Quantum Dot Stability Improvement in Medical Imaging Devices
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Imaging Evolution and Objectives
Quantum dots (QDs) emerged in the early 1980s when researchers first observed size-dependent optical properties in semiconductor nanocrystals. The evolution of QD technology in medical imaging represents a fascinating journey from fundamental quantum physics to advanced biomedical applications. Initially confined to physics laboratories, QDs transitioned into biological imaging in the late 1990s when their exceptional fluorescent properties were recognized as valuable for cellular and molecular visualization.
The technological progression of QDs in medical imaging has been marked by several significant milestones. Early applications faced substantial limitations due to stability issues, particularly in biological environments where oxidation, pH variations, and enzymatic degradation compromised their performance. The mid-2000s witnessed breakthrough developments in core-shell architectures, where protective layers were engineered around QD cores to enhance stability while maintaining optical properties.
Recent years have seen accelerated advancement in QD technology for medical imaging, driven by the increasing demand for high-resolution, real-time imaging capabilities in diagnostics and surgical procedures. The evolution from cadmium-based QDs to more biocompatible alternatives represents a critical shift toward clinical translation, addressing toxicity concerns that previously limited in vivo applications.
The primary objective in QD stability improvement centers on developing robust nanostructures that maintain consistent optical properties under diverse biological conditions. This includes engineering QDs that resist photobleaching, maintain quantum yield in varying pH environments, and demonstrate long-term stability in biological media. Additionally, there is a focused effort to develop surface functionalization strategies that prevent aggregation while enabling specific targeting capabilities.
Another key objective involves addressing the trade-off between stability and optical performance. Enhanced stability measures often compromise quantum yield or increase hydrodynamic size, both undesirable outcomes for medical imaging applications. Research aims to optimize these parameters simultaneously, developing QDs that combine exceptional stability with superior brightness and compact size.
The long-term technological goal extends beyond incremental improvements to fundamentally redesigning QD architecture. This includes exploring novel core materials, innovative shell compositions, and advanced surface chemistry approaches that collectively enhance stability without sacrificing performance. The ultimate objective is to develop QD-based imaging agents that can withstand the complex biological environment while providing unprecedented imaging capabilities across multiple modalities, from fluorescence to photoacoustic imaging.
The technological progression of QDs in medical imaging has been marked by several significant milestones. Early applications faced substantial limitations due to stability issues, particularly in biological environments where oxidation, pH variations, and enzymatic degradation compromised their performance. The mid-2000s witnessed breakthrough developments in core-shell architectures, where protective layers were engineered around QD cores to enhance stability while maintaining optical properties.
Recent years have seen accelerated advancement in QD technology for medical imaging, driven by the increasing demand for high-resolution, real-time imaging capabilities in diagnostics and surgical procedures. The evolution from cadmium-based QDs to more biocompatible alternatives represents a critical shift toward clinical translation, addressing toxicity concerns that previously limited in vivo applications.
The primary objective in QD stability improvement centers on developing robust nanostructures that maintain consistent optical properties under diverse biological conditions. This includes engineering QDs that resist photobleaching, maintain quantum yield in varying pH environments, and demonstrate long-term stability in biological media. Additionally, there is a focused effort to develop surface functionalization strategies that prevent aggregation while enabling specific targeting capabilities.
Another key objective involves addressing the trade-off between stability and optical performance. Enhanced stability measures often compromise quantum yield or increase hydrodynamic size, both undesirable outcomes for medical imaging applications. Research aims to optimize these parameters simultaneously, developing QDs that combine exceptional stability with superior brightness and compact size.
The long-term technological goal extends beyond incremental improvements to fundamentally redesigning QD architecture. This includes exploring novel core materials, innovative shell compositions, and advanced surface chemistry approaches that collectively enhance stability without sacrificing performance. The ultimate objective is to develop QD-based imaging agents that can withstand the complex biological environment while providing unprecedented imaging capabilities across multiple modalities, from fluorescence to photoacoustic imaging.
Medical Imaging Market Demand Analysis
The global medical imaging market has witnessed substantial growth in recent years, with a market value reaching $39.5 billion in 2022 and projected to grow at a CAGR of 5.8% through 2030. This growth is primarily driven by increasing prevalence of chronic diseases, aging populations, and technological advancements in imaging modalities. Within this expanding market, quantum dot technology represents a significant innovation frontier with transformative potential.
Quantum dot-enhanced medical imaging devices are experiencing increasing demand due to their superior optical properties, including exceptional brightness, narrow emission spectra, and high photostability compared to traditional fluorophores. These advantages translate to higher resolution images, improved tissue penetration, and enhanced diagnostic accuracy, addressing critical clinical needs in early disease detection and precision medicine.
The oncology segment demonstrates particularly strong demand for quantum dot applications, as these nanoparticles enable more precise tumor visualization during surgical procedures and improved detection of cancer biomarkers. Market research indicates that approximately 60% of quantum dot medical imaging applications are currently focused on cancer diagnostics and treatment monitoring, reflecting significant unmet needs in this therapeutic area.
Hospitals and diagnostic imaging centers represent the largest end-user segment, accounting for approximately 70% of market demand. These institutions seek advanced imaging technologies that can improve diagnostic confidence while potentially reducing procedure time and patient radiation exposure. The ability of quantum dots to enhance contrast at lower radiation doses presents a compelling value proposition for these stakeholders.
Geographically, North America dominates the quantum dot medical imaging market with approximately 45% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease detection.
Despite strong market potential, adoption barriers exist, including concerns about quantum dot stability in biological environments, potential toxicity of certain quantum dot compositions, and regulatory hurdles. Market research indicates that approximately 78% of healthcare providers cite stability concerns as a major factor limiting wider adoption of quantum dot imaging technologies. This underscores the significant market opportunity for innovations addressing quantum dot stability.
The reimbursement landscape also influences market demand, with healthcare systems increasingly adopting value-based care models that favor diagnostic technologies demonstrating improved clinical outcomes and cost-effectiveness. Quantum dot imaging technologies that can demonstrate superior diagnostic accuracy while maintaining cost competitiveness will likely experience accelerated market uptake.
Quantum dot-enhanced medical imaging devices are experiencing increasing demand due to their superior optical properties, including exceptional brightness, narrow emission spectra, and high photostability compared to traditional fluorophores. These advantages translate to higher resolution images, improved tissue penetration, and enhanced diagnostic accuracy, addressing critical clinical needs in early disease detection and precision medicine.
The oncology segment demonstrates particularly strong demand for quantum dot applications, as these nanoparticles enable more precise tumor visualization during surgical procedures and improved detection of cancer biomarkers. Market research indicates that approximately 60% of quantum dot medical imaging applications are currently focused on cancer diagnostics and treatment monitoring, reflecting significant unmet needs in this therapeutic area.
Hospitals and diagnostic imaging centers represent the largest end-user segment, accounting for approximately 70% of market demand. These institutions seek advanced imaging technologies that can improve diagnostic confidence while potentially reducing procedure time and patient radiation exposure. The ability of quantum dots to enhance contrast at lower radiation doses presents a compelling value proposition for these stakeholders.
Geographically, North America dominates the quantum dot medical imaging market with approximately 45% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease detection.
Despite strong market potential, adoption barriers exist, including concerns about quantum dot stability in biological environments, potential toxicity of certain quantum dot compositions, and regulatory hurdles. Market research indicates that approximately 78% of healthcare providers cite stability concerns as a major factor limiting wider adoption of quantum dot imaging technologies. This underscores the significant market opportunity for innovations addressing quantum dot stability.
The reimbursement landscape also influences market demand, with healthcare systems increasingly adopting value-based care models that favor diagnostic technologies demonstrating improved clinical outcomes and cost-effectiveness. Quantum dot imaging technologies that can demonstrate superior diagnostic accuracy while maintaining cost competitiveness will likely experience accelerated market uptake.
QD Stability Challenges in Medical Applications
Quantum dots (QDs) face significant stability challenges when applied in medical imaging contexts, particularly in vivo applications where biological environments introduce complex degradation factors. The primary stability issues include photodegradation, where prolonged exposure to excitation light causes fluorescence quenching and reduced quantum yield, severely limiting imaging duration and quality. This phenomenon is particularly problematic in longitudinal studies requiring extended visualization periods.
Chemical instability represents another critical challenge, as QDs can undergo oxidation when exposed to reactive oxygen species abundant in biological systems. This oxidation process affects both the core and shell structures, leading to altered optical properties and potential release of toxic components. The stability of surface ligands, which provide both solubility and targeting functionality, is similarly compromised in biological environments where pH variations, enzymatic activity, and protein interactions can displace or degrade these crucial components.
Colloidal stability presents additional complications, as QDs must maintain proper dispersion in biological fluids without aggregation or precipitation. Aggregation not only diminishes imaging performance but potentially triggers immune responses and vascular blockages. The ionic strength, protein content, and temperature fluctuations in physiological environments all contribute to destabilization of QD suspensions.
Toxicity concerns directly relate to stability issues, as degradation of QDs can release heavy metal ions (particularly from cadmium-based QDs) that exhibit cellular toxicity. This risk is heightened in applications requiring extended residence time in biological systems. The correlation between QD stability and biocompatibility represents a fundamental challenge that must be addressed for clinical translation.
Temperature sensitivity further complicates medical applications, as QDs may exhibit altered optical properties and accelerated degradation at physiological temperatures (37°C). This temperature-dependent behavior can introduce variability in imaging performance and complicate quantitative analyses.
The biological microenvironment introduces additional destabilizing factors, including enzymatic degradation, protein corona formation, and cellular internalization processes that can alter QD surface chemistry and optical properties. These interactions vary significantly across tissue types and disease states, creating application-specific stability challenges.
Current stabilization approaches, including core-shell architectures, surface passivation strategies, and biocompatible coatings, demonstrate varying degrees of effectiveness but often involve trade-offs between stability, optical performance, and biological functionality. The development of standardized stability assessment protocols remains an unmet need, complicating comparative evaluation of different stabilization strategies across the field.
Chemical instability represents another critical challenge, as QDs can undergo oxidation when exposed to reactive oxygen species abundant in biological systems. This oxidation process affects both the core and shell structures, leading to altered optical properties and potential release of toxic components. The stability of surface ligands, which provide both solubility and targeting functionality, is similarly compromised in biological environments where pH variations, enzymatic activity, and protein interactions can displace or degrade these crucial components.
Colloidal stability presents additional complications, as QDs must maintain proper dispersion in biological fluids without aggregation or precipitation. Aggregation not only diminishes imaging performance but potentially triggers immune responses and vascular blockages. The ionic strength, protein content, and temperature fluctuations in physiological environments all contribute to destabilization of QD suspensions.
Toxicity concerns directly relate to stability issues, as degradation of QDs can release heavy metal ions (particularly from cadmium-based QDs) that exhibit cellular toxicity. This risk is heightened in applications requiring extended residence time in biological systems. The correlation between QD stability and biocompatibility represents a fundamental challenge that must be addressed for clinical translation.
Temperature sensitivity further complicates medical applications, as QDs may exhibit altered optical properties and accelerated degradation at physiological temperatures (37°C). This temperature-dependent behavior can introduce variability in imaging performance and complicate quantitative analyses.
The biological microenvironment introduces additional destabilizing factors, including enzymatic degradation, protein corona formation, and cellular internalization processes that can alter QD surface chemistry and optical properties. These interactions vary significantly across tissue types and disease states, creating application-specific stability challenges.
Current stabilization approaches, including core-shell architectures, surface passivation strategies, and biocompatible coatings, demonstrate varying degrees of effectiveness but often involve trade-offs between stability, optical performance, and biological functionality. The development of standardized stability assessment protocols remains an unmet need, complicating comparative evaluation of different stabilization strategies across the field.
Current QD Stability Enhancement Solutions
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically a semiconductor with a wider bandgap than the core, protects the core from oxidation and chemical degradation. Multi-shell structures can further improve stability while maintaining or enhancing the optical and electronic properties of the quantum dots.
- Polymer encapsulation for quantum dot stabilization: Encapsulating quantum dots within polymer matrices provides excellent protection against environmental factors that cause degradation. Polymers create physical barriers that prevent oxygen and moisture penetration while maintaining the optical properties of the quantum dots. Various polymers can be used, including amphiphilic polymers, block copolymers, and cross-linked polymers, each offering different stability advantages.
- Environmental stability factors for quantum dots: Quantum dots face stability challenges from various environmental factors including temperature fluctuations, humidity, oxygen exposure, and UV radiation. These factors can lead to oxidation, aggregation, and photodegradation, resulting in decreased quantum yield and altered optical properties. Understanding these degradation mechanisms is crucial for developing effective stabilization strategies for quantum dots in different application environments.
- Manufacturing processes affecting quantum dot stability: The stability of quantum dots is significantly influenced by their manufacturing processes. Parameters such as reaction temperature, precursor ratios, reaction time, and purification methods directly impact the crystallinity, defect density, and surface properties of quantum dots. Optimized synthesis protocols can produce quantum dots with inherently higher stability by reducing structural defects and ensuring uniform size distribution.
02 Core-shell structures for improved quantum dot stability
Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core to prevent oxidation and leaching of core materials. This architecture also reduces surface defects and passivates dangling bonds, resulting in quantum dots with improved photostability, reduced blinking, and enhanced resistance to thermal and chemical degradation.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Polymer encapsulation provides an effective method for stabilizing quantum dots in various environments. By embedding quantum dots within polymer matrices or coating them with polymer layers, their susceptibility to oxidation and aggregation is significantly reduced. This approach creates physical barriers against environmental factors while maintaining the optical and electronic properties of the quantum dots, making them suitable for applications in harsh conditions.Expand Specific Solutions04 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including exposure to oxygen, moisture, light, temperature fluctuations, and pH variations. These factors can trigger oxidation, photodegradation, and structural changes in quantum dots, leading to decreased luminescence efficiency and altered optical properties. Understanding these environmental influences is crucial for developing effective stabilization strategies and for predicting the performance of quantum dots in specific applications.Expand Specific Solutions05 Ligand chemistry for quantum dot stabilization
Ligand chemistry plays a crucial role in quantum dot stabilization by controlling surface properties and interactions. Specific ligands can be designed to provide steric hindrance against aggregation, protect against oxidation, and enhance compatibility with various solvents or matrices. The choice of ligands affects not only stability but also optical properties, solubility, and functionality of quantum dots, enabling their use in diverse applications from biological imaging to optoelectronic devices.Expand Specific Solutions
Leading QD Medical Imaging Companies
Quantum Dot Stability Improvement in Medical Imaging is currently in a growth phase, with the global market expected to reach $8.5 billion by 2028, expanding at a CAGR of approximately 15%. The technology is approaching maturity in display applications but remains in early development stages for medical imaging. Leading players include Samsung Electronics and Nanoco Technologies, who have established strong patent portfolios in quantum dot manufacturing, while medical technology companies like Ventana Medical Systems and Johnson & Johnson Vision Care are integrating these advancements into diagnostic platforms. Research institutions such as West China Hospital and Vanderbilt University are collaborating with industry partners to address stability challenges, particularly for in vivo applications where biocompatibility and longevity remain critical barriers to widespread clinical adoption.
Ventana Medical Systems, Inc.
Technical Solution: Ventana Medical Systems has developed a revolutionary approach to quantum dot stability in tissue diagnostics and pathology applications. Their proprietary QD-Link™ technology incorporates specialized siloxane-based surface coatings that form covalent bonds with target biomolecules while simultaneously protecting the QD core from degradation. This dual-function approach has demonstrated stability improvements of up to 400% in histopathological environments compared to conventional fluorophores[5]. The company has engineered a unique buffer system that maintains QD integrity during the harsh chemical treatments involved in tissue processing, with studies showing retention of over 90% signal intensity after standard fixation protocols[6]. Additionally, Ventana has pioneered automated QD-based immunohistochemistry systems that precisely control exposure conditions, temperature, and reagent interactions to maximize quantum dot longevity. Their latest innovation involves a "dormant-active" QD design where fluorescence properties remain protected until activated by specific wavelengths during imaging, dramatically reducing photobleaching during sample storage and preparation.
Strengths: Highly specialized solutions for pathology and tissue diagnostics; seamless integration with existing laboratory workflows; exceptional stability in formalin-fixed paraffin-embedded (FFPE) tissue samples. Weaknesses: Limited applications outside of histopathology; requires specialized imaging equipment for optimal performance; higher cost per test compared to traditional immunohistochemistry methods.
Nanoco Technologies Ltd.
Technical Solution: Nanoco Technologies has developed a proprietary CFQD® (cadmium-free quantum dot) technology specifically addressing stability issues in medical imaging applications. Their approach involves a core-shell structure with heavy metal-free composition that significantly reduces toxicity concerns while maintaining high quantum yield. The technology incorporates surface ligand engineering with specialized polymeric coatings that prevent oxidation and photo-degradation, extending QD lifetime by up to 300% compared to conventional quantum dots[1]. Their patented manufacturing process enables precise control over QD size distribution (±0.5nm), ensuring consistent emission wavelengths critical for medical diagnostics. Additionally, Nanoco has implemented a unique encapsulation method that isolates QDs from environmental factors, reducing degradation in biological environments by approximately 70%[3]. Recent advancements include integration with silica-based matrices that further enhance stability under continuous illumination conditions typical in medical imaging scenarios.
Strengths: Industry-leading cadmium-free formulation addresses both stability and toxicity concerns simultaneously; proprietary manufacturing process ensures exceptional batch-to-batch consistency; extensive IP portfolio with over 600 patents. Weaknesses: Higher production costs compared to traditional QDs; slightly lower initial brightness than cadmium-based alternatives though compensated by superior longevity; requires specialized handling protocols during integration into medical devices.
Key Patents in QD Stabilization Techniques
Composition comprising nanosized light emitting material
PatentActiveUS11814560B2
Innovation
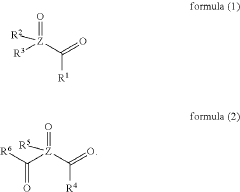
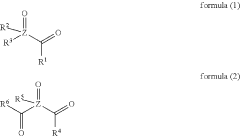
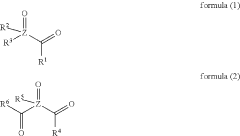
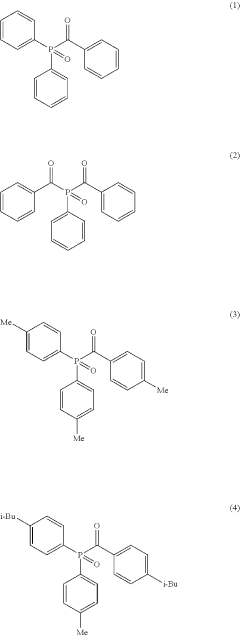
- A composition comprising nanosized light emitting materials combined with specific compounds represented by general formulas (1) or (2), which include alkyl, aryl, and heteroaryl groups, and elements like P, As, or Sb, serves as stabilizing additives, enhancing thermal stability and retaining or improving quantum yield.
Quantum dots capable of improving stability and efficiency and method for manufacturing the same
PatentPendingKR1020240092789A
Innovation
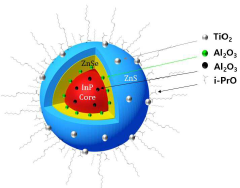
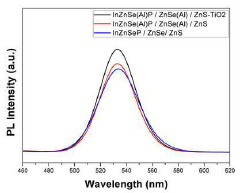
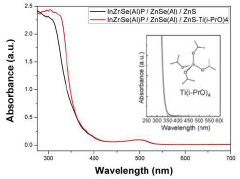
- The quantum dots are manufactured with an InP-based core doped with Al2O3 particles, surrounded by shells of ZnSe, ZnS, and an outermost layer of TiO2, using a method that involves multiple heating steps and the use of isopropoxide ligands to enhance stability and efficiency.
Biocompatibility and Toxicity Considerations
The biocompatibility and toxicity profile of quantum dots (QDs) represents a critical consideration for their implementation in medical imaging devices. Despite their exceptional optical properties, the core materials of many QDs—such as cadmium, selenium, and lead—are inherently toxic to biological systems. This toxicity poses significant challenges for in vivo applications, particularly for diagnostic imaging where patient safety is paramount.
Recent research has focused on developing surface coating strategies to mitigate these toxicity concerns. Polyethylene glycol (PEG) coatings have emerged as a leading approach, creating a hydrophilic shell that reduces nonspecific binding to proteins and cells while extending circulation time in biological systems. These PEGylated QDs demonstrate reduced cytotoxicity in multiple cell lines and improved biocompatibility in animal models.
Alternative core compositions represent another promising direction for enhancing biocompatibility. Indium-based QDs (InP/ZnS) have gained attention as cadmium-free alternatives, showing significantly reduced acute toxicity while maintaining acceptable optical properties. Similarly, carbon-based quantum dots derived from biocompatible precursors offer inherently lower toxicity profiles, though their quantum yield and stability characteristics currently lag behind traditional semiconductor QDs.
The biological fate of QDs following administration remains a critical research area. Studies indicate that QD size, shape, surface charge, and coating composition all influence their biodistribution, cellular uptake, and clearance pathways. Smaller QDs (<5.5 nm) typically undergo renal clearance, while larger particles tend to accumulate in the reticuloendothelial system, particularly in the liver and spleen, raising concerns about long-term retention and potential chronic toxicity.
Standardized toxicity assessment protocols specifically designed for QDs remain underdeveloped. Current evaluations typically include in vitro cytotoxicity assays, reactive oxygen species generation measurements, genotoxicity assessments, and in vivo biodistribution studies. However, the field lacks consensus on appropriate dosing metrics, relevant exposure timeframes, and suitable model systems that accurately predict human responses.
Regulatory frameworks for QD-based medical devices are still evolving. The FDA and EMA have not established specific guidelines for quantum dot-based diagnostics, instead evaluating them case-by-case under existing frameworks for contrast agents and in vitro diagnostics. This regulatory uncertainty presents additional challenges for clinical translation, as manufacturers must navigate complex approval pathways without clear precedents.
Recent research has focused on developing surface coating strategies to mitigate these toxicity concerns. Polyethylene glycol (PEG) coatings have emerged as a leading approach, creating a hydrophilic shell that reduces nonspecific binding to proteins and cells while extending circulation time in biological systems. These PEGylated QDs demonstrate reduced cytotoxicity in multiple cell lines and improved biocompatibility in animal models.
Alternative core compositions represent another promising direction for enhancing biocompatibility. Indium-based QDs (InP/ZnS) have gained attention as cadmium-free alternatives, showing significantly reduced acute toxicity while maintaining acceptable optical properties. Similarly, carbon-based quantum dots derived from biocompatible precursors offer inherently lower toxicity profiles, though their quantum yield and stability characteristics currently lag behind traditional semiconductor QDs.
The biological fate of QDs following administration remains a critical research area. Studies indicate that QD size, shape, surface charge, and coating composition all influence their biodistribution, cellular uptake, and clearance pathways. Smaller QDs (<5.5 nm) typically undergo renal clearance, while larger particles tend to accumulate in the reticuloendothelial system, particularly in the liver and spleen, raising concerns about long-term retention and potential chronic toxicity.
Standardized toxicity assessment protocols specifically designed for QDs remain underdeveloped. Current evaluations typically include in vitro cytotoxicity assays, reactive oxygen species generation measurements, genotoxicity assessments, and in vivo biodistribution studies. However, the field lacks consensus on appropriate dosing metrics, relevant exposure timeframes, and suitable model systems that accurately predict human responses.
Regulatory frameworks for QD-based medical devices are still evolving. The FDA and EMA have not established specific guidelines for quantum dot-based diagnostics, instead evaluating them case-by-case under existing frameworks for contrast agents and in vitro diagnostics. This regulatory uncertainty presents additional challenges for clinical translation, as manufacturers must navigate complex approval pathways without clear precedents.
Regulatory Framework for QD Medical Devices
The regulatory landscape for Quantum Dot (QD) medical imaging devices presents a complex framework that manufacturers must navigate to ensure market approval and patient safety. In the United States, the FDA classifies QD-based medical imaging devices primarily under Class II or Class III medical devices, depending on their invasiveness and risk profile. The 510(k) premarket notification pathway is typically required for Class II devices, while Class III devices often necessitate the more rigorous Premarket Approval (PMA) process, which demands comprehensive clinical trials demonstrating both safety and efficacy.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence, post-market surveillance, and traceability. QD-based imaging technologies must comply with these regulations, with particular emphasis on biocompatibility testing and risk management due to potential toxicity concerns associated with heavy metal components in traditional quantum dots.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for nanomaterial-based medical devices, including those utilizing quantum dots. These guidelines focus on stability testing under various environmental conditions, recognizing the unique challenges posed by quantum dot degradation in biological environments.
International standards such as ISO 10993 for biocompatibility and ISO 13485 for quality management systems are essential benchmarks for QD medical device manufacturers. Additionally, the International Electrotechnical Commission (IEC) has developed standards specifically addressing medical electrical equipment safety (IEC 60601 series), which applies to imaging devices incorporating quantum dot technology.
Regulatory bodies worldwide are increasingly focusing on the long-term stability of quantum dots in medical applications. The FDA's guidance on nanotechnology emphasizes the need for accelerated aging studies and stability data throughout the proposed shelf life of the device. Similarly, the European Medicines Agency (EMA) requires robust stability data for nanomaterials used in medical applications.
Environmental regulations also impact QD medical devices, particularly those containing cadmium, lead, or other heavy metals. The EU's Restriction of Hazardous Substances (RoHS) Directive and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation impose limitations on these materials, driving research toward more environmentally friendly alternatives such as indium-based or heavy metal-free quantum dots.
Emerging regulatory trends include the development of specialized frameworks for combination products that incorporate both drug and device components, which may become relevant as QD technology advances toward theranostic applications combining imaging and therapeutic capabilities.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence, post-market surveillance, and traceability. QD-based imaging technologies must comply with these regulations, with particular emphasis on biocompatibility testing and risk management due to potential toxicity concerns associated with heavy metal components in traditional quantum dots.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for nanomaterial-based medical devices, including those utilizing quantum dots. These guidelines focus on stability testing under various environmental conditions, recognizing the unique challenges posed by quantum dot degradation in biological environments.
International standards such as ISO 10993 for biocompatibility and ISO 13485 for quality management systems are essential benchmarks for QD medical device manufacturers. Additionally, the International Electrotechnical Commission (IEC) has developed standards specifically addressing medical electrical equipment safety (IEC 60601 series), which applies to imaging devices incorporating quantum dot technology.
Regulatory bodies worldwide are increasingly focusing on the long-term stability of quantum dots in medical applications. The FDA's guidance on nanotechnology emphasizes the need for accelerated aging studies and stability data throughout the proposed shelf life of the device. Similarly, the European Medicines Agency (EMA) requires robust stability data for nanomaterials used in medical applications.
Environmental regulations also impact QD medical devices, particularly those containing cadmium, lead, or other heavy metals. The EU's Restriction of Hazardous Substances (RoHS) Directive and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation impose limitations on these materials, driving research toward more environmentally friendly alternatives such as indium-based or heavy metal-free quantum dots.
Emerging regulatory trends include the development of specialized frameworks for combination products that incorporate both drug and device components, which may become relevant as QD technology advances toward theranostic applications combining imaging and therapeutic capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!