Regulatory Standards for Metal Oxide TFTs in Medical Devices
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Oxide TFT Technology Background and Objectives
Metal oxide thin-film transistors (TFTs) have emerged as a transformative technology in the electronics industry over the past two decades. Initially developed as an alternative to amorphous silicon (a-Si) TFTs, metal oxide semiconductors—particularly those based on indium gallium zinc oxide (IGZO)—have demonstrated superior electron mobility, stability, and transparency. The evolution of this technology began in the early 2000s with pioneering research at institutions in Japan and Korea, gradually progressing from laboratory curiosities to commercial applications primarily in display technologies.
The trajectory of metal oxide TFT development has been characterized by continuous improvements in material composition, deposition techniques, and device architectures. Early iterations faced challenges with stability and uniformity, but recent advancements have yielded devices with remarkable performance characteristics, including electron mobility exceeding 10 cm²/Vs, low off-state current, and excellent bias stress stability—properties particularly valuable for medical device applications.
In the medical device sector, metal oxide TFTs present unique opportunities for creating flexible, transparent, and biocompatible electronic interfaces. These properties enable the development of advanced patient monitoring systems, implantable devices, and diagnostic tools with enhanced functionality and reduced form factors. The inherent low-temperature processing compatibility of these materials further facilitates integration with temperature-sensitive substrates and components common in medical applications.
The primary technical objectives for metal oxide TFTs in medical devices center on several critical parameters. First, ensuring biocompatibility and long-term stability in physiological environments represents a fundamental requirement for any material interfacing with biological systems. Second, achieving ultra-low power consumption is essential for implantable and wearable medical technologies with limited energy resources. Third, developing robust encapsulation methods to protect these devices from bodily fluids while maintaining their electrical performance presents a significant engineering challenge.
Additionally, the technology aims to enable high-resolution sensing capabilities through improved signal-to-noise ratios and reduced pixel sizes in imaging arrays. For applications such as X-ray detectors and biosensors, enhancing the sensitivity and response time of metal oxide TFT-based systems remains a priority. The ultimate goal involves creating fully integrated systems that combine sensing, signal processing, and wireless communication capabilities on flexible substrates.
The regulatory landscape surrounding these devices adds another dimension to technology development objectives. As medical applications demand stringent safety and reliability standards, metal oxide TFT technology must evolve to meet specific regulatory requirements including biocompatibility testing protocols, electrical safety standards, and long-term reliability metrics defined by organizations such as the FDA, ISO, and IEC.
The trajectory of metal oxide TFT development has been characterized by continuous improvements in material composition, deposition techniques, and device architectures. Early iterations faced challenges with stability and uniformity, but recent advancements have yielded devices with remarkable performance characteristics, including electron mobility exceeding 10 cm²/Vs, low off-state current, and excellent bias stress stability—properties particularly valuable for medical device applications.
In the medical device sector, metal oxide TFTs present unique opportunities for creating flexible, transparent, and biocompatible electronic interfaces. These properties enable the development of advanced patient monitoring systems, implantable devices, and diagnostic tools with enhanced functionality and reduced form factors. The inherent low-temperature processing compatibility of these materials further facilitates integration with temperature-sensitive substrates and components common in medical applications.
The primary technical objectives for metal oxide TFTs in medical devices center on several critical parameters. First, ensuring biocompatibility and long-term stability in physiological environments represents a fundamental requirement for any material interfacing with biological systems. Second, achieving ultra-low power consumption is essential for implantable and wearable medical technologies with limited energy resources. Third, developing robust encapsulation methods to protect these devices from bodily fluids while maintaining their electrical performance presents a significant engineering challenge.
Additionally, the technology aims to enable high-resolution sensing capabilities through improved signal-to-noise ratios and reduced pixel sizes in imaging arrays. For applications such as X-ray detectors and biosensors, enhancing the sensitivity and response time of metal oxide TFT-based systems remains a priority. The ultimate goal involves creating fully integrated systems that combine sensing, signal processing, and wireless communication capabilities on flexible substrates.
The regulatory landscape surrounding these devices adds another dimension to technology development objectives. As medical applications demand stringent safety and reliability standards, metal oxide TFT technology must evolve to meet specific regulatory requirements including biocompatibility testing protocols, electrical safety standards, and long-term reliability metrics defined by organizations such as the FDA, ISO, and IEC.
Medical Device Market Requirements Analysis
The medical device market for metal oxide TFTs is experiencing significant growth, driven by increasing demand for advanced diagnostic and monitoring equipment. The global medical device market is projected to reach $612.7 billion by 2025, with a compound annual growth rate of 5.4%. Within this broader market, devices incorporating thin-film transistor technology represent a rapidly expanding segment, particularly in imaging systems, patient monitoring devices, and portable diagnostic equipment.
Medical device manufacturers are increasingly seeking components that can deliver enhanced performance while meeting stringent regulatory requirements. Metal oxide TFTs offer several advantages that align with these market demands, including higher electron mobility, better stability, and improved transparency compared to conventional amorphous silicon alternatives. These properties make them particularly suitable for medical imaging applications where high resolution and reliability are paramount.
The market requirements for metal oxide TFTs in medical devices are multifaceted. Durability and reliability stand as primary concerns, with devices expected to maintain consistent performance over extended operational lifespans, often exceeding 5-7 years in clinical settings. Additionally, medical environments demand materials that can withstand repeated sterilization procedures without degradation of performance characteristics.
Biocompatibility represents another critical market requirement. As these components may be incorporated into devices with direct or indirect patient contact, manufacturers must ensure materials comply with ISO 10993 standards for biocompatibility. This includes testing for cytotoxicity, sensitization, and irritation potential of all materials used in the fabrication process.
Energy efficiency has emerged as an increasingly important market driver, particularly for portable and wearable medical devices. Metal oxide TFTs offer lower power consumption compared to traditional technologies, extending battery life and reducing heat generation—both crucial factors in medical applications where patient comfort and device reliability are essential.
The market also demands scalable manufacturing processes that can maintain consistent quality while meeting production volume requirements. As healthcare systems globally push for cost containment, manufacturers seek technologies that can be produced efficiently without compromising performance or regulatory compliance.
Interoperability with existing medical systems represents another significant market requirement. New devices incorporating metal oxide TFT technology must seamlessly integrate with hospital information systems, electronic health records, and other medical equipment. This necessitates standardized interfaces and communication protocols that comply with healthcare IT standards such as HL7 and DICOM.
Medical device manufacturers are increasingly seeking components that can deliver enhanced performance while meeting stringent regulatory requirements. Metal oxide TFTs offer several advantages that align with these market demands, including higher electron mobility, better stability, and improved transparency compared to conventional amorphous silicon alternatives. These properties make them particularly suitable for medical imaging applications where high resolution and reliability are paramount.
The market requirements for metal oxide TFTs in medical devices are multifaceted. Durability and reliability stand as primary concerns, with devices expected to maintain consistent performance over extended operational lifespans, often exceeding 5-7 years in clinical settings. Additionally, medical environments demand materials that can withstand repeated sterilization procedures without degradation of performance characteristics.
Biocompatibility represents another critical market requirement. As these components may be incorporated into devices with direct or indirect patient contact, manufacturers must ensure materials comply with ISO 10993 standards for biocompatibility. This includes testing for cytotoxicity, sensitization, and irritation potential of all materials used in the fabrication process.
Energy efficiency has emerged as an increasingly important market driver, particularly for portable and wearable medical devices. Metal oxide TFTs offer lower power consumption compared to traditional technologies, extending battery life and reducing heat generation—both crucial factors in medical applications where patient comfort and device reliability are essential.
The market also demands scalable manufacturing processes that can maintain consistent quality while meeting production volume requirements. As healthcare systems globally push for cost containment, manufacturers seek technologies that can be produced efficiently without compromising performance or regulatory compliance.
Interoperability with existing medical systems represents another significant market requirement. New devices incorporating metal oxide TFT technology must seamlessly integrate with hospital information systems, electronic health records, and other medical equipment. This necessitates standardized interfaces and communication protocols that comply with healthcare IT standards such as HL7 and DICOM.
Current Status and Challenges of Metal Oxide TFTs
Metal oxide thin-film transistors (TFTs) have emerged as promising candidates for various medical device applications due to their unique combination of electrical performance, optical transparency, and mechanical flexibility. However, the current landscape presents a complex mix of technological achievements and persistent challenges that must be addressed for widespread adoption in medical contexts.
The global development status of metal oxide TFTs shows significant regional variations. Research institutions and companies in East Asia, particularly in South Korea, Japan, and Taiwan, have established leadership positions in metal oxide semiconductor technology, with substantial patent portfolios and manufacturing capabilities. European research centers have focused on novel applications in flexible electronics and biosensing, while North American entities have emphasized integration with existing silicon-based technologies and specialized medical applications.
From a technical perspective, several critical challenges currently limit the implementation of metal oxide TFTs in medical devices. Stability issues remain paramount, particularly in physiological environments where exposure to moisture, ions, and varying temperatures can degrade performance over time. The bias stress instability of these devices, especially under continuous operation conditions typical in medical monitoring, presents reliability concerns that must be addressed through materials engineering or circuit design compensation techniques.
Uniformity in large-area fabrication represents another significant hurdle. While laboratory demonstrations have shown excellent performance metrics, translating these results to manufacturing scales with consistent device-to-device performance remains difficult. This challenge is particularly acute for medical applications where device reliability and reproducibility are non-negotiable requirements.
Biocompatibility considerations create additional complexity. The potential leaching of metal ions from oxide semiconductors like IGZO (indium-gallium-zinc oxide) or ZnO into surrounding tissues requires thorough investigation. Current research indicates generally favorable biocompatibility profiles, but long-term studies and standardized testing protocols specific to metal oxide TFTs in medical contexts are still developing.
The regulatory landscape presents perhaps the most formidable barrier to commercialization. Medical device regulations vary significantly across regions, with FDA, EU MDR, and other frameworks imposing different requirements for materials characterization, reliability testing, and risk assessment. The novel nature of metal oxide TFTs means that clear regulatory pathways have not been fully established, creating uncertainty for manufacturers and extending development timelines.
Interface engineering between the metal oxide semiconductor and biological systems represents an emerging research frontier. Creating stable, low-impedance connections between electronic components and biological tissues or fluids remains challenging, particularly for implantable or long-term wearable applications where biofouling can compromise device performance.
The global development status of metal oxide TFTs shows significant regional variations. Research institutions and companies in East Asia, particularly in South Korea, Japan, and Taiwan, have established leadership positions in metal oxide semiconductor technology, with substantial patent portfolios and manufacturing capabilities. European research centers have focused on novel applications in flexible electronics and biosensing, while North American entities have emphasized integration with existing silicon-based technologies and specialized medical applications.
From a technical perspective, several critical challenges currently limit the implementation of metal oxide TFTs in medical devices. Stability issues remain paramount, particularly in physiological environments where exposure to moisture, ions, and varying temperatures can degrade performance over time. The bias stress instability of these devices, especially under continuous operation conditions typical in medical monitoring, presents reliability concerns that must be addressed through materials engineering or circuit design compensation techniques.
Uniformity in large-area fabrication represents another significant hurdle. While laboratory demonstrations have shown excellent performance metrics, translating these results to manufacturing scales with consistent device-to-device performance remains difficult. This challenge is particularly acute for medical applications where device reliability and reproducibility are non-negotiable requirements.
Biocompatibility considerations create additional complexity. The potential leaching of metal ions from oxide semiconductors like IGZO (indium-gallium-zinc oxide) or ZnO into surrounding tissues requires thorough investigation. Current research indicates generally favorable biocompatibility profiles, but long-term studies and standardized testing protocols specific to metal oxide TFTs in medical contexts are still developing.
The regulatory landscape presents perhaps the most formidable barrier to commercialization. Medical device regulations vary significantly across regions, with FDA, EU MDR, and other frameworks imposing different requirements for materials characterization, reliability testing, and risk assessment. The novel nature of metal oxide TFTs means that clear regulatory pathways have not been fully established, creating uncertainty for manufacturers and extending development timelines.
Interface engineering between the metal oxide semiconductor and biological systems represents an emerging research frontier. Creating stable, low-impedance connections between electronic components and biological tissues or fluids remains challenging, particularly for implantable or long-term wearable applications where biofouling can compromise device performance.
Current Regulatory Compliance Solutions
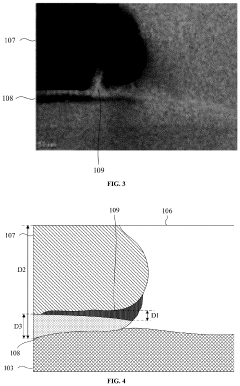
01 Metal oxide semiconductor materials for TFTs
Various metal oxide semiconductor materials can be used in thin-film transistors (TFTs) to achieve specific electrical properties. These materials include zinc oxide (ZnO), indium gallium zinc oxide (IGZO), and other metal oxide combinations that offer advantages such as high electron mobility, good stability, and transparency. The selection of appropriate metal oxide materials is crucial for optimizing TFT performance in display and electronic applications.- Metal oxide semiconductor materials for TFTs: Various metal oxide semiconductor materials are used in thin-film transistors (TFTs) to achieve specific electrical properties. These materials include zinc oxide (ZnO), indium gallium zinc oxide (IGZO), and other metal oxide combinations that offer advantages such as high electron mobility, good stability, and transparency. The selection of metal oxide materials significantly impacts the performance characteristics of the TFT, including switching speed, power consumption, and reliability.
- Fabrication methods for metal oxide TFTs: Various fabrication techniques are employed to manufacture metal oxide TFTs with optimized performance. These methods include sputtering, atomic layer deposition (ALD), solution processing, and annealing treatments. The fabrication process significantly influences the crystallinity, defect density, and interface quality of the metal oxide layer, which in turn affects the electrical characteristics of the TFT. Advanced fabrication methods aim to achieve uniform deposition, precise thickness control, and reduced defect concentration.
- Device structures and architectures for metal oxide TFTs: Different structural configurations are implemented in metal oxide TFTs to enhance performance and functionality. These include bottom-gate, top-gate, dual-gate, and self-aligned architectures. The device structure affects key parameters such as channel length, gate control efficiency, parasitic capacitance, and contact resistance. Advanced architectures incorporate features like passivation layers, etch-stop layers, and optimized electrode configurations to improve stability and reduce leakage currents.
- Integration of metal oxide TFTs in display technologies: Metal oxide TFTs are widely integrated into various display technologies, including LCD, OLED, and flexible displays. The integration involves addressing challenges related to pixel circuits, backplane design, and driving schemes. Metal oxide TFTs offer advantages for display applications due to their transparency, high mobility, and low off-current characteristics. Advanced integration approaches focus on achieving high resolution, uniform brightness, fast response time, and compatibility with large-area manufacturing processes.
- Performance enhancement and stability improvement techniques: Various methods are employed to enhance the performance and stability of metal oxide TFTs. These include doping with specific elements, interface engineering, passivation treatments, and bias stress compensation techniques. Performance enhancement focuses on improving parameters such as mobility, threshold voltage stability, subthreshold swing, and resistance to environmental factors. Advanced approaches address issues like oxygen vacancy formation, charge trapping, and bias-induced degradation to achieve long-term operational stability.
02 Fabrication methods for metal oxide TFTs
Various fabrication techniques are employed to manufacture metal oxide TFTs, including sputtering, atomic layer deposition (ALD), solution processing, and chemical vapor deposition (CVD). These methods control the deposition of metal oxide layers with precise thickness and composition, which directly affects transistor performance. Post-deposition treatments such as annealing are often used to improve crystallinity and electrical properties of the metal oxide semiconductor layer.Expand Specific Solutions03 Device structures and architectures for metal oxide TFTs
Different structural configurations are used in metal oxide TFTs, including bottom-gate, top-gate, staggered, and coplanar architectures. Each structure offers specific advantages in terms of performance, manufacturing complexity, and integration capability. The selection of appropriate gate dielectrics, electrode materials, and channel layer thickness significantly impacts the electrical characteristics and stability of the TFTs. Advanced structures may incorporate additional layers for passivation or to enhance specific properties.Expand Specific Solutions04 Performance enhancement techniques for metal oxide TFTs
Various methods are employed to improve the performance of metal oxide TFTs, including doping with specific elements to control carrier concentration, interface engineering to reduce trap states, and multilayer channel structures to optimize charge transport. Techniques such as hydrogen or nitrogen incorporation can passivate defects and enhance stability. Post-fabrication treatments like plasma exposure or specific annealing conditions can also significantly improve mobility, threshold voltage stability, and overall device reliability.Expand Specific Solutions05 Applications of metal oxide TFTs in displays and electronics
Metal oxide TFTs are widely used in various applications including active-matrix displays (LCDs, OLEDs), transparent electronics, flexible devices, and large-area sensor arrays. Their unique combination of high mobility, optical transparency, and low-temperature processability makes them suitable for next-generation display technologies and wearable electronics. Integration strategies for metal oxide TFTs with other components in complex circuits enable advanced functionalities in consumer electronics, medical devices, and industrial systems.Expand Specific Solutions
Key Industry Players in Medical TFT Manufacturing
The regulatory landscape for Metal Oxide TFTs in medical devices is evolving within a growing market characterized by increasing adoption of advanced display technologies in healthcare applications. The industry is currently in a transitional phase from early adoption to mainstream implementation, with market size expanding as medical device manufacturers recognize the benefits of Metal Oxide TFT technology. Leading players like BOE Technology Group, TCL China Star Optoelectronics, and Canon have achieved moderate technical maturity, while companies such as Applied Materials and Tianma Microelectronics are advancing manufacturing processes. Research institutions including Interuniversitair Micro-Electronica Centrum (IMEC), South China University of Technology, and IIT Madras are contributing to technological innovations. Regulatory frameworks are still developing, with companies like Carestream Health navigating compliance requirements for medical-grade implementations.
BOE Technology Group Co., Ltd.
Technical Solution: BOE Technology has developed specialized metal oxide TFT technologies for medical display applications that comply with international regulatory standards. Their medical-grade IGZO TFT panels are manufactured in ISO 13485 certified facilities with comprehensive traceability systems that document compliance with FDA Quality System Regulations. BOE's technology incorporates specialized pixel designs that maintain consistent luminance and contrast ratios within the parameters required for diagnostic medical displays under DICOM standards. Their manufacturing process includes enhanced aging tests that simulate the extended operational lifetimes required for medical equipment, with statistical process control methods that ensure consistent performance across production batches. BOE has implemented specialized testing protocols for their medical TFT panels that verify compliance with IEC 60601-1 electrical safety standards and electromagnetic compatibility requirements, with particular attention to leakage current limitations and isolation requirements.
Strengths: Vertical integration capabilities from TFT manufacturing to complete display systems; large-scale production capacity with established quality management systems. Weaknesses: Less specialized in medical-only applications compared to dedicated medical technology companies; regulatory expertise varies across different international markets.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed comprehensive manufacturing solutions for medical-grade metal oxide TFTs that meet stringent regulatory standards. Their AKT PECVD (Plasma-Enhanced Chemical Vapor Deposition) systems are specifically configured to produce high-performance IGZO TFTs with the stability and uniformity required for medical applications. The company's manufacturing processes incorporate in-line quality control systems that monitor critical parameters affecting biocompatibility and electrical safety, ensuring compliance with ISO 13485 for medical device manufacturing. Applied Materials' equipment enables precise control of metal oxide film composition and thickness, critical for maintaining consistent performance within FDA-approved specifications. Their end-to-end contamination control protocols address both particulate and molecular contamination concerns specific to medical device regulations, with documented validation procedures that support manufacturers' regulatory submissions.
Strengths: Industry-leading manufacturing equipment with proven capability to produce medical-grade TFTs at scale; comprehensive process control systems that support regulatory documentation requirements. Weaknesses: Requires significant capital investment; complex implementation process requiring specialized expertise in both semiconductor manufacturing and medical device regulations.
Critical Technical Standards and Certifications
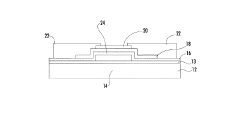
Metal oxide thin film transistor with excellent temperature stability
PatentInactiveJP2016502278A
Innovation
- Implementing a passivation material with a conduction band at least 0.5 eV higher than the metal oxide semiconductor's conduction band to transfer electrons away from the interface, reducing oxygen loss and improving stability by selecting materials like Ta2O5, TiO2, or other insulating oxides that are less susceptible to reduction.
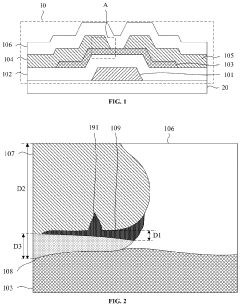
Metal-oxide thin-film transistor and method for fabricating same, display panel, and display device
PatentActiveUS20240194747A1
Innovation
- A metal-oxide thin-film transistor design with a laminated structure including a bulk metal layer and an electrode protection layer, where a metal-oxide layer with a thickness not exceeding 2% of the source or drain electrode thickness is inserted between the electrode protection layer and the bulk metal layer, controlling the oxygen content and reducing the formation of hydrogen bonds in the passivation layer to enhance stability.
Biocompatibility and Safety Considerations
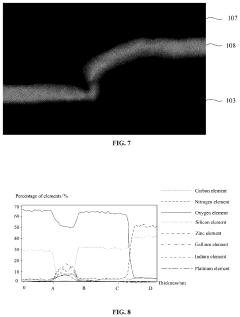
The biocompatibility of metal oxide TFTs in medical devices represents a critical consideration for regulatory compliance and patient safety. These thin-film transistors utilize materials such as indium gallium zinc oxide (IGZO), zinc oxide (ZnO), and indium tin oxide (ITO), which must undergo rigorous testing to ensure they do not elicit adverse biological responses when in contact with human tissues or bodily fluids. ISO 10993 series standards provide the framework for biological evaluation of medical devices, with particular emphasis on cytotoxicity, sensitization, and irritation testing for materials that may have direct or indirect patient contact.
Metal oxide materials present unique biocompatibility challenges due to potential ion leaching under certain conditions. Studies have shown that zinc and indium ions can be released in aqueous environments, necessitating comprehensive leachable and extractable testing protocols. The FDA guidance document on biocompatibility testing recommends manufacturers conduct exhaustive chemical characterization to identify potential leachables before proceeding with biological testing.
Encapsulation technologies play a crucial role in mitigating biocompatibility concerns. High-quality passivation layers using biocompatible materials such as parylene-C or medical-grade silicones can effectively isolate metal oxide components from biological environments. The effectiveness of these barrier layers must be validated through accelerated aging studies simulating the device's intended lifetime under physiological conditions.
Safety considerations extend beyond biocompatibility to include electrical safety parameters. Metal oxide TFTs must comply with IEC 60601-1 standards for medical electrical equipment, with particular attention to leakage current limitations. The semiconductor properties of metal oxides can change under prolonged electrical stress or radiation exposure, potentially affecting device safety profiles over time.
Sterilization compatibility represents another critical safety consideration. Common sterilization methods including ethylene oxide, gamma radiation, and autoclave processing can potentially alter the electrical characteristics of metal oxide TFTs. Manufacturers must validate that their devices maintain performance specifications and safety parameters after multiple sterilization cycles appropriate for the intended clinical application.
Long-term implantable devices incorporating metal oxide TFTs face additional scrutiny regarding chronic toxicity and carcinogenicity. The EU Medical Device Regulation (MDR) and the FDA's premarket approval process require extensive in vivo testing for such applications, with particular focus on local tissue response and systemic effects over extended periods. Manufacturers must develop comprehensive biological risk assessment plans that address both known hazards and theoretical concerns related to novel material applications.
Metal oxide materials present unique biocompatibility challenges due to potential ion leaching under certain conditions. Studies have shown that zinc and indium ions can be released in aqueous environments, necessitating comprehensive leachable and extractable testing protocols. The FDA guidance document on biocompatibility testing recommends manufacturers conduct exhaustive chemical characterization to identify potential leachables before proceeding with biological testing.
Encapsulation technologies play a crucial role in mitigating biocompatibility concerns. High-quality passivation layers using biocompatible materials such as parylene-C or medical-grade silicones can effectively isolate metal oxide components from biological environments. The effectiveness of these barrier layers must be validated through accelerated aging studies simulating the device's intended lifetime under physiological conditions.
Safety considerations extend beyond biocompatibility to include electrical safety parameters. Metal oxide TFTs must comply with IEC 60601-1 standards for medical electrical equipment, with particular attention to leakage current limitations. The semiconductor properties of metal oxides can change under prolonged electrical stress or radiation exposure, potentially affecting device safety profiles over time.
Sterilization compatibility represents another critical safety consideration. Common sterilization methods including ethylene oxide, gamma radiation, and autoclave processing can potentially alter the electrical characteristics of metal oxide TFTs. Manufacturers must validate that their devices maintain performance specifications and safety parameters after multiple sterilization cycles appropriate for the intended clinical application.
Long-term implantable devices incorporating metal oxide TFTs face additional scrutiny regarding chronic toxicity and carcinogenicity. The EU Medical Device Regulation (MDR) and the FDA's premarket approval process require extensive in vivo testing for such applications, with particular focus on local tissue response and systemic effects over extended periods. Manufacturers must develop comprehensive biological risk assessment plans that address both known hazards and theoretical concerns related to novel material applications.
Global Regulatory Harmonization Trends
The global regulatory landscape for medical devices incorporating metal oxide TFTs is moving toward greater harmonization, with significant progress in recent years. The International Medical Device Regulators Forum (IMDRF) has been instrumental in establishing frameworks that bridge regulatory differences across major markets including the US, EU, Japan, China, and other key regions. These efforts aim to reduce redundant testing requirements and streamline approval processes while maintaining rigorous safety standards.
FDA and EU MDR representatives have initiated collaborative working groups specifically addressing novel semiconductor technologies in medical applications, with metal oxide TFTs receiving particular attention due to their increasing adoption in flexible medical displays and sensors. The 2022 International Harmonization Summit produced preliminary consensus documents outlining common testing protocols for TFT-based medical interfaces, representing a significant step toward unified standards.
The Medical Technology Harmonization Coalition (MTHC) has developed a standardized risk classification system for semiconductor components in medical devices that has been adopted by regulatory bodies in 17 countries. This system specifically addresses metal oxide TFT technology with considerations for biocompatibility, electrical safety, and long-term stability requirements that transcend national boundaries.
Mutual Recognition Agreements (MRAs) between major regulatory authorities have expanded to include quality management system audits for facilities manufacturing metal oxide TFT components for medical applications. This development has reduced regulatory burdens for manufacturers operating across multiple markets, with an estimated 30% reduction in compliance costs according to industry reports.
The ISO/IEC Joint Technical Committee has established Working Group 12, focused exclusively on developing harmonized testing methodologies for next-generation display technologies in healthcare settings. Their forthcoming standard ISO/IEC 24786 (expected 2024) will provide unified testing protocols for metal oxide TFT displays used in diagnostic imaging and patient monitoring systems, addressing a critical gap in current regulatory frameworks.
Challenges to complete harmonization persist, particularly regarding electromagnetic compatibility requirements and radiation hardness standards for metal oxide TFTs in different regions. The Asia-Pacific Medical Technology Forum has proposed a regional consensus approach that may serve as a template for broader international alignment, with particular focus on accelerated aging protocols and environmental stress testing methodologies relevant to metal oxide semiconductor stability in medical environments.
FDA and EU MDR representatives have initiated collaborative working groups specifically addressing novel semiconductor technologies in medical applications, with metal oxide TFTs receiving particular attention due to their increasing adoption in flexible medical displays and sensors. The 2022 International Harmonization Summit produced preliminary consensus documents outlining common testing protocols for TFT-based medical interfaces, representing a significant step toward unified standards.
The Medical Technology Harmonization Coalition (MTHC) has developed a standardized risk classification system for semiconductor components in medical devices that has been adopted by regulatory bodies in 17 countries. This system specifically addresses metal oxide TFT technology with considerations for biocompatibility, electrical safety, and long-term stability requirements that transcend national boundaries.
Mutual Recognition Agreements (MRAs) between major regulatory authorities have expanded to include quality management system audits for facilities manufacturing metal oxide TFT components for medical applications. This development has reduced regulatory burdens for manufacturers operating across multiple markets, with an estimated 30% reduction in compliance costs according to industry reports.
The ISO/IEC Joint Technical Committee has established Working Group 12, focused exclusively on developing harmonized testing methodologies for next-generation display technologies in healthcare settings. Their forthcoming standard ISO/IEC 24786 (expected 2024) will provide unified testing protocols for metal oxide TFT displays used in diagnostic imaging and patient monitoring systems, addressing a critical gap in current regulatory frameworks.
Challenges to complete harmonization persist, particularly regarding electromagnetic compatibility requirements and radiation hardness standards for metal oxide TFTs in different regions. The Asia-Pacific Medical Technology Forum has proposed a regional consensus approach that may serve as a template for broader international alignment, with particular focus on accelerated aging protocols and environmental stress testing methodologies relevant to metal oxide semiconductor stability in medical environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!