Reduce Noise in NMR Spectra with Deconvolution Techniques
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Spectroscopy Noise Reduction Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, becoming an indispensable analytical tool in chemistry, biochemistry, and medical diagnostics. The technique leverages the magnetic properties of certain atomic nuclei to provide detailed structural information about molecules. However, NMR spectra are inherently susceptible to various noise sources that can obscure valuable data and complicate interpretation.
The evolution of NMR technology has been marked by continuous efforts to enhance signal quality and reduce noise interference. Early NMR instruments operated at low magnetic field strengths, resulting in limited resolution and significant noise challenges. The introduction of superconducting magnets in the 1960s and subsequent advances in hardware design have progressively improved signal-to-noise ratios, yet noise remains a persistent challenge in modern applications.
Noise in NMR spectra originates from multiple sources, including thermal noise from electronic components, environmental electromagnetic interference, sample impurities, and inherent quantum mechanical limitations. These noise factors become particularly problematic in applications requiring high sensitivity, such as metabolomics, protein structure determination, and medical imaging, where weak signals must be distinguished from background noise.
Deconvolution techniques represent a mathematical approach to noise reduction that has gained significant traction in recent years. These methods aim to separate overlapping signals and extract meaningful information from noisy spectra through computational processing. The advancement of these techniques has paralleled developments in computing power and algorithm sophistication, enabling increasingly effective noise reduction strategies.
The primary technical objectives for noise reduction in NMR spectroscopy using deconvolution techniques include: enhancing spectral resolution to distinguish closely spaced signals; improving detection limits for low-concentration analytes; preserving quantitative accuracy for reliable concentration measurements; and developing automated processing workflows that reduce subjective interpretation biases.
Recent trends indicate a shift toward machine learning and artificial intelligence approaches for noise reduction, which can adapt to specific noise patterns and spectral characteristics. These emerging methodologies promise to overcome limitations of traditional deconvolution techniques by learning optimal noise reduction parameters from large datasets of spectra.
The ultimate goal of this technical research is to develop robust, adaptable deconvolution methodologies that can effectively reduce noise across diverse NMR applications while maintaining spectral integrity. Success in this domain would significantly expand the utility of NMR spectroscopy in challenging environments and for complex samples, potentially enabling new applications in fields ranging from pharmaceutical development to clinical diagnostics.
The evolution of NMR technology has been marked by continuous efforts to enhance signal quality and reduce noise interference. Early NMR instruments operated at low magnetic field strengths, resulting in limited resolution and significant noise challenges. The introduction of superconducting magnets in the 1960s and subsequent advances in hardware design have progressively improved signal-to-noise ratios, yet noise remains a persistent challenge in modern applications.
Noise in NMR spectra originates from multiple sources, including thermal noise from electronic components, environmental electromagnetic interference, sample impurities, and inherent quantum mechanical limitations. These noise factors become particularly problematic in applications requiring high sensitivity, such as metabolomics, protein structure determination, and medical imaging, where weak signals must be distinguished from background noise.
Deconvolution techniques represent a mathematical approach to noise reduction that has gained significant traction in recent years. These methods aim to separate overlapping signals and extract meaningful information from noisy spectra through computational processing. The advancement of these techniques has paralleled developments in computing power and algorithm sophistication, enabling increasingly effective noise reduction strategies.
The primary technical objectives for noise reduction in NMR spectroscopy using deconvolution techniques include: enhancing spectral resolution to distinguish closely spaced signals; improving detection limits for low-concentration analytes; preserving quantitative accuracy for reliable concentration measurements; and developing automated processing workflows that reduce subjective interpretation biases.
Recent trends indicate a shift toward machine learning and artificial intelligence approaches for noise reduction, which can adapt to specific noise patterns and spectral characteristics. These emerging methodologies promise to overcome limitations of traditional deconvolution techniques by learning optimal noise reduction parameters from large datasets of spectra.
The ultimate goal of this technical research is to develop robust, adaptable deconvolution methodologies that can effectively reduce noise across diverse NMR applications while maintaining spectral integrity. Success in this domain would significantly expand the utility of NMR spectroscopy in challenging environments and for complex samples, potentially enabling new applications in fields ranging from pharmaceutical development to clinical diagnostics.
Market Applications and Demand for High-Resolution NMR
Nuclear Magnetic Resonance (NMR) spectroscopy with enhanced resolution capabilities has witnessed significant market growth across multiple sectors. The global NMR market, valued at approximately $2.3 billion in 2022, is projected to reach $3.5 billion by 2028, with high-resolution applications driving substantial portions of this expansion.
The pharmaceutical industry represents the largest market segment for high-resolution NMR, accounting for nearly 40% of the total market share. Drug discovery and development processes heavily rely on precise molecular structure determination, where noise reduction through deconvolution techniques has become essential for identifying novel therapeutic compounds and understanding drug-target interactions at the molecular level.
Biotechnology companies increasingly demand advanced NMR solutions for protein structure analysis and metabolomics studies. The ability to obtain clean, high-resolution spectra directly impacts research outcomes in areas such as enzyme engineering and biomarker discovery, creating a specialized market segment growing at 8.7% annually.
Academic and research institutions constitute another significant market, particularly as funding for structural biology and materials science increases. These institutions often serve as early adopters of innovative deconvolution algorithms and noise reduction methodologies, establishing proof-of-concept before commercial implementation.
The food and beverage industry has emerged as a rapidly expanding application area, utilizing high-resolution NMR for authentication of premium products, detection of adulterants, and quality control. Companies are willing to invest in advanced analytical technologies that can provide definitive chemical fingerprints of their products, driving demand for noise-reduced spectral analysis.
Environmental monitoring represents a growing market opportunity, with regulatory agencies and testing laboratories requiring increasingly sensitive detection of contaminants in soil, water, and air samples. The ability to identify trace compounds in complex environmental matrices depends critically on effective noise reduction techniques.
The petrochemical industry continues to be a stable market for high-resolution NMR, using it for composition analysis of complex hydrocarbon mixtures and quality control in production processes. Enhanced spectral resolution directly translates to improved process efficiency and product quality.
Market trends indicate increasing demand for automated data processing solutions that incorporate advanced deconvolution algorithms, with particular emphasis on cloud-based platforms that can handle large spectral datasets. This shift reflects the broader industry movement toward integrated analytical workflows and data-driven decision making across all application sectors.
The pharmaceutical industry represents the largest market segment for high-resolution NMR, accounting for nearly 40% of the total market share. Drug discovery and development processes heavily rely on precise molecular structure determination, where noise reduction through deconvolution techniques has become essential for identifying novel therapeutic compounds and understanding drug-target interactions at the molecular level.
Biotechnology companies increasingly demand advanced NMR solutions for protein structure analysis and metabolomics studies. The ability to obtain clean, high-resolution spectra directly impacts research outcomes in areas such as enzyme engineering and biomarker discovery, creating a specialized market segment growing at 8.7% annually.
Academic and research institutions constitute another significant market, particularly as funding for structural biology and materials science increases. These institutions often serve as early adopters of innovative deconvolution algorithms and noise reduction methodologies, establishing proof-of-concept before commercial implementation.
The food and beverage industry has emerged as a rapidly expanding application area, utilizing high-resolution NMR for authentication of premium products, detection of adulterants, and quality control. Companies are willing to invest in advanced analytical technologies that can provide definitive chemical fingerprints of their products, driving demand for noise-reduced spectral analysis.
Environmental monitoring represents a growing market opportunity, with regulatory agencies and testing laboratories requiring increasingly sensitive detection of contaminants in soil, water, and air samples. The ability to identify trace compounds in complex environmental matrices depends critically on effective noise reduction techniques.
The petrochemical industry continues to be a stable market for high-resolution NMR, using it for composition analysis of complex hydrocarbon mixtures and quality control in production processes. Enhanced spectral resolution directly translates to improved process efficiency and product quality.
Market trends indicate increasing demand for automated data processing solutions that incorporate advanced deconvolution algorithms, with particular emphasis on cloud-based platforms that can handle large spectral datasets. This shift reflects the broader industry movement toward integrated analytical workflows and data-driven decision making across all application sectors.
Current Challenges in NMR Signal Processing
Nuclear Magnetic Resonance (NMR) spectroscopy represents one of the most powerful analytical techniques in modern chemistry, biochemistry, and materials science. However, despite its widespread application, NMR signal processing continues to face significant challenges that limit its full potential. The primary obstacle remains the inherently low signal-to-noise ratio (SNR) characteristic of NMR experiments, particularly when analyzing samples with low concentration or nuclei with low natural abundance.
Traditional noise reduction methods such as signal averaging require extended acquisition times, which may not be feasible for unstable samples or time-sensitive experiments. Additionally, the presence of overlapping peaks in complex mixtures creates substantial difficulties in accurate peak identification and quantification, especially in biomolecular NMR where spectral congestion is common.
Hardware limitations contribute significantly to current challenges. Magnetic field inhomogeneities introduce line broadening and phase distortions that complicate spectral interpretation. Even with advanced shimming techniques, achieving perfect field homogeneity remains elusive, particularly for heterogeneous samples or those with susceptibility variations.
Digital signal processing artifacts represent another critical challenge. The discrete Fourier transform, fundamental to NMR data processing, introduces truncation artifacts and can exacerbate noise-related issues. Window functions applied to reduce these artifacts often come at the cost of decreased spectral resolution, creating a difficult trade-off for analysts.
Phase correction presents persistent difficulties, especially for complex spectra with overlapping resonances. Manual phase correction remains subjective and time-consuming, while automated algorithms may fail for spectra with significant baseline distortions or phase-shifted multiplets.
Baseline distortions, arising from instrumental imperfections, broad resonances from macromolecules, or solvent effects, complicate accurate integration and quantification. Current correction methods often require manual intervention and may introduce artificial features that affect data interpretation.
The increasing dimensionality of modern NMR experiments (3D, 4D, and beyond) exponentially increases data complexity and processing requirements. Handling these multidimensional datasets demands sophisticated algorithms and substantial computational resources, creating bottlenecks in data analysis workflows.
Non-uniform sampling (NUS) techniques, while reducing acquisition times, introduce reconstruction artifacts that require advanced processing methods. The optimal balance between sampling sparsity and spectral quality remains an active area of research, with no universally accepted solution.
Finally, the lack of standardized processing protocols across different NMR platforms and laboratories hampers reproducibility and data sharing. Variations in processing parameters can lead to significantly different spectral appearances and interpretations, complicating multi-center studies and meta-analyses.
Traditional noise reduction methods such as signal averaging require extended acquisition times, which may not be feasible for unstable samples or time-sensitive experiments. Additionally, the presence of overlapping peaks in complex mixtures creates substantial difficulties in accurate peak identification and quantification, especially in biomolecular NMR where spectral congestion is common.
Hardware limitations contribute significantly to current challenges. Magnetic field inhomogeneities introduce line broadening and phase distortions that complicate spectral interpretation. Even with advanced shimming techniques, achieving perfect field homogeneity remains elusive, particularly for heterogeneous samples or those with susceptibility variations.
Digital signal processing artifacts represent another critical challenge. The discrete Fourier transform, fundamental to NMR data processing, introduces truncation artifacts and can exacerbate noise-related issues. Window functions applied to reduce these artifacts often come at the cost of decreased spectral resolution, creating a difficult trade-off for analysts.
Phase correction presents persistent difficulties, especially for complex spectra with overlapping resonances. Manual phase correction remains subjective and time-consuming, while automated algorithms may fail for spectra with significant baseline distortions or phase-shifted multiplets.
Baseline distortions, arising from instrumental imperfections, broad resonances from macromolecules, or solvent effects, complicate accurate integration and quantification. Current correction methods often require manual intervention and may introduce artificial features that affect data interpretation.
The increasing dimensionality of modern NMR experiments (3D, 4D, and beyond) exponentially increases data complexity and processing requirements. Handling these multidimensional datasets demands sophisticated algorithms and substantial computational resources, creating bottlenecks in data analysis workflows.
Non-uniform sampling (NUS) techniques, while reducing acquisition times, introduce reconstruction artifacts that require advanced processing methods. The optimal balance between sampling sparsity and spectral quality remains an active area of research, with no universally accepted solution.
Finally, the lack of standardized processing protocols across different NMR platforms and laboratories hampers reproducibility and data sharing. Variations in processing parameters can lead to significantly different spectral appearances and interpretations, complicating multi-center studies and meta-analyses.
State-of-the-Art Deconvolution Algorithms
01 Signal processing algorithms for NMR noise reduction
Various signal processing algorithms can be applied to NMR spectra to reduce noise and enhance signal quality. These techniques include digital filtering, wavelet transforms, and advanced mathematical models that can separate noise from meaningful signals. By implementing these algorithms, researchers can achieve cleaner spectra with improved signal-to-noise ratios, making it easier to identify and quantify chemical compounds in complex mixtures.- Signal processing algorithms for NMR noise reduction: Various signal processing algorithms can be applied to NMR spectra to reduce noise and improve signal quality. These techniques include digital filtering, wavelet transforms, and Fourier-based methods that can separate signal from noise components. Advanced computational approaches help in enhancing spectral resolution by minimizing baseline distortions and random fluctuations, resulting in cleaner spectra for more accurate analysis and interpretation.
- Hardware-based noise suppression techniques: Hardware modifications and improvements in NMR instrumentation can significantly reduce noise at the acquisition stage. These include optimized probe designs, improved RF coils, cryogenic cooling systems, and advanced shielding techniques. Hardware-based approaches focus on minimizing electronic noise, thermal noise, and environmental interferences before digital processing, providing cleaner raw data for subsequent deconvolution and analysis.
- Machine learning and AI for spectral deconvolution: Machine learning and artificial intelligence techniques are increasingly applied to NMR spectral deconvolution to handle complex noise patterns. These approaches use neural networks, deep learning algorithms, and pattern recognition to distinguish between meaningful signals and noise. AI-based methods can adaptively learn from datasets to improve deconvolution accuracy, especially in complex mixtures where traditional methods may fail due to overlapping peaks or variable noise profiles.
- Time-domain deconvolution methods: Time-domain approaches to NMR deconvolution work directly with the free induction decay (FID) signal before Fourier transformation. These methods include maximum entropy reconstruction, linear prediction, and parametric modeling techniques that can effectively separate overlapping resonances and reduce noise artifacts. By addressing noise in the time domain, these approaches can preserve signal characteristics that might otherwise be distorted during frequency-domain processing.
- Multi-dimensional NMR deconvolution techniques: Specialized deconvolution methods for multi-dimensional NMR data address the unique challenges of noise in higher-dimensional spectra. These techniques include non-uniform sampling, projection reconstruction, and covariance processing that can effectively reduce noise while maintaining spectral resolution. Multi-dimensional approaches exploit correlations between different spectral dimensions to distinguish genuine signals from random noise, enabling more accurate analysis of complex molecular structures.
02 Hardware-based noise reduction techniques
Hardware modifications and improvements can significantly reduce noise in NMR spectroscopy. These include optimized probe designs, improved RF coils, cryogenic cooling systems, and advanced shielding techniques. Such hardware enhancements minimize environmental interference and electronic noise, resulting in cleaner baseline signals and improved spectral resolution, which is particularly valuable when analyzing samples with low concentration analytes.Expand Specific Solutions03 Automated deconvolution methods for overlapping peaks
Automated deconvolution methods help separate overlapping peaks in complex NMR spectra. These techniques employ mathematical models and algorithms to identify and resolve individual spectral components that would otherwise appear as merged signals. Machine learning and artificial intelligence approaches can further enhance these methods by recognizing patterns in spectral data. Such automated approaches improve the accuracy of quantitative analysis and structural elucidation in complex mixtures.Expand Specific Solutions04 Time-domain signal processing for NMR deconvolution
Time-domain signal processing techniques focus on analyzing and manipulating the free induction decay (FID) signal before Fourier transformation. These methods include linear prediction, maximum entropy methods, and other time-domain fitting algorithms that can extract more information from the raw data. By processing signals in the time domain, these techniques can effectively reduce noise, enhance resolution, and recover signals that might be lost in conventional frequency-domain analysis.Expand Specific Solutions05 Multi-dimensional NMR deconvolution techniques
Multi-dimensional NMR deconvolution techniques address the challenges of analyzing complex 2D, 3D, or higher-dimensional NMR data. These methods employ specialized algorithms designed to handle the increased complexity and data volume of multi-dimensional experiments. By effectively separating overlapping signals across multiple dimensions, these techniques provide enhanced structural information and improved spectral resolution, particularly valuable for analyzing complex biomolecules like proteins and nucleic acids.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The NMR spectra deconvolution technology market is currently in a growth phase, with increasing adoption across pharmaceutical, chemical, and medical research sectors. The global market size for advanced NMR analysis techniques is estimated at $1.2-1.5 billion, expanding at approximately 5-7% annually. Technologically, the field shows varying maturity levels, with established players like Bruker and JEOL leading commercial applications while academic institutions drive innovation. Jilin University, Zhejiang University, and University of Virginia are advancing fundamental algorithmic approaches, while companies like Huawei and Samsung Electronics contribute computational solutions. Medical-focused entities including Mayo Foundation and Shanghai United Imaging Intelligence are adapting these techniques for clinical applications. The convergence of traditional NMR expertise with AI-driven signal processing represents the frontier of development in this space.
Jilin University
Technical Solution: Jilin University has developed innovative deconvolution techniques for NMR spectroscopy focusing on solid-state NMR applications. Their approach centers on a hybrid methodology combining maximum entropy methods with iterative time-domain deconvolution. The university's research team has created algorithms that specifically address the challenges of overlapping peaks and baseline distortions common in complex biological samples. Their technique implements a two-stage process: first applying wavelet transforms to separate noise from signal components across multiple scales, then employing constrained deconvolution that preserves known physical constraints of the spectral data[2]. Jilin's method incorporates reference deconvolution using internal standards to correct for instrumental imperfections, particularly effective for removing artifacts in heteronuclear correlation experiments. Their algorithms utilize statistical validation approaches to optimize deconvolution parameters automatically, reducing user bias and improving reproducibility[5]. The university has demonstrated particular success in applications involving protein structure determination and metabolomics, where signal overlap and noise present significant challenges to accurate interpretation.
Strengths: Particularly effective for solid-state NMR applications where noise challenges are most severe; strong theoretical foundation based on fundamental NMR physics; extensively validated through peer-reviewed research. Weaknesses: Implementation requires significant NMR expertise; primarily developed for research rather than routine clinical applications; may require customization for specific experimental setups.
Koninklijke Philips NV
Technical Solution: Philips has pioneered advanced deconvolution techniques for NMR spectroscopy that focus on clinical applications. Their approach combines traditional mathematical deconvolution with artificial intelligence to enhance spectral quality. The company's proprietary algorithms implement a multi-stage deconvolution process that first identifies noise characteristics through statistical analysis, then applies targeted filtering techniques to remove specific noise types while preserving signal integrity. Philips' solution incorporates reference deconvolution methods that utilize internal reference standards to correct for instrumental imperfections and environmental variations[2]. Their technology employs wavelet-based decomposition to separate noise from signal across different frequency scales, allowing for more precise noise identification and removal. The system also features adaptive processing parameters that automatically adjust based on sample characteristics and acquisition conditions, optimizing noise reduction for each specific application[4]. Philips has integrated these deconvolution techniques into their clinical NMR systems to improve diagnostic accuracy.
Strengths: Highly optimized for clinical diagnostic applications; seamless integration with existing Philips MR systems; extensive validation in medical settings ensures reliability. Weaknesses: Proprietary nature limits customization for research applications; primarily designed for specific Philips hardware configurations; computational demands may require dedicated processing hardware.
Key Patents and Publications in NMR Signal Enhancement
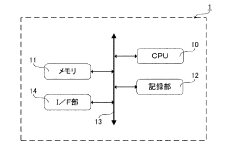
Measurement system and noise reduction method for nmr spectra
PatentActiveJPWO2016098845A1
Innovation
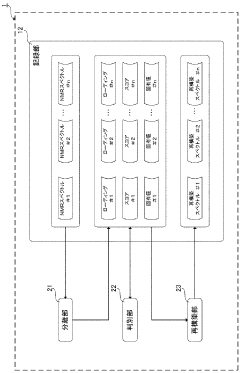
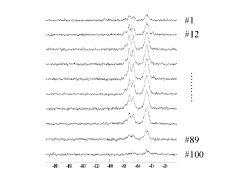
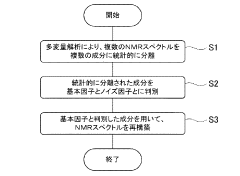
- A noise reduction method involving multivariate analysis, such as principal component analysis, to separate and discriminate effective components from noise components in NMR spectra, reconstructing the spectrum with reduced noise.
Method for removing noise from nuclear magnetic resonance signals and images
PatentInactiveUS7253627B1
Innovation
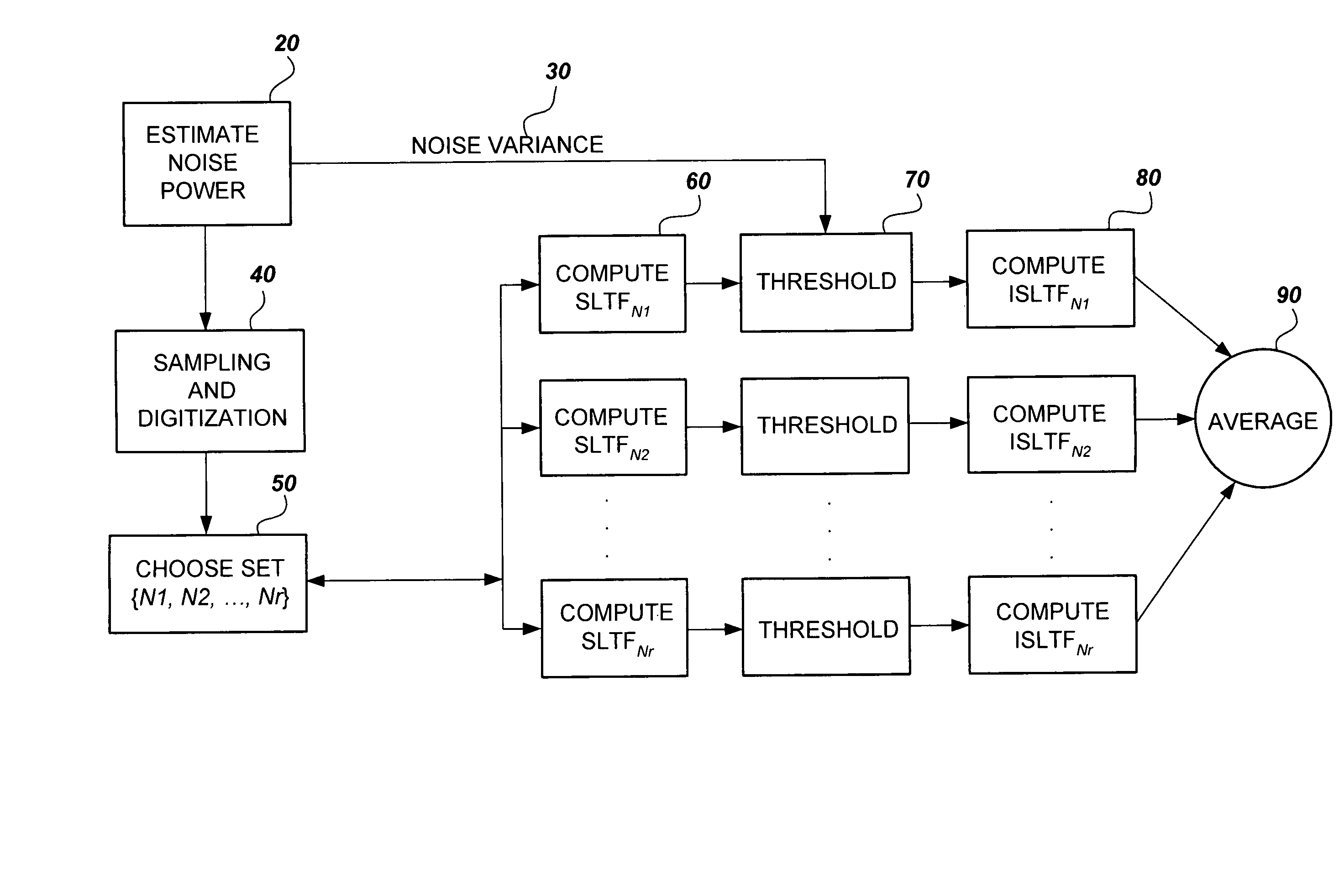
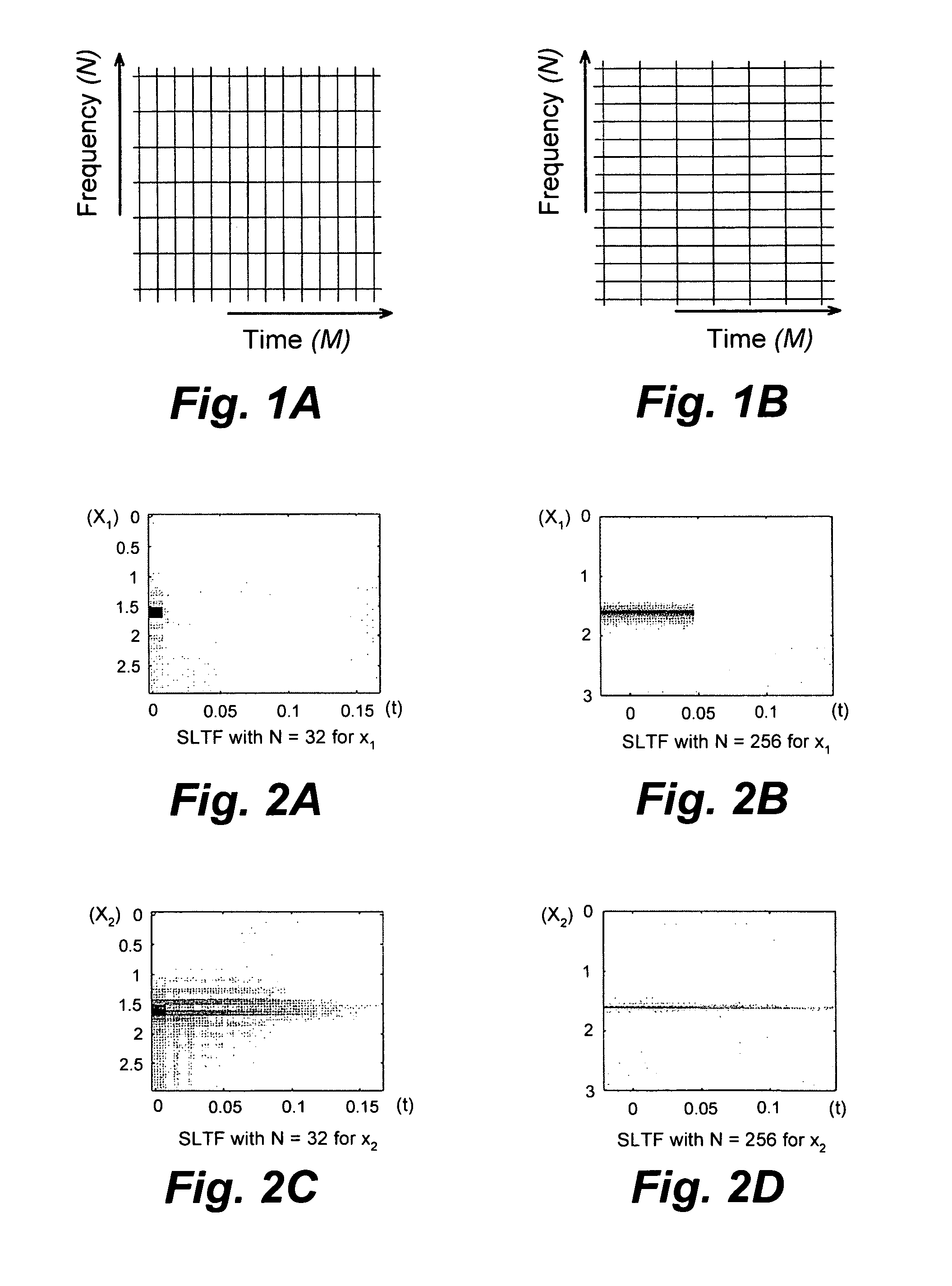
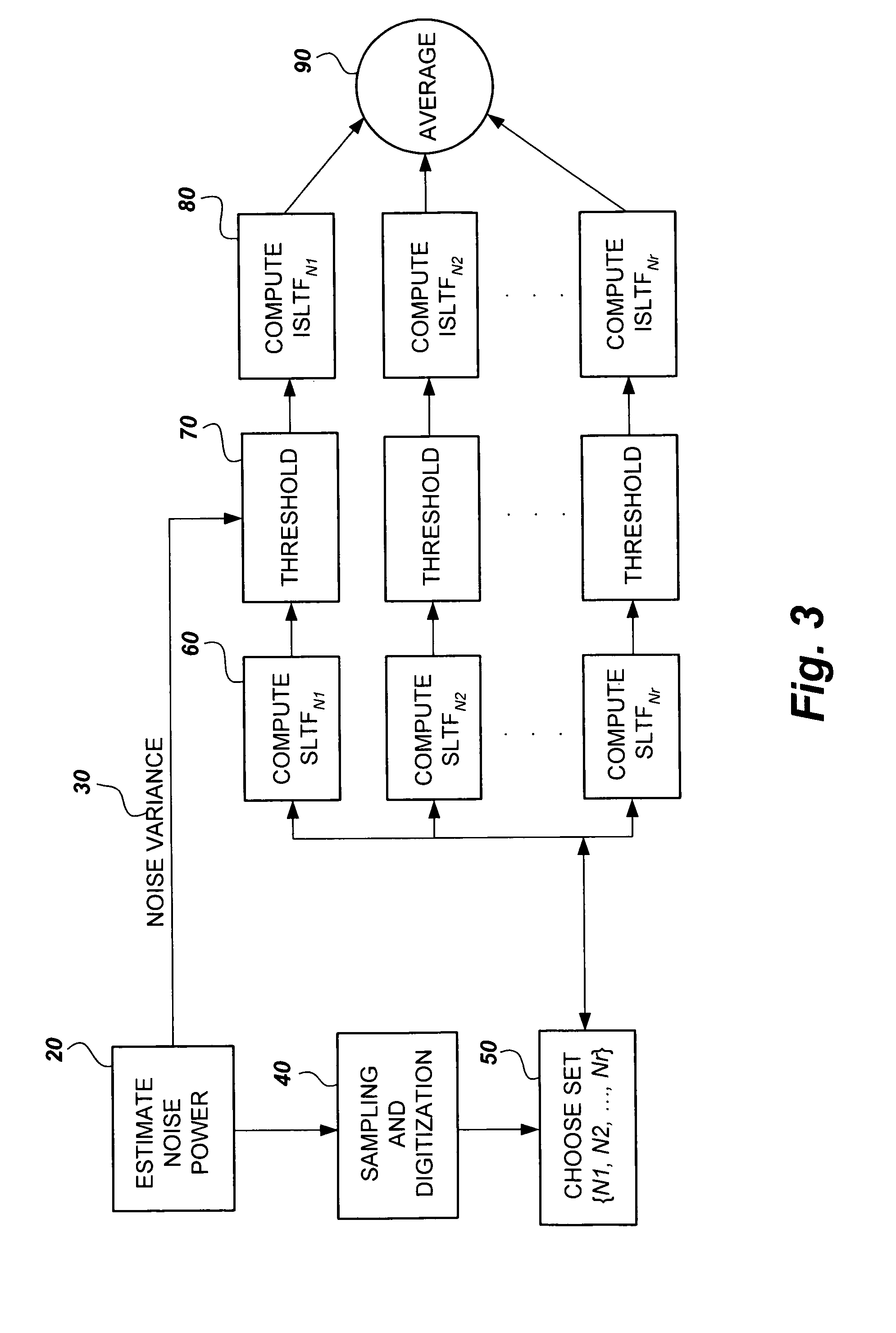
- The method employs stable linear time-frequency (SLTF) transforms to denoise NMR signals by calculating noise variance, applying thresholding techniques to transform coefficients, and averaging denoised signals across multiple SLTF domains to enhance signal-to-noise ratio (SNR) without extending acquisition time.
Hardware-Software Integration for Optimal NMR Performance
The integration of hardware and software components represents a critical frontier in optimizing NMR spectroscopy performance, particularly when implementing deconvolution techniques for noise reduction. Modern NMR systems require sophisticated coordination between physical instrumentation and computational algorithms to achieve optimal signal processing outcomes.
Hardware considerations begin with the magnet system itself, where stability and field homogeneity directly impact spectral quality. Advanced superconducting magnets with field strengths of 600-1000 MHz provide the foundation for high-resolution spectra, while cryogenic probe technology has dramatically improved signal-to-noise ratios by reducing thermal noise at the detection stage.
Signal acquisition hardware, including analog-to-digital converters (ADCs), must operate with sufficient sampling rates and bit depths to capture the full dynamic range of NMR signals without introducing quantization noise. Latest-generation ADCs with 24-bit resolution and sampling rates exceeding 5 MHz have become essential for preserving signal fidelity prior to deconvolution processing.
On the software side, real-time processing capabilities have evolved to handle increasingly complex deconvolution algorithms. Modern NMR consoles incorporate field-programmable gate arrays (FPGAs) and dedicated digital signal processors that can implement sophisticated filtering and deconvolution operations during acquisition, rather than solely in post-processing.
The interface between hardware and software components requires careful optimization of data transfer protocols. High-speed interfaces utilizing PCIe 4.0 or similar technologies ensure that data bottlenecks do not compromise the performance of deconvolution algorithms, which often require iterative processing of large datasets.
Calibration systems represent another critical integration point, where software routines must accurately characterize hardware performance parameters to inform deconvolution models. Automated shimming procedures, for example, provide essential information about field inhomogeneities that can be incorporated into deconvolution algorithms.
Recent innovations include integrated machine learning approaches that adaptively optimize hardware parameters based on real-time analysis of spectral quality. These systems can dynamically adjust gradient strengths, pulse sequences, and receiver gain settings to maximize the effectiveness of subsequent deconvolution processing.
The development of standardized APIs and hardware abstraction layers has facilitated more seamless integration between vendor-specific hardware and third-party deconvolution software. This ecosystem approach allows specialized deconvolution techniques to be deployed across diverse hardware platforms while maintaining consistent performance characteristics.
Hardware considerations begin with the magnet system itself, where stability and field homogeneity directly impact spectral quality. Advanced superconducting magnets with field strengths of 600-1000 MHz provide the foundation for high-resolution spectra, while cryogenic probe technology has dramatically improved signal-to-noise ratios by reducing thermal noise at the detection stage.
Signal acquisition hardware, including analog-to-digital converters (ADCs), must operate with sufficient sampling rates and bit depths to capture the full dynamic range of NMR signals without introducing quantization noise. Latest-generation ADCs with 24-bit resolution and sampling rates exceeding 5 MHz have become essential for preserving signal fidelity prior to deconvolution processing.
On the software side, real-time processing capabilities have evolved to handle increasingly complex deconvolution algorithms. Modern NMR consoles incorporate field-programmable gate arrays (FPGAs) and dedicated digital signal processors that can implement sophisticated filtering and deconvolution operations during acquisition, rather than solely in post-processing.
The interface between hardware and software components requires careful optimization of data transfer protocols. High-speed interfaces utilizing PCIe 4.0 or similar technologies ensure that data bottlenecks do not compromise the performance of deconvolution algorithms, which often require iterative processing of large datasets.
Calibration systems represent another critical integration point, where software routines must accurately characterize hardware performance parameters to inform deconvolution models. Automated shimming procedures, for example, provide essential information about field inhomogeneities that can be incorporated into deconvolution algorithms.
Recent innovations include integrated machine learning approaches that adaptively optimize hardware parameters based on real-time analysis of spectral quality. These systems can dynamically adjust gradient strengths, pulse sequences, and receiver gain settings to maximize the effectiveness of subsequent deconvolution processing.
The development of standardized APIs and hardware abstraction layers has facilitated more seamless integration between vendor-specific hardware and third-party deconvolution software. This ecosystem approach allows specialized deconvolution techniques to be deployed across diverse hardware platforms while maintaining consistent performance characteristics.
Validation Metrics and Quality Control Standards
Validation of deconvolution techniques for NMR spectra noise reduction requires robust metrics and quality control standards to ensure reliability and reproducibility. Signal-to-Noise Ratio (SNR) serves as a fundamental metric, quantifying the improvement in spectral quality by comparing pre- and post-deconvolution SNR values. Effective deconvolution should demonstrate at least a 30-50% enhancement in SNR without introducing artificial features.
Resolution Enhancement Factor (REF) measures the ability to distinguish closely spaced peaks, calculated by comparing peak width at half-maximum before and after processing. Industry standards typically require a minimum REF of 1.5 for clinical applications and 2.0 for research-grade analyses, with higher values indicating superior performance.
Residual analysis provides critical insights by examining the difference between original and reconstructed spectra. The residual should exhibit random noise characteristics without systematic patterns, with root mean square deviation (RMSD) values below 5% of the maximum signal intensity for acceptable deconvolution quality.
Peak position accuracy represents another essential validation parameter, with maximum allowable deviations of ±0.01 ppm for quantitative applications. This ensures that deconvolution does not introduce artificial shifts that could lead to misidentification of chemical compounds.
Reproducibility testing must be conducted through multiple iterations with varying noise levels, demonstrating consistent results within 95% confidence intervals. This typically involves Monte Carlo simulations with synthetic noise addition to evaluate algorithm stability under different conditions.
For clinical and pharmaceutical applications, regulatory bodies including FDA and EMA have established specific validation protocols requiring documentation of algorithm parameters, validation datasets, and performance metrics. These standards mandate that deconvolution methods demonstrate at least 98% specificity and 95% sensitivity in peak detection compared to manual expert analysis.
Benchmark datasets containing spectra with known ground truth have been developed by organizations such as NIST and IUPAC, providing standardized testing platforms for algorithm validation. Performance on these datasets allows for objective comparison between different deconvolution approaches and establishes minimum quality thresholds for specific applications.
Resolution Enhancement Factor (REF) measures the ability to distinguish closely spaced peaks, calculated by comparing peak width at half-maximum before and after processing. Industry standards typically require a minimum REF of 1.5 for clinical applications and 2.0 for research-grade analyses, with higher values indicating superior performance.
Residual analysis provides critical insights by examining the difference between original and reconstructed spectra. The residual should exhibit random noise characteristics without systematic patterns, with root mean square deviation (RMSD) values below 5% of the maximum signal intensity for acceptable deconvolution quality.
Peak position accuracy represents another essential validation parameter, with maximum allowable deviations of ±0.01 ppm for quantitative applications. This ensures that deconvolution does not introduce artificial shifts that could lead to misidentification of chemical compounds.
Reproducibility testing must be conducted through multiple iterations with varying noise levels, demonstrating consistent results within 95% confidence intervals. This typically involves Monte Carlo simulations with synthetic noise addition to evaluate algorithm stability under different conditions.
For clinical and pharmaceutical applications, regulatory bodies including FDA and EMA have established specific validation protocols requiring documentation of algorithm parameters, validation datasets, and performance metrics. These standards mandate that deconvolution methods demonstrate at least 98% specificity and 95% sensitivity in peak detection compared to manual expert analysis.
Benchmark datasets containing spectra with known ground truth have been developed by organizations such as NIST and IUPAC, providing standardized testing platforms for algorithm validation. Performance on these datasets allows for objective comparison between different deconvolution approaches and establishes minimum quality thresholds for specific applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!