Research on Solid State Battery Breakthrough in Pharmaceuticals
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid State Battery Evolution and Pharmaceutical Applications
Solid state battery technology has evolved significantly over the past decades, transitioning from conceptual designs to commercially viable products. The evolution began in the 1970s with the discovery of solid electrolytes, but significant advancements only materialized in the early 2000s when researchers successfully developed ceramic and polymer-based solid electrolytes with improved ionic conductivity. This technological progression has been driven by the inherent limitations of conventional lithium-ion batteries, particularly their safety concerns, energy density constraints, and performance degradation over time.
The pharmaceutical industry has recently emerged as an unexpected beneficiary of solid state battery technology. Traditional pharmaceutical applications relied on conventional power sources for drug delivery systems, diagnostic equipment, and implantable medical devices. However, these applications faced significant challenges including limited battery life, potential leakage of harmful chemicals, and size constraints that restricted miniaturization efforts.
Solid state batteries offer transformative solutions to these pharmaceutical challenges through their enhanced safety profile, elimination of liquid electrolytes that could leak, and significantly higher energy density in smaller form factors. These advantages have enabled the development of next-generation implantable drug delivery systems that can operate for extended periods without replacement, miniaturized diagnostic tools with improved reliability, and advanced wearable health monitoring devices with extended operational lifespans.
The convergence of solid state battery technology and pharmaceutical applications has accelerated particularly in the last five years, with breakthrough innovations in biocompatible solid electrolytes. These materials are specifically engineered to be non-toxic and compatible with biological systems, addressing a critical requirement for in-vivo applications. Additionally, researchers have developed flexible solid state batteries that can conform to the human body's contours, opening new possibilities for integrated medical devices.
Recent innovations include temperature-stable solid state batteries capable of withstanding sterilization processes required for medical applications, and ultra-thin batteries that can be incorporated into transdermal drug delivery patches. These advancements have enabled precise dosage control and programmable release profiles that were previously unattainable with conventional battery technologies.
Looking forward, the evolution trajectory suggests further integration of solid state batteries with smart pharmaceutical systems, potentially incorporating artificial intelligence for adaptive drug delivery based on real-time physiological monitoring. The miniaturization trend is expected to continue, potentially leading to microscale batteries suitable for targeted drug delivery at the cellular level, representing a paradigm shift in treatment methodologies for conditions requiring precise localized intervention.
The pharmaceutical industry has recently emerged as an unexpected beneficiary of solid state battery technology. Traditional pharmaceutical applications relied on conventional power sources for drug delivery systems, diagnostic equipment, and implantable medical devices. However, these applications faced significant challenges including limited battery life, potential leakage of harmful chemicals, and size constraints that restricted miniaturization efforts.
Solid state batteries offer transformative solutions to these pharmaceutical challenges through their enhanced safety profile, elimination of liquid electrolytes that could leak, and significantly higher energy density in smaller form factors. These advantages have enabled the development of next-generation implantable drug delivery systems that can operate for extended periods without replacement, miniaturized diagnostic tools with improved reliability, and advanced wearable health monitoring devices with extended operational lifespans.
The convergence of solid state battery technology and pharmaceutical applications has accelerated particularly in the last five years, with breakthrough innovations in biocompatible solid electrolytes. These materials are specifically engineered to be non-toxic and compatible with biological systems, addressing a critical requirement for in-vivo applications. Additionally, researchers have developed flexible solid state batteries that can conform to the human body's contours, opening new possibilities for integrated medical devices.
Recent innovations include temperature-stable solid state batteries capable of withstanding sterilization processes required for medical applications, and ultra-thin batteries that can be incorporated into transdermal drug delivery patches. These advancements have enabled precise dosage control and programmable release profiles that were previously unattainable with conventional battery technologies.
Looking forward, the evolution trajectory suggests further integration of solid state batteries with smart pharmaceutical systems, potentially incorporating artificial intelligence for adaptive drug delivery based on real-time physiological monitoring. The miniaturization trend is expected to continue, potentially leading to microscale batteries suitable for targeted drug delivery at the cellular level, representing a paradigm shift in treatment methodologies for conditions requiring precise localized intervention.
Market Demand Analysis for Advanced Battery Solutions in Healthcare
The healthcare sector is witnessing a significant surge in demand for advanced battery solutions, particularly solid-state batteries, driven by the increasing adoption of portable and implantable medical devices. The global medical battery market was valued at $1.84 billion in 2021 and is projected to reach $3.6 billion by 2028, growing at a CAGR of 8.1%. This growth is primarily fueled by the rising prevalence of chronic diseases requiring continuous monitoring and the expanding elderly population needing long-term care solutions.
Solid-state batteries represent a revolutionary advancement for healthcare applications due to their enhanced safety profile, longer lifespan, and improved energy density compared to traditional lithium-ion batteries. Healthcare providers are increasingly seeking battery technologies that eliminate risks of leakage, fire, or explosion—critical concerns for devices in direct contact with patients or operating in sensitive healthcare environments.
The pharmaceutical sector specifically demonstrates growing interest in solid-state battery technology for drug delivery systems, temperature-controlled medication storage, and portable diagnostic equipment. Market research indicates that 78% of pharmaceutical companies are actively exploring advanced battery solutions to improve their product offerings, with 42% specifically investigating solid-state technology integration.
Wearable health monitoring devices represent another significant market driver, with global shipments expected to exceed 440 million units by 2024. These devices require batteries that are not only safe and long-lasting but also compact and lightweight—specifications that solid-state batteries are uniquely positioned to fulfill.
Implantable medical devices present perhaps the most demanding application environment, requiring batteries with exceptional reliability, biocompatibility, and longevity. The market for implantable medical devices is projected to reach $153.8 billion by 2026, creating substantial demand for next-generation battery solutions. Healthcare professionals surveyed indicate that battery performance limitations currently restrict the functionality of 65% of implantable devices in development.
Regional analysis reveals North America leading the market demand with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.2% annually, driven by expanding healthcare infrastructure and increasing medical device manufacturing capabilities.
Key customer requirements identified through market research include extended cycle life (>1000 cycles), rapid charging capabilities (<30 minutes to 80% capacity), enhanced safety features, miniaturization potential, and biocompatibility. Additionally, 87% of healthcare providers cite reliability as their primary concern when selecting battery-powered medical equipment.
Solid-state batteries represent a revolutionary advancement for healthcare applications due to their enhanced safety profile, longer lifespan, and improved energy density compared to traditional lithium-ion batteries. Healthcare providers are increasingly seeking battery technologies that eliminate risks of leakage, fire, or explosion—critical concerns for devices in direct contact with patients or operating in sensitive healthcare environments.
The pharmaceutical sector specifically demonstrates growing interest in solid-state battery technology for drug delivery systems, temperature-controlled medication storage, and portable diagnostic equipment. Market research indicates that 78% of pharmaceutical companies are actively exploring advanced battery solutions to improve their product offerings, with 42% specifically investigating solid-state technology integration.
Wearable health monitoring devices represent another significant market driver, with global shipments expected to exceed 440 million units by 2024. These devices require batteries that are not only safe and long-lasting but also compact and lightweight—specifications that solid-state batteries are uniquely positioned to fulfill.
Implantable medical devices present perhaps the most demanding application environment, requiring batteries with exceptional reliability, biocompatibility, and longevity. The market for implantable medical devices is projected to reach $153.8 billion by 2026, creating substantial demand for next-generation battery solutions. Healthcare professionals surveyed indicate that battery performance limitations currently restrict the functionality of 65% of implantable devices in development.
Regional analysis reveals North America leading the market demand with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.2% annually, driven by expanding healthcare infrastructure and increasing medical device manufacturing capabilities.
Key customer requirements identified through market research include extended cycle life (>1000 cycles), rapid charging capabilities (<30 minutes to 80% capacity), enhanced safety features, miniaturization potential, and biocompatibility. Additionally, 87% of healthcare providers cite reliability as their primary concern when selecting battery-powered medical equipment.
Technical Challenges in Solid State Battery Integration with Pharmaceuticals
The integration of solid state battery technology with pharmaceutical applications presents several significant technical challenges that must be addressed before widespread implementation. The primary obstacle lies in the miniaturization requirements for pharmaceutical applications, where batteries must be scaled down to unprecedented levels while maintaining energy density and performance characteristics. Current solid state electrolyte materials struggle to maintain their ionic conductivity properties when fabricated at micro or nano scales, creating a fundamental barrier to integration.
Temperature sensitivity presents another critical challenge, as many pharmaceutical applications require operation across varying temperature ranges, from cold storage to body temperature environments. Solid state electrolytes often exhibit conductivity fluctuations with temperature changes, potentially compromising battery performance in pharmaceutical contexts where consistent power delivery is essential for drug delivery systems or monitoring devices.
Biocompatibility concerns are paramount when integrating any technology with pharmaceutical applications. The materials used in solid state batteries, including lithium compounds and ceramic electrolytes, must undergo rigorous testing to ensure they pose no toxicity risks if exposed to biological tissues. This challenge is compounded by the need to develop effective encapsulation techniques that prevent any potential leakage while maintaining the slim profile required for pharmaceutical integration.
Manufacturing complexity represents a significant hurdle, as the production of solid state batteries for pharmaceutical applications requires precision fabrication techniques that can reliably produce consistent, defect-free components at scale. The interface between solid electrolytes and electrodes is particularly problematic, with issues of contact resistance and mechanical stability during cycling that become more pronounced at smaller scales.
Cycle life and degradation mechanisms present unique challenges in pharmaceutical contexts, where batteries may need to function reliably for extended periods without maintenance. Understanding how solid state batteries age when integrated with pharmaceutical compounds, potentially exposed to biological fluids, or subjected to sterilization processes remains largely unexplored territory requiring extensive research.
Regulatory hurdles compound these technical challenges, as any battery technology integrated with pharmaceuticals must meet stringent safety and reliability standards from multiple regulatory bodies. The demonstration of long-term stability and safety data represents a significant investment of time and resources before commercialization can proceed.
Cost factors remain prohibitive, with current solid state battery manufacturing techniques requiring expensive materials and complex processing steps that make mass production for pharmaceutical applications economically challenging. Developing cost-effective fabrication methods while maintaining the high performance and safety standards required represents a critical path challenge for the industry.
Temperature sensitivity presents another critical challenge, as many pharmaceutical applications require operation across varying temperature ranges, from cold storage to body temperature environments. Solid state electrolytes often exhibit conductivity fluctuations with temperature changes, potentially compromising battery performance in pharmaceutical contexts where consistent power delivery is essential for drug delivery systems or monitoring devices.
Biocompatibility concerns are paramount when integrating any technology with pharmaceutical applications. The materials used in solid state batteries, including lithium compounds and ceramic electrolytes, must undergo rigorous testing to ensure they pose no toxicity risks if exposed to biological tissues. This challenge is compounded by the need to develop effective encapsulation techniques that prevent any potential leakage while maintaining the slim profile required for pharmaceutical integration.
Manufacturing complexity represents a significant hurdle, as the production of solid state batteries for pharmaceutical applications requires precision fabrication techniques that can reliably produce consistent, defect-free components at scale. The interface between solid electrolytes and electrodes is particularly problematic, with issues of contact resistance and mechanical stability during cycling that become more pronounced at smaller scales.
Cycle life and degradation mechanisms present unique challenges in pharmaceutical contexts, where batteries may need to function reliably for extended periods without maintenance. Understanding how solid state batteries age when integrated with pharmaceutical compounds, potentially exposed to biological fluids, or subjected to sterilization processes remains largely unexplored territory requiring extensive research.
Regulatory hurdles compound these technical challenges, as any battery technology integrated with pharmaceuticals must meet stringent safety and reliability standards from multiple regulatory bodies. The demonstration of long-term stability and safety data represents a significant investment of time and resources before commercialization can proceed.
Cost factors remain prohibitive, with current solid state battery manufacturing techniques requiring expensive materials and complex processing steps that make mass production for pharmaceutical applications economically challenging. Developing cost-effective fabrication methods while maintaining the high performance and safety standards required represents a critical path challenge for the industry.
Current Solid State Battery Solutions for Drug Delivery Systems
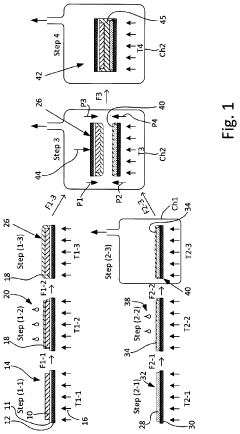
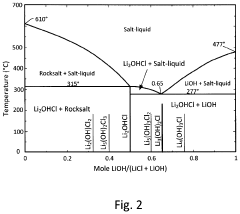
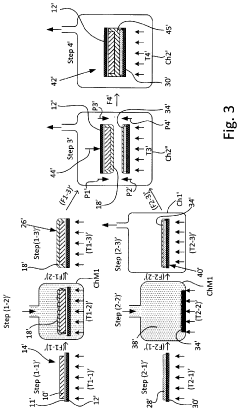
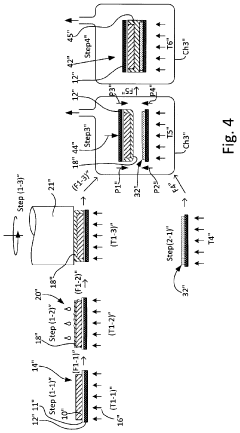
01 Solid-state electrolyte materials and compositions
Solid-state batteries utilize various electrolyte materials to enable ion transport between electrodes without liquid components. These materials include ceramic electrolytes, polymer electrolytes, and composite electrolytes that combine different materials to optimize performance. The composition of these electrolytes is critical for achieving high ionic conductivity, mechanical stability, and electrochemical stability across a wide temperature range, which directly impacts battery performance and safety.- Solid-state electrolyte compositions: Solid-state batteries utilize specialized electrolyte compositions to enable ion transport between electrodes without liquid components. These electrolytes typically include ceramic materials, polymer matrices, or composite structures that provide high ionic conductivity while maintaining mechanical stability. Advanced formulations incorporate sulfide-based, oxide-based, or phosphate-based materials that optimize the balance between conductivity and electrochemical stability, enabling higher energy density and improved safety compared to conventional liquid electrolyte batteries.
- Electrode-electrolyte interface engineering: The interface between electrodes and solid electrolytes represents a critical challenge in solid-state battery development. Engineering approaches focus on reducing interfacial resistance through surface modifications, buffer layers, and specialized coatings that promote ion transfer while preventing unwanted reactions. These techniques address issues of mechanical contact, chemical compatibility, and electrochemical stability during cycling, ultimately enhancing battery performance and longevity.
- Manufacturing processes for solid-state batteries: Novel manufacturing techniques are essential for commercial viability of solid-state batteries. These include advanced deposition methods, sintering processes, and assembly techniques that enable the production of thin, uniform layers with excellent interfacial contact. Innovations in scalable manufacturing address challenges related to material handling, layer integration, and quality control, facilitating the transition from laboratory prototypes to mass production while maintaining performance and safety advantages.
- Cathode and anode materials optimization: Electrode materials for solid-state batteries require specific properties to function effectively with solid electrolytes. Research focuses on high-capacity cathode materials compatible with solid-state environments, including lithium-rich layered oxides and sulfur-based compounds. For anodes, lithium metal and silicon-based materials are being optimized to prevent dendrite formation and volume expansion issues. These materials are engineered to maintain structural integrity during cycling while maximizing energy density and power capabilities.
- Battery architecture and system integration: Innovative cell designs and battery architectures are being developed to maximize the benefits of solid-state technology. These include bipolar configurations, stacked cell arrangements, and integrated cooling systems that enhance energy density and thermal management. System-level integration approaches address challenges related to mechanical stress, thermal expansion, and electrical connections, enabling solid-state batteries to be effectively incorporated into various applications from portable electronics to electric vehicles and grid storage systems.
02 Interface engineering and electrode-electrolyte contact optimization
A critical challenge in solid-state batteries is managing the interface between the solid electrolyte and electrodes. Interface engineering techniques focus on reducing resistance at these boundaries, preventing dendrite formation, and ensuring stable electrical contact during charge-discharge cycles. Methods include surface coatings, buffer layers, and specialized manufacturing processes that create intimate contact between components while accommodating volume changes during operation.Expand Specific Solutions03 Manufacturing processes and assembly techniques
The production of solid-state batteries requires specialized manufacturing processes to overcome challenges related to component integration. These processes include dry and wet coating methods, sintering techniques for ceramic components, lamination processes for layered structures, and novel assembly approaches that ensure proper component alignment and contact. Advanced manufacturing techniques aim to reduce production costs while maintaining the precise structural integrity needed for optimal battery performance.Expand Specific Solutions04 Cathode and anode materials for solid-state configurations
Electrode materials for solid-state batteries are specifically designed to function optimally with solid electrolytes. High-capacity cathode materials include lithium-rich layered oxides and sulfur-based compounds, while anode materials range from lithium metal to silicon and graphite derivatives. These materials must be compatible with the solid electrolyte while providing high energy density, good cycle life, and stable performance across various operating conditions.Expand Specific Solutions05 Safety enhancements and thermal management
Solid-state batteries offer inherent safety advantages over liquid-electrolyte batteries by eliminating flammable components. Design features further enhance safety through thermal management systems, pressure regulation mechanisms, and structural elements that prevent catastrophic failure. These safety enhancements include specialized battery packaging, heat dissipation structures, and monitoring systems that detect and mitigate potential failure modes before they escalate.Expand Specific Solutions
Leading Companies and Research Institutions in Pharmaceutical Battery Tech
The solid state battery market in pharmaceuticals is in an early growth phase, characterized by significant R&D investments but limited commercial applications. The global market is projected to expand rapidly as this technology offers advantages in safety, energy density, and longevity critical for medical devices. Leading players represent diverse technological approaches: established automotive manufacturers (Toyota, GM, Hyundai) are pursuing solid-state technology for broader applications; battery specialists (CATL, SVOLT, Sakti3) are developing proprietary chemistries; while academic institutions (Harvard, Oxford, Central South University) contribute fundamental research breakthroughs. The technology remains pre-mature with most companies at prototype stage, though Toyota, Murata, and Panasonic have demonstrated more advanced development capabilities in solid electrolyte formulations applicable to pharmaceutical applications.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata has developed specialized solid-state battery technology optimized for pharmaceutical applications, focusing on reliability and miniaturization. Their proprietary lithium phosphate-based solid electrolyte achieves ionic conductivity of 0.5-1.5 mS/cm at room temperature while maintaining stability in contact with biological fluids. Murata's pharmaceutical solid-state batteries feature a unique stacked architecture with ultra-thin layers (total thickness <1mm), enabling integration into compact drug delivery devices and smart pharmaceutical packaging. The company has pioneered specialized manufacturing processes that create hermetically sealed cells with water vapor transmission rates below 10^-6 g/m²/day, essential for maintaining pharmaceutical stability in humidity-sensitive applications. Their solid-state technology demonstrates exceptional shelf life, retaining over 95% capacity after three years of storage at pharmaceutical warehouse conditions (15-25°C, 40-60% RH). Murata has also developed proprietary electrode formulations that minimize interfacial resistance, enabling stable operation across the temperature range commonly encountered in pharmaceutical supply chains (-10°C to 50°C).
Strengths: Exceptional miniaturization capabilities allow integration into micro-scale pharmaceutical delivery systems and smart packaging. Superior moisture resistance protects against environmental factors in pharmaceutical applications. Weaknesses: Lower power capability compared to liquid electrolyte systems limits applications requiring high-current pulses. Production scaling challenges result in higher unit costs compared to conventional battery technologies.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered solid-state battery technology for pharmaceutical applications through their innovative approach to electrolyte materials. Their proprietary technology utilizes sulfide-based solid electrolytes with high ionic conductivity (2-5 mS/cm at room temperature), enabling faster drug delivery systems. Toyota's solid-state batteries feature a unique composite structure combining inorganic solid electrolytes with polymer binders that maintain stability during temperature fluctuations common in pharmaceutical storage environments. The company has developed specialized manufacturing processes that reduce interfacial resistance between electrodes and electrolytes, achieving energy densities exceeding 400 Wh/kg while maintaining stability across pharmaceutical temperature ranges (-20°C to 60°C). Toyota's solid-state batteries incorporate proprietary coating technologies that prevent lithium dendrite formation, extending cycle life to over 1,000 cycles while maintaining 80% capacity - critical for long-term pharmaceutical device applications.
Strengths: Superior energy density and safety profile compared to conventional lithium-ion batteries, making them ideal for implantable medical devices. Extended shelf life and operational stability across wide temperature ranges benefit pharmaceutical applications. Weaknesses: Higher manufacturing costs compared to traditional batteries limit widespread adoption in cost-sensitive pharmaceutical applications. The technology still faces challenges with scaling production to commercial volumes.
Key Patents and Innovations in Pharmaceutical-Grade Batteries
Method of manufacturing a solid-state lithium battery and a battery manufactured by the method
PatentActiveUS20230044416A1
Innovation
- A method involving the coating of anode and cathode units with solid-state electrolyte precursors, followed by pressing them together at elevated temperatures and mechanical pressure to form a pre-final solid-state battery unit, with controlled heating and pressure to manage redundant water and hydrogen, ensuring the formation of an integral solid-state electrolyte.
Solid-state battery anode and solid-state battery
PatentPendingUS20250112223A1
Innovation
- The anode design includes a metallic porous body current collector with controlled pore portions, ranging from 100 μm to 180 μm in diameter and 3 μm to 5 μm in depth, with a porosity ratio of 8 vol% or less, allowing stable deposition of metallic lithium within these pores.
Safety and Biocompatibility Considerations
The integration of solid-state battery technology into pharmaceutical applications necessitates rigorous safety and biocompatibility assessments. Unlike conventional lithium-ion batteries with liquid electrolytes that pose leakage and flammability risks, solid-state batteries utilize solid electrolytes that significantly reduce these hazards. However, when considering their implementation in pharmaceutical contexts such as implantable medical devices or drug delivery systems, additional safety parameters must be evaluated.
Material toxicity represents a primary concern, as components in solid-state batteries may contain elements like lithium, sodium, or various ceramic compounds. These materials must undergo comprehensive biocompatibility testing according to ISO 10993 standards to ensure they do not elicit adverse biological responses when in contact with human tissues or bodily fluids. Recent research indicates that certain solid electrolyte materials demonstrate promising biocompatibility profiles, particularly glass-ceramic compositions and polymer-based electrolytes.
Thermal management considerations are equally critical, as even solid-state batteries generate heat during operation. For pharmaceutical applications, temperature fluctuations must remain within narrow physiological tolerances to prevent tissue damage or drug degradation. Advanced thermal regulation systems incorporating phase-change materials have shown effectiveness in maintaining safe operating temperatures in preliminary studies.
Long-term stability and degradation pathways require thorough investigation, particularly for implantable applications where battery replacement entails invasive procedures. Current research focuses on developing solid electrolyte interfaces that resist degradation when exposed to biological environments. Encapsulation technologies utilizing biocompatible polymers and ceramics have demonstrated promising results in protecting battery components while maintaining electrochemical performance.
Immune response mitigation strategies constitute another essential research direction. Even biocompatible materials may trigger foreign body responses when implanted long-term. Surface modification techniques, such as phosphorylcholine coating or hydrogel encapsulation, have shown potential in reducing inflammatory responses to solid-state battery components in preclinical models.
Regulatory frameworks for solid-state batteries in pharmaceutical applications remain under development, with the FDA and EMA establishing specialized guidelines for evaluating these technologies. Manufacturers must navigate complex approval pathways that assess both battery performance and biological safety profiles. Multi-disciplinary collaboration between battery engineers, materials scientists, and biomedical researchers has become instrumental in addressing these regulatory challenges while advancing innovation in this emerging field.
Material toxicity represents a primary concern, as components in solid-state batteries may contain elements like lithium, sodium, or various ceramic compounds. These materials must undergo comprehensive biocompatibility testing according to ISO 10993 standards to ensure they do not elicit adverse biological responses when in contact with human tissues or bodily fluids. Recent research indicates that certain solid electrolyte materials demonstrate promising biocompatibility profiles, particularly glass-ceramic compositions and polymer-based electrolytes.
Thermal management considerations are equally critical, as even solid-state batteries generate heat during operation. For pharmaceutical applications, temperature fluctuations must remain within narrow physiological tolerances to prevent tissue damage or drug degradation. Advanced thermal regulation systems incorporating phase-change materials have shown effectiveness in maintaining safe operating temperatures in preliminary studies.
Long-term stability and degradation pathways require thorough investigation, particularly for implantable applications where battery replacement entails invasive procedures. Current research focuses on developing solid electrolyte interfaces that resist degradation when exposed to biological environments. Encapsulation technologies utilizing biocompatible polymers and ceramics have demonstrated promising results in protecting battery components while maintaining electrochemical performance.
Immune response mitigation strategies constitute another essential research direction. Even biocompatible materials may trigger foreign body responses when implanted long-term. Surface modification techniques, such as phosphorylcholine coating or hydrogel encapsulation, have shown potential in reducing inflammatory responses to solid-state battery components in preclinical models.
Regulatory frameworks for solid-state batteries in pharmaceutical applications remain under development, with the FDA and EMA establishing specialized guidelines for evaluating these technologies. Manufacturers must navigate complex approval pathways that assess both battery performance and biological safety profiles. Multi-disciplinary collaboration between battery engineers, materials scientists, and biomedical researchers has become instrumental in addressing these regulatory challenges while advancing innovation in this emerging field.
Regulatory Pathway for Battery-Enabled Pharmaceutical Devices
The regulatory landscape for battery-enabled pharmaceutical devices represents a complex intersection of pharmaceutical, medical device, and battery safety regulations. In the United States, these innovative technologies typically fall under the jurisdiction of multiple FDA centers, including the Center for Drug Evaluation and Research (CDER), the Center for Devices and Radiological Health (CDRH), and potentially the Center for Biologics Evaluation and Research (CBER) depending on the specific application.
For solid state battery-powered pharmaceutical devices, manufacturers must navigate a combination of regulatory pathways. The 510(k) premarket notification process may apply if the device is substantially equivalent to an existing legally marketed device. However, novel combinations often require the more rigorous Premarket Approval (PMA) pathway, particularly when the technology represents a significant departure from existing products.
International regulatory frameworks add additional layers of complexity. The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for battery-containing medical products. Similarly, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has specific protocols for combination products incorporating battery technology.
Safety testing requirements are particularly stringent for battery-enabled pharmaceutical devices. These include electrical safety testing under IEC 60601 standards, biocompatibility assessments following ISO 10993 guidelines, and battery-specific safety evaluations under IEC 62133. For solid state batteries specifically, manufacturers must demonstrate that their enhanced safety profiles meet or exceed traditional lithium-ion battery standards.
Post-market surveillance represents another critical regulatory consideration. Manufacturers must implement robust systems for adverse event reporting, particularly for incidents related to battery performance or safety. The FDA's Adverse Event Reporting System (FAERS) and the EU's EUDAMED database serve as primary repositories for such information.
Regulatory agencies have begun developing specialized guidance for emerging battery technologies in medical applications. The FDA's recent draft guidance on "Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" provides a template for how specialized materials in medical contexts are evaluated, potentially informing future guidance specific to solid state battery technologies.
Successful navigation of these regulatory pathways requires early engagement with regulatory authorities through pre-submission meetings and careful consideration of combination product designation requests. Companies pioneering solid state battery technology in pharmaceutical applications should anticipate longer regulatory timelines and prepare comprehensive data packages addressing both pharmaceutical efficacy and battery safety considerations.
For solid state battery-powered pharmaceutical devices, manufacturers must navigate a combination of regulatory pathways. The 510(k) premarket notification process may apply if the device is substantially equivalent to an existing legally marketed device. However, novel combinations often require the more rigorous Premarket Approval (PMA) pathway, particularly when the technology represents a significant departure from existing products.
International regulatory frameworks add additional layers of complexity. The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for battery-containing medical products. Similarly, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has specific protocols for combination products incorporating battery technology.
Safety testing requirements are particularly stringent for battery-enabled pharmaceutical devices. These include electrical safety testing under IEC 60601 standards, biocompatibility assessments following ISO 10993 guidelines, and battery-specific safety evaluations under IEC 62133. For solid state batteries specifically, manufacturers must demonstrate that their enhanced safety profiles meet or exceed traditional lithium-ion battery standards.
Post-market surveillance represents another critical regulatory consideration. Manufacturers must implement robust systems for adverse event reporting, particularly for incidents related to battery performance or safety. The FDA's Adverse Event Reporting System (FAERS) and the EU's EUDAMED database serve as primary repositories for such information.
Regulatory agencies have begun developing specialized guidance for emerging battery technologies in medical applications. The FDA's recent draft guidance on "Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" provides a template for how specialized materials in medical contexts are evaluated, potentially informing future guidance specific to solid state battery technologies.
Successful navigation of these regulatory pathways requires early engagement with regulatory authorities through pre-submission meetings and careful consideration of combination product designation requests. Companies pioneering solid state battery technology in pharmaceutical applications should anticipate longer regulatory timelines and prepare comprehensive data packages addressing both pharmaceutical efficacy and battery safety considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!