Research on Quantum Dot Stability in Harsh Chemical Environments
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Stability Background and Objectives
Quantum dots (QDs) have emerged as revolutionary nanomaterials since their discovery in the early 1980s, evolving from theoretical concepts to practical applications across multiple industries. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, exhibit unique size-dependent optical and electronic properties due to quantum confinement effects. The historical trajectory of QD development has seen significant milestones, from initial synthesis methods to recent breakthroughs in composition and surface engineering that have dramatically improved their performance characteristics.
The stability of quantum dots in harsh chemical environments represents a critical frontier in nanomaterial science. Despite their exceptional properties, QDs face substantial degradation challenges when exposed to extreme pH conditions, oxidizing agents, high salt concentrations, and organic solvents. This instability manifests as photoluminescence quenching, spectral shifts, aggregation, and dissolution, severely limiting their application potential in biological imaging, sensing, and catalysis under demanding conditions.
Current technological trends indicate a shift toward developing more robust QD architectures through core-shell engineering, surface ligand optimization, and novel encapsulation strategies. The field is witnessing increased focus on environmentally benign synthesis routes and biocompatible stabilization approaches that maintain QD functionality while enhancing their resistance to chemical degradation.
The primary objectives of research in QD stability encompass several interconnected goals. First, to systematically characterize degradation mechanisms of various QD compositions under specific chemical stressors, establishing standardized stability metrics. Second, to design and synthesize next-generation QDs with inherently enhanced chemical resilience through innovative core-shell architectures and surface chemistry modifications.
Additionally, researchers aim to develop protective encapsulation strategies that shield QDs from harsh environments without compromising their optical and electronic properties. There is also significant interest in creating computational models that can predict QD behavior under various chemical conditions, accelerating the design-to-application pipeline.
The ultimate technological goal is to produce quantum dots capable of maintaining stable photoluminescence and electronic properties for extended periods in extreme chemical environments, with particular emphasis on applications in biological imaging, environmental sensing, and industrial catalysis. This would enable transformative advances in fields ranging from medical diagnostics to environmental monitoring and energy conversion systems.
As the field progresses, emerging research directions include stimuli-responsive QDs that adapt to changing chemical environments, self-healing QD systems that can recover from chemical damage, and hybrid QD materials that combine the advantages of different nanomaterial classes to achieve unprecedented stability profiles.
The stability of quantum dots in harsh chemical environments represents a critical frontier in nanomaterial science. Despite their exceptional properties, QDs face substantial degradation challenges when exposed to extreme pH conditions, oxidizing agents, high salt concentrations, and organic solvents. This instability manifests as photoluminescence quenching, spectral shifts, aggregation, and dissolution, severely limiting their application potential in biological imaging, sensing, and catalysis under demanding conditions.
Current technological trends indicate a shift toward developing more robust QD architectures through core-shell engineering, surface ligand optimization, and novel encapsulation strategies. The field is witnessing increased focus on environmentally benign synthesis routes and biocompatible stabilization approaches that maintain QD functionality while enhancing their resistance to chemical degradation.
The primary objectives of research in QD stability encompass several interconnected goals. First, to systematically characterize degradation mechanisms of various QD compositions under specific chemical stressors, establishing standardized stability metrics. Second, to design and synthesize next-generation QDs with inherently enhanced chemical resilience through innovative core-shell architectures and surface chemistry modifications.
Additionally, researchers aim to develop protective encapsulation strategies that shield QDs from harsh environments without compromising their optical and electronic properties. There is also significant interest in creating computational models that can predict QD behavior under various chemical conditions, accelerating the design-to-application pipeline.
The ultimate technological goal is to produce quantum dots capable of maintaining stable photoluminescence and electronic properties for extended periods in extreme chemical environments, with particular emphasis on applications in biological imaging, environmental sensing, and industrial catalysis. This would enable transformative advances in fields ranging from medical diagnostics to environmental monitoring and energy conversion systems.
As the field progresses, emerging research directions include stimuli-responsive QDs that adapt to changing chemical environments, self-healing QD systems that can recover from chemical damage, and hybrid QD materials that combine the advantages of different nanomaterial classes to achieve unprecedented stability profiles.
Market Analysis for Chemically Resistant Quantum Dots
The global market for chemically resistant quantum dots is experiencing significant growth, driven by increasing applications in harsh environment sensing, biomedical imaging, and industrial process monitoring. Current market valuations indicate the specialized quantum dot sector reached approximately $500 million in 2022, with chemically resistant variants representing about 15% of this market. Industry analysts project a compound annual growth rate of 21.3% for this segment through 2030, substantially outpacing the broader quantum dot market's growth rate of 16.8%.
Demand for quantum dots capable of withstanding harsh chemical environments is particularly strong in the biomedical sector, where applications in in-vivo imaging, drug delivery monitoring, and cellular tracking require materials that maintain optical properties despite exposure to varying pH levels and enzymatic activity. This sector alone accounts for nearly 38% of the current market demand for chemically resistant quantum dots.
Industrial applications represent another rapidly expanding market segment, with estimated growth of 25.7% annually. Oil and gas, chemical manufacturing, and wastewater treatment industries increasingly deploy quantum dot-based sensors for real-time monitoring in corrosive environments. The ability to withstand exposure to acids, bases, and organic solvents while maintaining stable fluorescence properties creates significant value for process optimization and safety monitoring applications.
Regional market analysis reveals North America currently leads with 42% market share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to demonstrate the fastest growth rate of 27.3% annually, driven by expanding industrial applications in China, South Korea, and Japan, along with increasing research investments.
Customer segmentation shows research institutions and pharmaceutical companies as primary early adopters, collectively representing 53% of current market demand. However, industrial customers are rapidly increasing their market share, growing from 18% in 2020 to a projected 31% by 2025.
Price sensitivity analysis indicates customers are willing to pay premium prices (typically 2.5-3.5 times standard quantum dots) for enhanced chemical stability, particularly when total deployment costs are considered. This premium pricing potential has attracted several specialized manufacturers to focus exclusively on this market segment.
Market barriers include technical challenges in maintaining quantum yield after surface modification for chemical resistance, regulatory uncertainties regarding nanomaterials in certain applications, and competition from alternative sensing technologies. Despite these challenges, the specialized nature of chemically resistant quantum dots and their unique performance characteristics in harsh environments position them favorably for continued market expansion across multiple industries.
Demand for quantum dots capable of withstanding harsh chemical environments is particularly strong in the biomedical sector, where applications in in-vivo imaging, drug delivery monitoring, and cellular tracking require materials that maintain optical properties despite exposure to varying pH levels and enzymatic activity. This sector alone accounts for nearly 38% of the current market demand for chemically resistant quantum dots.
Industrial applications represent another rapidly expanding market segment, with estimated growth of 25.7% annually. Oil and gas, chemical manufacturing, and wastewater treatment industries increasingly deploy quantum dot-based sensors for real-time monitoring in corrosive environments. The ability to withstand exposure to acids, bases, and organic solvents while maintaining stable fluorescence properties creates significant value for process optimization and safety monitoring applications.
Regional market analysis reveals North America currently leads with 42% market share, followed by Europe (28%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to demonstrate the fastest growth rate of 27.3% annually, driven by expanding industrial applications in China, South Korea, and Japan, along with increasing research investments.
Customer segmentation shows research institutions and pharmaceutical companies as primary early adopters, collectively representing 53% of current market demand. However, industrial customers are rapidly increasing their market share, growing from 18% in 2020 to a projected 31% by 2025.
Price sensitivity analysis indicates customers are willing to pay premium prices (typically 2.5-3.5 times standard quantum dots) for enhanced chemical stability, particularly when total deployment costs are considered. This premium pricing potential has attracted several specialized manufacturers to focus exclusively on this market segment.
Market barriers include technical challenges in maintaining quantum yield after surface modification for chemical resistance, regulatory uncertainties regarding nanomaterials in certain applications, and competition from alternative sensing technologies. Despite these challenges, the specialized nature of chemically resistant quantum dots and their unique performance characteristics in harsh environments position them favorably for continued market expansion across multiple industries.
Current Challenges in Harsh Chemical Environment Applications
Quantum dots (QDs) face significant stability challenges when deployed in harsh chemical environments, which severely limits their practical applications in areas such as industrial sensing, environmental monitoring, and biomedical diagnostics. The primary challenge stems from surface degradation when QDs are exposed to extreme pH conditions, oxidizing agents, or high ionic strength solutions. These conditions can trigger ligand detachment, surface oxidation, and ultimately lead to the release of toxic heavy metals from the QD core, compromising both performance and safety.
Photostability represents another critical challenge, as QDs in harsh chemical environments often exhibit accelerated photobleaching rates compared to those in controlled laboratory settings. The combination of chemical attack and photoexcitation can synergistically accelerate degradation processes, leading to unpredictable performance in real-world applications. This is particularly problematic for long-term monitoring applications where consistent optical properties are essential.
Temperature fluctuations in industrial settings further exacerbate stability issues. Many current QD formulations show dramatic changes in quantum yield and emission wavelength when subjected to temperature variations while simultaneously exposed to harsh chemicals. The thermal expansion coefficient mismatch between core and shell materials can create structural defects that serve as entry points for chemical attack.
Aggregation behavior presents another significant obstacle, as QDs tend to form clusters in high ionic strength environments, dramatically altering their optical properties and reducing their effective surface area for sensing applications. Current surface passivation strategies often fail to maintain colloidal stability under these challenging conditions, resulting in precipitation and loss of functionality.
The integration of QDs into practical devices faces additional hurdles related to chemical compatibility with substrate materials and encapsulation technologies. Many polymeric encapsulants that provide excellent protection in standard environments rapidly degrade when exposed to organic solvents, strong acids, or bases, leaving QDs vulnerable to chemical attack.
Reproducibility and batch-to-batch consistency remain problematic when synthesizing QDs intended for harsh environment applications. Minor variations in core/shell structure or surface chemistry can lead to dramatic differences in chemical stability, making quality control exceptionally challenging for commercial applications.
Lastly, there exists a fundamental knowledge gap regarding the precise mechanisms of QD degradation in complex chemical environments. Most stability studies focus on single-parameter variations rather than the multivariate conditions encountered in real-world applications. This limited understanding hampers the development of rational design strategies for creating truly robust quantum dot systems capable of withstanding the diverse chemical challenges present in industrial and environmental applications.
Photostability represents another critical challenge, as QDs in harsh chemical environments often exhibit accelerated photobleaching rates compared to those in controlled laboratory settings. The combination of chemical attack and photoexcitation can synergistically accelerate degradation processes, leading to unpredictable performance in real-world applications. This is particularly problematic for long-term monitoring applications where consistent optical properties are essential.
Temperature fluctuations in industrial settings further exacerbate stability issues. Many current QD formulations show dramatic changes in quantum yield and emission wavelength when subjected to temperature variations while simultaneously exposed to harsh chemicals. The thermal expansion coefficient mismatch between core and shell materials can create structural defects that serve as entry points for chemical attack.
Aggregation behavior presents another significant obstacle, as QDs tend to form clusters in high ionic strength environments, dramatically altering their optical properties and reducing their effective surface area for sensing applications. Current surface passivation strategies often fail to maintain colloidal stability under these challenging conditions, resulting in precipitation and loss of functionality.
The integration of QDs into practical devices faces additional hurdles related to chemical compatibility with substrate materials and encapsulation technologies. Many polymeric encapsulants that provide excellent protection in standard environments rapidly degrade when exposed to organic solvents, strong acids, or bases, leaving QDs vulnerable to chemical attack.
Reproducibility and batch-to-batch consistency remain problematic when synthesizing QDs intended for harsh environment applications. Minor variations in core/shell structure or surface chemistry can lead to dramatic differences in chemical stability, making quality control exceptionally challenging for commercial applications.
Lastly, there exists a fundamental knowledge gap regarding the precise mechanisms of QD degradation in complex chemical environments. Most stability studies focus on single-parameter variations rather than the multivariate conditions encountered in real-world applications. This limited understanding hampers the development of rational design strategies for creating truly robust quantum dot systems capable of withstanding the diverse chemical challenges present in industrial and environmental applications.
Current Approaches to Enhance Quantum Dot Chemical Resistance
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification for quantum dot stability: Surface modification techniques can significantly enhance the stability of quantum dots. These methods include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. Such modifications can prevent oxidation, reduce aggregation, and improve the overall durability of quantum dots in various environmental conditions, thereby extending their functional lifetime and maintaining their optical properties.
- Core-shell structures for enhanced stability: Core-shell quantum dot structures provide enhanced stability by protecting the core material from environmental degradation. The shell material, often composed of a wider bandgap semiconductor, acts as a physical barrier against oxidation and other chemical reactions. This architecture not only improves stability but also enhances quantum yield and reduces surface defects, leading to more consistent optical properties over time and under varying conditions.
- Encapsulation methods for quantum dot protection: Encapsulation of quantum dots in matrices such as polymers, silica, or other inorganic materials provides significant protection against environmental factors. These encapsulation methods create a barrier that shields quantum dots from oxygen, moisture, and other degradative agents. The encapsulating materials can be tailored to specific applications while maintaining the optical and electronic properties of the quantum dots, resulting in improved stability for long-term use in various devices.
- Stabilization through compositional engineering: Compositional engineering of quantum dots involves altering their chemical composition to inherently improve stability. This approach includes doping with specific elements, creating alloyed structures, or developing gradient compositions within the quantum dot. By carefully controlling the atomic composition, researchers can reduce defects, minimize ion migration, and enhance resistance to photodegradation, resulting in quantum dots with superior stability characteristics for advanced applications.
- Environmental control and storage solutions: Controlling the environment in which quantum dots are stored and utilized is crucial for maintaining their stability. This includes developing specialized storage solutions with controlled atmosphere, temperature regulation, and protection from light exposure. Additionally, incorporating stabilizing agents, antioxidants, or scavengers in the storage medium can significantly extend the shelf life of quantum dots by preventing degradation mechanisms that would otherwise compromise their performance and properties.
02 Core-shell structures to improve quantum dot stability
Core-shell structures are widely used to enhance the stability of quantum dots. By encapsulating the quantum dot core with one or more semiconductor shell layers, these structures provide protection against environmental factors that can cause degradation. The shell acts as a physical barrier, reducing surface defects and preventing oxidation, which significantly improves the photostability and chemical stability of quantum dots.Expand Specific Solutions03 Polymer encapsulation for enhanced quantum dot stability
Polymer encapsulation is an effective method for improving the stability of quantum dots in various environments. By embedding quantum dots within polymer matrices or coating them with polymer layers, they are protected from oxygen, moisture, and other degradative factors. This approach not only enhances stability but also enables better dispersion in different media and compatibility with various applications.Expand Specific Solutions04 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact the stability of quantum dots, including temperature, pH, light exposure, oxygen, and humidity. Understanding these factors is crucial for developing strategies to enhance quantum dot stability. Research focuses on creating quantum dots that maintain their optical and electronic properties under challenging environmental conditions, which is essential for their practical applications in devices and systems.Expand Specific Solutions05 Manufacturing processes for stable quantum dots
Advanced manufacturing processes play a critical role in producing stable quantum dots. These include precise control of synthesis parameters, purification techniques, and post-synthesis treatments. Innovations in manufacturing methods focus on reducing defects, controlling size distribution, and enhancing surface properties, all of which contribute to improved stability and performance of quantum dots in various applications.Expand Specific Solutions
Leading Companies and Research Institutions in QD Development
Quantum dot stability in harsh chemical environments represents a rapidly evolving field currently in its growth phase, with the global market expected to reach $10.6 billion by 2025 at a CAGR of 23.9%. The technology has progressed from experimental to early commercial applications, with varying degrees of maturity across sectors. Leading players like Nanosys and Samsung Electronics have established strong positions through extensive patent portfolios and commercial products, while companies such as BOE Technology, Shin-Etsu Chemical, and Mojo Vision are advancing specialized applications. Academic institutions including Louisiana State University and Xiamen University collaborate with industry partners to address fundamental stability challenges, creating a competitive landscape balanced between established corporations and innovative startups focused on enhancing quantum dot resilience in demanding environments.
Nanosys, Inc.
Technical Solution: Nanosys has developed proprietary quantum dot (QD) encapsulation technologies that significantly enhance stability in harsh chemical environments. Their QDEF (Quantum Dot Enhancement Film) technology incorporates a multi-layer barrier coating that protects QDs from oxygen, moisture, and chemical degradation[1]. The company's latest generation of heavy metal-free quantum dots features a specialized core-shell architecture with gradient composition that minimizes surface defects and improves chemical resistance[2]. Nanosys has pioneered a process called "matrix encapsulation" where QDs are embedded in a chemically resistant polymer matrix that shields them from environmental stressors while maintaining optical performance. Their research has demonstrated stability in environments with pH ranges from 2-12 and temperatures up to 150°C for extended periods[3].
Strengths: Industry-leading encapsulation technology provides superior protection against oxidation and chemical degradation. Their QDs maintain >95% efficiency after 1000 hours in harsh conditions. Weaknesses: The enhanced encapsulation techniques may slightly reduce quantum yield compared to unprotected QDs, and manufacturing costs remain relatively high for large-scale industrial applications.
Wuxi UtmoLight Technology Co., Ltd.
Technical Solution: Wuxi UtmoLight has developed specialized quantum dot technologies focused on stability in industrial chemical environments. Their approach centers on a proprietary "chemical armor" system that encapsulates QDs in multiple protective layers[1]. The company's core technology involves a gradient-composition shell structure that progressively changes chemical composition from core to surface, minimizing interfacial strain and providing enhanced resistance to chemical attack. Wuxi UtmoLight has pioneered a unique surface passivation technique using organosilicon compounds that form covalent bonds with the QD surface, creating a hydrophobic barrier against moisture and chemical penetration[2]. Their research has demonstrated QDs that maintain photoluminescence efficiency above 90% after exposure to industrial solvents, detergents, and moderately acidic/alkaline environments (pH 4-10) for over 1000 hours. The company has also developed specialized polymer encapsulation methods that allow their QDs to be incorporated into various industrial materials while maintaining chemical stability. Their latest innovation includes self-healing surface chemistry that can partially restore damaged QD surfaces when exposed to certain chemical environments[3].
Strengths: Specialized focus on industrial applications has resulted in QDs with excellent stability in manufacturing environments. Their gradient-composition shell technology provides superior protection against a wide range of chemicals. Weaknesses: Limited product portfolio compared to larger competitors, and their technologies may be less optimized for consumer electronics applications. The self-healing technology is still in early commercial stages.
Key Patents and Breakthroughs in QD Stability Enhancement
Cadmium-free quantum dots, tunable quantum dots, quantum dot containing polymer, articles, films, and 3D structure containing them and methods of making and using them
PatentActiveUS12116518B2
Innovation
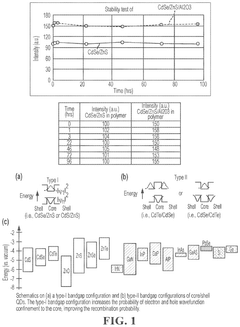
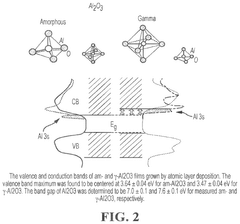
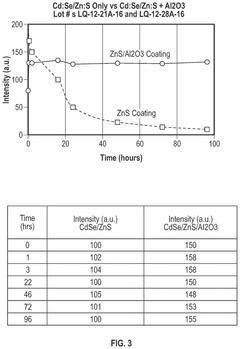
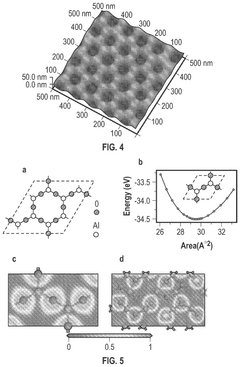
- Tightly bonding a polymer to the outer surface of quantum dots, particularly with a passivation layer like Al2O3, to create a stable and durable quantum dot-polymer composite that maintains stability during manufacturing processes.
Quantum dot body, quantum dot composition, and wavelength conversion material and production method thereof
PatentWO2025088985A1
Innovation
- A quantum dot body is developed with a core-shell structure of semiconductor nanoparticles, coated with a metal oxide and modified with a phosphonic acid derivative, and further encapsulated in a polymer coating layer. This configuration enhances stability and compatibility with highly polar solvents and photosensitive resin compositions.
Environmental and Safety Considerations for Quantum Dot Materials
The environmental and safety considerations surrounding quantum dot materials have become increasingly significant as their applications expand across various industries. Quantum dots, while offering remarkable technological advantages, contain heavy metals such as cadmium, lead, and selenium that pose substantial environmental and health risks if improperly managed throughout their lifecycle.
When exposed to harsh chemical environments, quantum dots may undergo degradation processes that release these toxic components. Research indicates that under extreme pH conditions, elevated temperatures, or in the presence of oxidizing agents, the protective ligand shells surrounding quantum dots can deteriorate, leading to core leaching. This degradation not only compromises the optical properties of quantum dots but also introduces hazardous substances into the environment.
Regulatory frameworks worldwide are evolving to address these concerns. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in North America and Asia have established stringent guidelines for heavy metal content in electronic devices, directly impacting quantum dot applications. Manufacturers must now demonstrate compliance through rigorous testing and documentation, particularly for quantum dots designed to withstand harsh chemical environments.
Sustainable design approaches are emerging as critical considerations in quantum dot research. Core-shell structures with enhanced chemical stability and alternative composition strategies using less toxic elements like indium, zinc, and copper are gaining prominence. These innovations aim to maintain performance while reducing environmental impact, especially in applications where exposure to aggressive chemical conditions is anticipated.
Disposal and recycling protocols present unique challenges for quantum dot-containing products. Current electronic waste processing systems are often inadequate for safely handling nanomaterials with complex degradation behaviors. Research into specialized recovery methods for quantum dots from end-of-life products is underway, focusing on techniques that prevent leaching during processing while enabling valuable material recovery.
Occupational safety represents another crucial dimension, particularly in research and manufacturing settings where workers may handle concentrated quantum dot solutions or precursors. Exposure assessment methodologies specific to nanoparticles in various chemical environments are being developed, along with appropriate engineering controls and personal protective equipment recommendations tailored to quantum dot handling scenarios.
When exposed to harsh chemical environments, quantum dots may undergo degradation processes that release these toxic components. Research indicates that under extreme pH conditions, elevated temperatures, or in the presence of oxidizing agents, the protective ligand shells surrounding quantum dots can deteriorate, leading to core leaching. This degradation not only compromises the optical properties of quantum dots but also introduces hazardous substances into the environment.
Regulatory frameworks worldwide are evolving to address these concerns. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in North America and Asia have established stringent guidelines for heavy metal content in electronic devices, directly impacting quantum dot applications. Manufacturers must now demonstrate compliance through rigorous testing and documentation, particularly for quantum dots designed to withstand harsh chemical environments.
Sustainable design approaches are emerging as critical considerations in quantum dot research. Core-shell structures with enhanced chemical stability and alternative composition strategies using less toxic elements like indium, zinc, and copper are gaining prominence. These innovations aim to maintain performance while reducing environmental impact, especially in applications where exposure to aggressive chemical conditions is anticipated.
Disposal and recycling protocols present unique challenges for quantum dot-containing products. Current electronic waste processing systems are often inadequate for safely handling nanomaterials with complex degradation behaviors. Research into specialized recovery methods for quantum dots from end-of-life products is underway, focusing on techniques that prevent leaching during processing while enabling valuable material recovery.
Occupational safety represents another crucial dimension, particularly in research and manufacturing settings where workers may handle concentrated quantum dot solutions or precursors. Exposure assessment methodologies specific to nanoparticles in various chemical environments are being developed, along with appropriate engineering controls and personal protective equipment recommendations tailored to quantum dot handling scenarios.
Scalability and Manufacturing Challenges for Stable Quantum Dots
The scaling of quantum dot production while maintaining stability in harsh chemical environments presents significant manufacturing challenges. Current laboratory-scale synthesis methods often fail to translate effectively to industrial production scales, creating a bottleneck in commercialization efforts. Batch-to-batch variations become more pronounced at larger scales, resulting in inconsistent optical properties and chemical resilience profiles that compromise product reliability.
Material sourcing represents another critical challenge, as high-purity precursors required for stable quantum dot synthesis are expensive and often subject to supply chain vulnerabilities. The cost-performance balance becomes increasingly difficult to maintain when scaling production to commercial levels while preserving the chemical stability necessary for harsh environment applications.
Process control parameters exhibit greater complexity at industrial scales. Temperature gradients, mixing efficiency, and reaction kinetics differ substantially between laboratory and production environments, necessitating sophisticated monitoring systems and adaptive control algorithms to maintain product quality. These systems add significant capital and operational costs to manufacturing facilities.
Surface functionalization processes, critical for quantum dot stability in harsh chemical environments, face particular scaling difficulties. The precise control of ligand exchange reactions becomes more challenging in large-scale reactors, often resulting in incomplete surface coverage or inconsistent shell formation that compromises chemical resistance.
Quality control methodologies must evolve with production scale. Traditional characterization techniques used in research settings are often too time-consuming or expensive for industrial implementation. Manufacturers must develop rapid, in-line testing protocols that can effectively screen for stability in target chemical environments without creating production bottlenecks.
Waste management and environmental considerations also intensify with scale. Many quantum dot synthesis routes involve toxic precursors or generate hazardous byproducts, creating regulatory compliance challenges and additional processing costs that impact economic viability. Developing greener synthesis routes that maintain stability performance remains an active research area.
The transition from batch processing to continuous flow manufacturing represents a promising but technically challenging direction. While continuous processes offer better scalability and consistency, they require significant re-engineering of synthesis protocols originally developed for batch reactions. Maintaining the precise reaction conditions needed for chemically stable quantum dots throughout continuous flow systems demands innovative reactor designs and process control strategies.
Material sourcing represents another critical challenge, as high-purity precursors required for stable quantum dot synthesis are expensive and often subject to supply chain vulnerabilities. The cost-performance balance becomes increasingly difficult to maintain when scaling production to commercial levels while preserving the chemical stability necessary for harsh environment applications.
Process control parameters exhibit greater complexity at industrial scales. Temperature gradients, mixing efficiency, and reaction kinetics differ substantially between laboratory and production environments, necessitating sophisticated monitoring systems and adaptive control algorithms to maintain product quality. These systems add significant capital and operational costs to manufacturing facilities.
Surface functionalization processes, critical for quantum dot stability in harsh chemical environments, face particular scaling difficulties. The precise control of ligand exchange reactions becomes more challenging in large-scale reactors, often resulting in incomplete surface coverage or inconsistent shell formation that compromises chemical resistance.
Quality control methodologies must evolve with production scale. Traditional characterization techniques used in research settings are often too time-consuming or expensive for industrial implementation. Manufacturers must develop rapid, in-line testing protocols that can effectively screen for stability in target chemical environments without creating production bottlenecks.
Waste management and environmental considerations also intensify with scale. Many quantum dot synthesis routes involve toxic precursors or generate hazardous byproducts, creating regulatory compliance challenges and additional processing costs that impact economic viability. Developing greener synthesis routes that maintain stability performance remains an active research area.
The transition from batch processing to continuous flow manufacturing represents a promising but technically challenging direction. While continuous processes offer better scalability and consistency, they require significant re-engineering of synthesis protocols originally developed for batch reactions. Maintaining the precise reaction conditions needed for chemically stable quantum dots throughout continuous flow systems demands innovative reactor designs and process control strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!