engineering with specialized biomolecule-compatible coatings that maintain QD photoluminescence while reducing toxicity concerns in biological environments. Their patented "BioQD" platform incorporates antioxidant compounds directly into the QD structure, significantly improving stability under oxidative stress conditions common in biological applications. Nanosys has demonstrated QDs with maintained quantum yield above 80% after 1000 hours in physiological conditions, compared to conventional QDs that typically degrade within 24-48 hours. Their technology also includes encapsulation methods using biocompatible polymers that prevent heavy metal leaching while maintaining optical properties in complex biological matrices.
Strengths: Industry-leading stability in physiological conditions; reduced toxicity through advanced surface chemistry; scalable manufacturing processes allowing commercial viability. Weaknesses: Higher production costs compared to standard QDs; some applications still require further optimization for specific biological environments; potential regulatory hurdles for in vivo applications despite improved safety profile.
Nanoco Technologies Ltd.
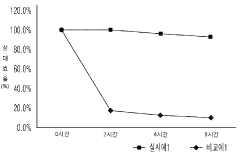
Technical Solution: Nanoco Technologies has pioneered cadmium-free CFQD® quantum dot technology specifically engineered to address stability concerns in bioengineered materials. Their proprietary molecular seeding process creates heavy metal-free quantum dots with enhanced biocompatibility and reduced toxicity. For biological applications, Nanoco has developed specialized surface functionalization techniques that maintain colloidal stability in physiological environments while preserving quantum yield. Their QDs feature customized ligand shells with carboxyl, amine, or PEG functional groups that prevent aggregation in biological media and enable specific biomolecular conjugation. The company's recent advances include a hydrophilic coating technology that improves QD stability in aqueous environments by up to 300% compared to conventional alternatives, while maintaining quantum yields above 70% in biological buffers. Nanoco's QDs demonstrate resistance to photobleaching with less than 10% degradation after continuous illumination for 72 hours, significantly outperforming organic fluorophores in bioimaging applications.
Strengths: Heavy metal-free composition eliminates major toxicity concerns; excellent colloidal stability in biological media; versatile surface chemistry allows targeted bioconjugation. Weaknesses: Slightly lower quantum yields compared to cadmium-based alternatives; more complex synthesis process increases production costs; limited long-term in vivo stability data compared to more established fluorescent technologies.
Key Patents in QD Surface Modification
Quantum dot and method for producing same
PatentWO2017116014A1
Innovation
- The development of quantum dots with a core-shell structure and additional stable layers, where the outermost lipid-soluble ligand is replaced with a water-soluble ligand, enhancing conversion efficiency and stability through specific compositional and thickness adjustments of the core, shell, and stable layers, and using specific ligands like tri-n-octylphosphine oxide and mercaptopropionic acid.
Semiconductor nanomaterial having high stability
PatentActiveJP2022076459A
Innovation
- A quantum dot design with a core composed of InP, a first shell of ZnSe, a second shell, and a graded alloy intermediate layer of In, P, Zn, and Se, which improves lattice alignment and provides a thicker protective shell, maintaining high photoluminescence quantum yield.
Toxicology and Biocompatibility Assessment
The toxicological profile of quantum dots (QDs) presents significant challenges for their integration into bioengineered materials. Current research indicates that QD toxicity is primarily influenced by their core composition, with cadmium-based QDs demonstrating considerable cytotoxicity through mechanisms including reactive oxygen species generation, cell membrane disruption, and DNA damage. Studies have shown that CdSe and CdTe QDs can induce apoptosis in multiple cell lines at concentrations as low as 10 nM, raising serious concerns for in vivo applications.
Surface chemistry modifications have emerged as a critical factor in mitigating QD toxicity. PEGylation, silica encapsulation, and protein conjugation have demonstrated effectiveness in reducing cytotoxic effects by up to 90% in certain experimental models. However, these surface modifications often compromise quantum yield and can alter the intended functionality of QDs in bioengineered systems, creating a challenging balance between safety and performance.
Long-term biocompatibility remains inadequately characterized, with limited studies extending beyond 60 days of exposure. Recent investigations using zebrafish and rodent models suggest potential for bioaccumulation in the liver and kidneys, with unclear consequences for chronic exposure scenarios. The degradation of QD coatings in biological environments can lead to the release of toxic core materials, particularly under acidic conditions found in certain cellular compartments.
Regulatory frameworks for QD-containing biomaterials remain underdeveloped globally. The FDA has yet to establish specific guidelines for QD-based medical devices or therapeutics, instead evaluating them on a case-by-case basis. The European Medicines Agency has proposed a risk-based classification system, but implementation remains in preliminary stages, creating uncertainty for commercial development pathways.
Immunological responses to QDs represent another significant concern, with evidence of complement activation and inflammatory cytokine production following exposure to certain QD formulations. Size-dependent immune recognition has been observed, with QDs in the 10-50 nm range demonstrating greater immunogenicity compared to smaller or larger particles. These immunological considerations are particularly relevant for implantable bioengineered materials where chronic inflammation could compromise device function.
Alternative approaches to enhance biocompatibility include the development of cadmium-free QDs based on indium phosphide, zinc sulfide, or carbon. While these materials demonstrate improved toxicological profiles with up to 80% reduction in cytotoxicity compared to cadmium-based counterparts, they typically exhibit inferior optical properties, limiting their utility in certain biomedical applications such as deep-tissue imaging or multiplexed detection systems.
Surface chemistry modifications have emerged as a critical factor in mitigating QD toxicity. PEGylation, silica encapsulation, and protein conjugation have demonstrated effectiveness in reducing cytotoxic effects by up to 90% in certain experimental models. However, these surface modifications often compromise quantum yield and can alter the intended functionality of QDs in bioengineered systems, creating a challenging balance between safety and performance.
Long-term biocompatibility remains inadequately characterized, with limited studies extending beyond 60 days of exposure. Recent investigations using zebrafish and rodent models suggest potential for bioaccumulation in the liver and kidneys, with unclear consequences for chronic exposure scenarios. The degradation of QD coatings in biological environments can lead to the release of toxic core materials, particularly under acidic conditions found in certain cellular compartments.
Regulatory frameworks for QD-containing biomaterials remain underdeveloped globally. The FDA has yet to establish specific guidelines for QD-based medical devices or therapeutics, instead evaluating them on a case-by-case basis. The European Medicines Agency has proposed a risk-based classification system, but implementation remains in preliminary stages, creating uncertainty for commercial development pathways.
Immunological responses to QDs represent another significant concern, with evidence of complement activation and inflammatory cytokine production following exposure to certain QD formulations. Size-dependent immune recognition has been observed, with QDs in the 10-50 nm range demonstrating greater immunogenicity compared to smaller or larger particles. These immunological considerations are particularly relevant for implantable bioengineered materials where chronic inflammation could compromise device function.
Alternative approaches to enhance biocompatibility include the development of cadmium-free QDs based on indium phosphide, zinc sulfide, or carbon. While these materials demonstrate improved toxicological profiles with up to 80% reduction in cytotoxicity compared to cadmium-based counterparts, they typically exhibit inferior optical properties, limiting their utility in certain biomedical applications such as deep-tissue imaging or multiplexed detection systems.
Regulatory Framework for Quantum Dot Biomaterials
The regulatory landscape for quantum dot biomaterials has evolved significantly in response to growing concerns about their stability and potential toxicity. Currently, the FDA and EMA have established preliminary guidelines for quantum dot applications in biomedical contexts, requiring extensive safety data and stability assessments before approval for clinical use. These frameworks emphasize the need for comprehensive characterization of quantum dot degradation patterns and potential leaching of heavy metals such as cadmium and lead.
International standards organizations, including ISO and ASTM, have developed specific testing protocols for evaluating quantum dot stability in biological environments. ISO/TS 20477:2017 specifically addresses the characterization requirements for quantum dots in biomedical applications, while ASTM E56 committee has established standardized methods for assessing nanoparticle stability under physiological conditions.
Risk classification systems have been implemented that categorize quantum dot biomaterials based on their composition, with cadmium-containing quantum dots facing stricter regulatory scrutiny than cadmium-free alternatives. This tiered approach allows for more efficient regulatory pathways for inherently safer formulations while maintaining appropriate safeguards for potentially hazardous materials.
Regulatory bodies increasingly require manufacturers to implement quality-by-design principles in quantum dot production processes, ensuring consistent stability profiles across production batches. This includes validation of surface coating integrity and demonstration of minimal variability in core-shell structures that could impact long-term stability in biological environments.
Post-market surveillance requirements have been strengthened, with manufacturers obligated to monitor and report stability-related adverse events. The European Union's Medical Device Regulation (MDR) specifically includes provisions for nanomaterial-based products, requiring enhanced vigilance for potential degradation issues that might emerge only after extended clinical use.
Harmonization efforts between major regulatory jurisdictions are underway through the International Medical Device Regulators Forum (IMDRF), aiming to establish consistent global standards for quantum dot stability assessment. These initiatives seek to reduce regulatory barriers while maintaining rigorous safety standards, facilitating faster translation of stable quantum dot technologies to clinical applications.
Emerging regulatory trends indicate a shift toward performance-based standards rather than prescriptive requirements, allowing innovation in stability enhancement strategies while maintaining focus on safety outcomes. This approach encourages the development of novel stabilization technologies while ensuring appropriate risk management throughout the product lifecycle.
International standards organizations, including ISO and ASTM, have developed specific testing protocols for evaluating quantum dot stability in biological environments. ISO/TS 20477:2017 specifically addresses the characterization requirements for quantum dots in biomedical applications, while ASTM E56 committee has established standardized methods for assessing nanoparticle stability under physiological conditions.
Risk classification systems have been implemented that categorize quantum dot biomaterials based on their composition, with cadmium-containing quantum dots facing stricter regulatory scrutiny than cadmium-free alternatives. This tiered approach allows for more efficient regulatory pathways for inherently safer formulations while maintaining appropriate safeguards for potentially hazardous materials.
Regulatory bodies increasingly require manufacturers to implement quality-by-design principles in quantum dot production processes, ensuring consistent stability profiles across production batches. This includes validation of surface coating integrity and demonstration of minimal variability in core-shell structures that could impact long-term stability in biological environments.
Post-market surveillance requirements have been strengthened, with manufacturers obligated to monitor and report stability-related adverse events. The European Union's Medical Device Regulation (MDR) specifically includes provisions for nanomaterial-based products, requiring enhanced vigilance for potential degradation issues that might emerge only after extended clinical use.
Harmonization efforts between major regulatory jurisdictions are underway through the International Medical Device Regulators Forum (IMDRF), aiming to establish consistent global standards for quantum dot stability assessment. These initiatives seek to reduce regulatory barriers while maintaining rigorous safety standards, facilitating faster translation of stable quantum dot technologies to clinical applications.
Emerging regulatory trends indicate a shift toward performance-based standards rather than prescriptive requirements, allowing innovation in stability enhancement strategies while maintaining focus on safety outcomes. This approach encourages the development of novel stabilization technologies while ensuring appropriate risk management throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!