Solving Overlapping Peaks in NMR Through Multidimensional Approaches
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Spectroscopy Evolution and Research Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, transforming from a technique primarily used for structural elucidation of simple molecules to a sophisticated analytical method capable of investigating complex biological systems. The initial development focused on one-dimensional (1D) NMR techniques, which provided valuable information about molecular structure but faced significant limitations when analyzing complex mixtures due to signal overlap.
The 1970s marked a pivotal turning point with the introduction of two-dimensional (2D) NMR methods by Richard Ernst, who later received the Nobel Prize for his contributions. This innovation fundamentally changed the landscape of NMR spectroscopy by distributing spectral information across two frequency dimensions, thereby significantly enhancing resolution and enabling the analysis of more complex molecular systems.
Further technological advancements in the 1980s and 1990s led to the development of higher-dimensional NMR techniques (3D, 4D, and beyond), which have become essential tools in structural biology, particularly for protein structure determination. These multidimensional approaches have been instrumental in addressing the persistent challenge of overlapping peaks, which remains one of the most significant obstacles in NMR analysis.
The evolution of NMR hardware has paralleled methodological developments, with higher field strengths, cryogenic probe technology, and advanced pulse sequence designs collectively enhancing sensitivity and resolution. Modern NMR spectrometers operating at field strengths of 1 GHz and above represent the cutting edge of this technology, offering unprecedented resolution for complex mixture analysis.
Recent years have witnessed the integration of NMR with computational methods, including machine learning and artificial intelligence, to further address the overlapping peaks problem. These hybrid approaches leverage pattern recognition and statistical analysis to deconvolute complex spectra and extract meaningful structural information from seemingly uninterpretable data.
The primary objective of current research in multidimensional NMR approaches is to develop robust methodologies that can effectively resolve overlapping signals in complex mixtures without requiring excessive measurement time or sample quantities. This includes optimizing pulse sequences, exploring novel sampling strategies such as non-uniform sampling (NUS), and developing advanced computational algorithms for spectral reconstruction and analysis.
Additional research goals include enhancing the accessibility of multidimensional NMR techniques for routine analytical applications, improving quantitative accuracy in the presence of overlapping signals, and developing specialized approaches for specific classes of compounds or biological systems where peak overlap presents particular challenges.
The 1970s marked a pivotal turning point with the introduction of two-dimensional (2D) NMR methods by Richard Ernst, who later received the Nobel Prize for his contributions. This innovation fundamentally changed the landscape of NMR spectroscopy by distributing spectral information across two frequency dimensions, thereby significantly enhancing resolution and enabling the analysis of more complex molecular systems.
Further technological advancements in the 1980s and 1990s led to the development of higher-dimensional NMR techniques (3D, 4D, and beyond), which have become essential tools in structural biology, particularly for protein structure determination. These multidimensional approaches have been instrumental in addressing the persistent challenge of overlapping peaks, which remains one of the most significant obstacles in NMR analysis.
The evolution of NMR hardware has paralleled methodological developments, with higher field strengths, cryogenic probe technology, and advanced pulse sequence designs collectively enhancing sensitivity and resolution. Modern NMR spectrometers operating at field strengths of 1 GHz and above represent the cutting edge of this technology, offering unprecedented resolution for complex mixture analysis.
Recent years have witnessed the integration of NMR with computational methods, including machine learning and artificial intelligence, to further address the overlapping peaks problem. These hybrid approaches leverage pattern recognition and statistical analysis to deconvolute complex spectra and extract meaningful structural information from seemingly uninterpretable data.
The primary objective of current research in multidimensional NMR approaches is to develop robust methodologies that can effectively resolve overlapping signals in complex mixtures without requiring excessive measurement time or sample quantities. This includes optimizing pulse sequences, exploring novel sampling strategies such as non-uniform sampling (NUS), and developing advanced computational algorithms for spectral reconstruction and analysis.
Additional research goals include enhancing the accessibility of multidimensional NMR techniques for routine analytical applications, improving quantitative accuracy in the presence of overlapping signals, and developing specialized approaches for specific classes of compounds or biological systems where peak overlap presents particular challenges.
Market Applications and Demand for High-Resolution NMR
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved into an indispensable analytical tool across multiple industries, with high-resolution capabilities driving significant market demand. The global NMR market is currently valued at over 2.5 billion USD, with projections indicating growth to reach 3.2 billion USD by 2027, representing a compound annual growth rate of approximately 3.8%.
The pharmaceutical and biotechnology sectors constitute the largest market segment for high-resolution NMR technologies, accounting for nearly 40% of the total market share. These industries rely heavily on advanced NMR techniques for drug discovery, development, and quality control processes. The ability to resolve overlapping peaks through multidimensional approaches has become particularly crucial for analyzing complex biological macromolecules, including proteins and nucleic acids, which are central to modern drug development pipelines.
Food and beverage industries represent another significant market segment, where high-resolution NMR is increasingly employed for authentication, quality assessment, and detection of adulterants. The growing consumer demand for transparency in food composition has accelerated the adoption of sophisticated analytical methods capable of providing detailed molecular fingerprints of food products.
Academic and research institutions continue to drive innovation in NMR applications, creating a steady demand for cutting-edge instrumentation and methodologies. Government funding for research in structural biology, metabolomics, and materials science has further stimulated market growth in this segment.
The petrochemical industry utilizes high-resolution NMR for characterizing complex hydrocarbon mixtures, optimizing refining processes, and developing new materials. The ability to distinguish between closely related chemical structures through multidimensional NMR approaches translates directly into economic benefits through improved process efficiency and product quality.
Emerging applications in environmental monitoring, forensic science, and clinical diagnostics are expanding the market reach of high-resolution NMR technologies. Particularly in metabolomics and personalized medicine, the demand for techniques capable of resolving complex biological samples with overlapping spectral features has seen remarkable growth.
Geographically, North America dominates the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and India, along with the expansion of pharmaceutical and biotechnology sectors in these countries.
The market demand is increasingly shifting toward more accessible, user-friendly systems that can deliver high-resolution capabilities without requiring extensive specialized training. This trend is opening new market segments among smaller research facilities, quality control laboratories, and educational institutions previously limited by technical expertise requirements.
The pharmaceutical and biotechnology sectors constitute the largest market segment for high-resolution NMR technologies, accounting for nearly 40% of the total market share. These industries rely heavily on advanced NMR techniques for drug discovery, development, and quality control processes. The ability to resolve overlapping peaks through multidimensional approaches has become particularly crucial for analyzing complex biological macromolecules, including proteins and nucleic acids, which are central to modern drug development pipelines.
Food and beverage industries represent another significant market segment, where high-resolution NMR is increasingly employed for authentication, quality assessment, and detection of adulterants. The growing consumer demand for transparency in food composition has accelerated the adoption of sophisticated analytical methods capable of providing detailed molecular fingerprints of food products.
Academic and research institutions continue to drive innovation in NMR applications, creating a steady demand for cutting-edge instrumentation and methodologies. Government funding for research in structural biology, metabolomics, and materials science has further stimulated market growth in this segment.
The petrochemical industry utilizes high-resolution NMR for characterizing complex hydrocarbon mixtures, optimizing refining processes, and developing new materials. The ability to distinguish between closely related chemical structures through multidimensional NMR approaches translates directly into economic benefits through improved process efficiency and product quality.
Emerging applications in environmental monitoring, forensic science, and clinical diagnostics are expanding the market reach of high-resolution NMR technologies. Particularly in metabolomics and personalized medicine, the demand for techniques capable of resolving complex biological samples with overlapping spectral features has seen remarkable growth.
Geographically, North America dominates the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments in China, Japan, and India, along with the expansion of pharmaceutical and biotechnology sectors in these countries.
The market demand is increasingly shifting toward more accessible, user-friendly systems that can deliver high-resolution capabilities without requiring extensive specialized training. This trend is opening new market segments among smaller research facilities, quality control laboratories, and educational institutions previously limited by technical expertise requirements.
Current Limitations in NMR Peak Resolution
Nuclear Magnetic Resonance (NMR) spectroscopy faces significant challenges in peak resolution, particularly when analyzing complex molecular structures. The primary limitation stems from signal overlap in one-dimensional spectra, where peaks from different nuclei coincide at similar chemical shift values. This phenomenon becomes increasingly problematic as molecular complexity increases, with biomolecules, polymers, and natural products being especially challenging.
Resolution limitations are fundamentally tied to the physical constraints of NMR instrumentation. Even with high-field magnets (up to 1.2 GHz), the natural linewidth of signals and the finite difference in chemical shifts between nuclei create inherent barriers to complete peak separation. This is exacerbated by factors such as magnetic field inhomogeneity, which broadens peaks and further reduces resolution.
Digital resolution constraints also impact peak separation capabilities. The number of data points collected during acquisition directly affects the digital resolution of the resulting spectrum. While increasing acquisition time can improve resolution, this approach is limited by relaxation processes and sample stability considerations, creating a practical ceiling for resolution enhancement through this method.
Sample-related factors contribute significantly to resolution challenges. Molecular dynamics, including conformational exchange and aggregation, can lead to peak broadening. Viscosity effects in concentrated samples or macromolecular systems further degrade spectral quality by affecting molecular tumbling rates and relaxation properties.
The presence of paramagnetic species, either as impurities or as integral components of the sample, introduces additional line-broadening effects through enhanced relaxation mechanisms. This particularly impacts metalloproteins and organometallic compounds, where the paramagnetic centers are essential to the molecular structure but detrimental to spectral resolution.
Traditional processing techniques like apodization functions offer limited improvement. While window functions can enhance resolution, they invariably introduce artifacts or signal-to-noise ratio penalties, representing a fundamental trade-off rather than a solution to the underlying problem.
Quantitative analysis becomes unreliable when peaks overlap, as accurate integration of individual signals becomes impossible. This severely impacts applications requiring precise concentration measurements or reaction monitoring, limiting the analytical power of NMR in complex mixtures.
These limitations collectively create a significant barrier to the application of NMR in fields requiring detailed structural analysis of complex systems, driving the need for multidimensional approaches that can distribute spectral information across additional frequency dimensions to alleviate overlap problems.
Resolution limitations are fundamentally tied to the physical constraints of NMR instrumentation. Even with high-field magnets (up to 1.2 GHz), the natural linewidth of signals and the finite difference in chemical shifts between nuclei create inherent barriers to complete peak separation. This is exacerbated by factors such as magnetic field inhomogeneity, which broadens peaks and further reduces resolution.
Digital resolution constraints also impact peak separation capabilities. The number of data points collected during acquisition directly affects the digital resolution of the resulting spectrum. While increasing acquisition time can improve resolution, this approach is limited by relaxation processes and sample stability considerations, creating a practical ceiling for resolution enhancement through this method.
Sample-related factors contribute significantly to resolution challenges. Molecular dynamics, including conformational exchange and aggregation, can lead to peak broadening. Viscosity effects in concentrated samples or macromolecular systems further degrade spectral quality by affecting molecular tumbling rates and relaxation properties.
The presence of paramagnetic species, either as impurities or as integral components of the sample, introduces additional line-broadening effects through enhanced relaxation mechanisms. This particularly impacts metalloproteins and organometallic compounds, where the paramagnetic centers are essential to the molecular structure but detrimental to spectral resolution.
Traditional processing techniques like apodization functions offer limited improvement. While window functions can enhance resolution, they invariably introduce artifacts or signal-to-noise ratio penalties, representing a fundamental trade-off rather than a solution to the underlying problem.
Quantitative analysis becomes unreliable when peaks overlap, as accurate integration of individual signals becomes impossible. This severely impacts applications requiring precise concentration measurements or reaction monitoring, limiting the analytical power of NMR in complex mixtures.
These limitations collectively create a significant barrier to the application of NMR in fields requiring detailed structural analysis of complex systems, driving the need for multidimensional approaches that can distribute spectral information across additional frequency dimensions to alleviate overlap problems.
Contemporary Multidimensional NMR Methodologies
01 Advanced signal processing techniques for resolving overlapping peaks
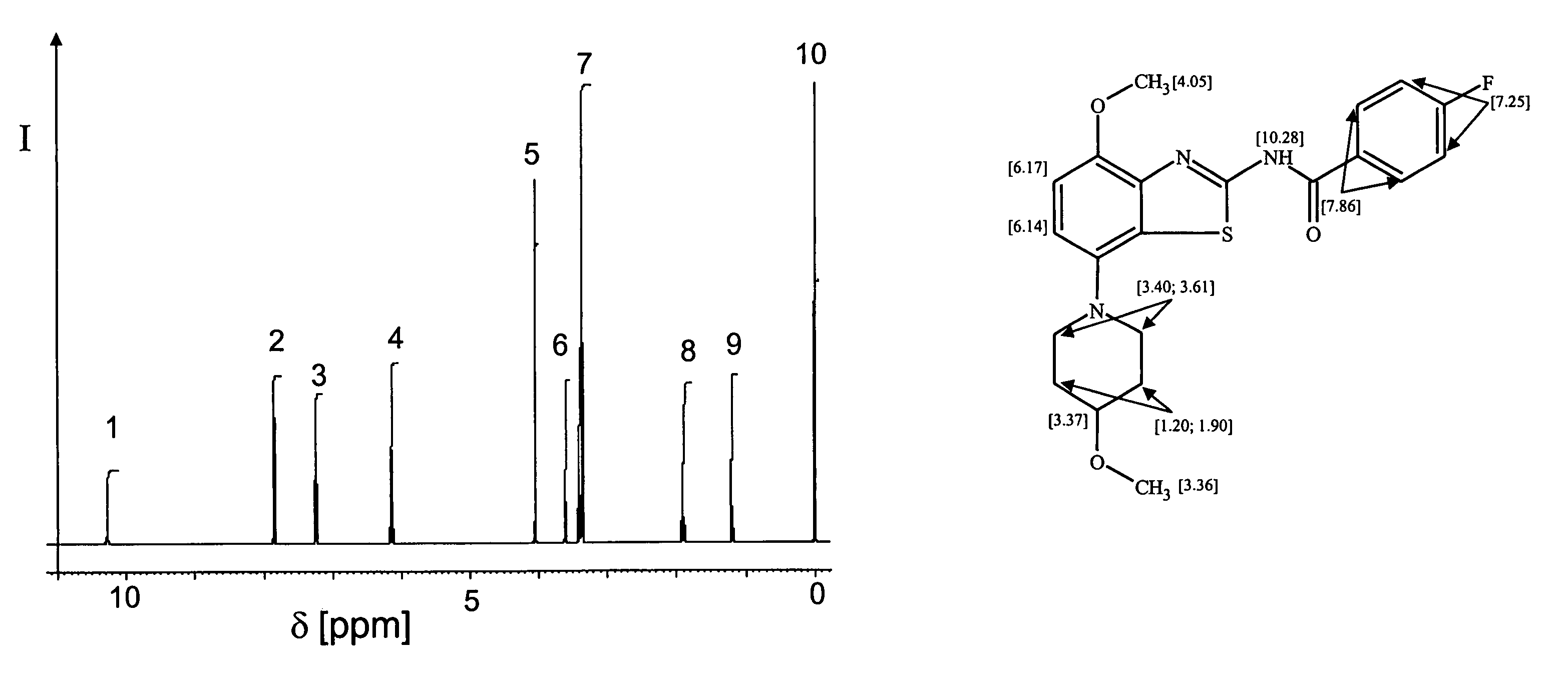
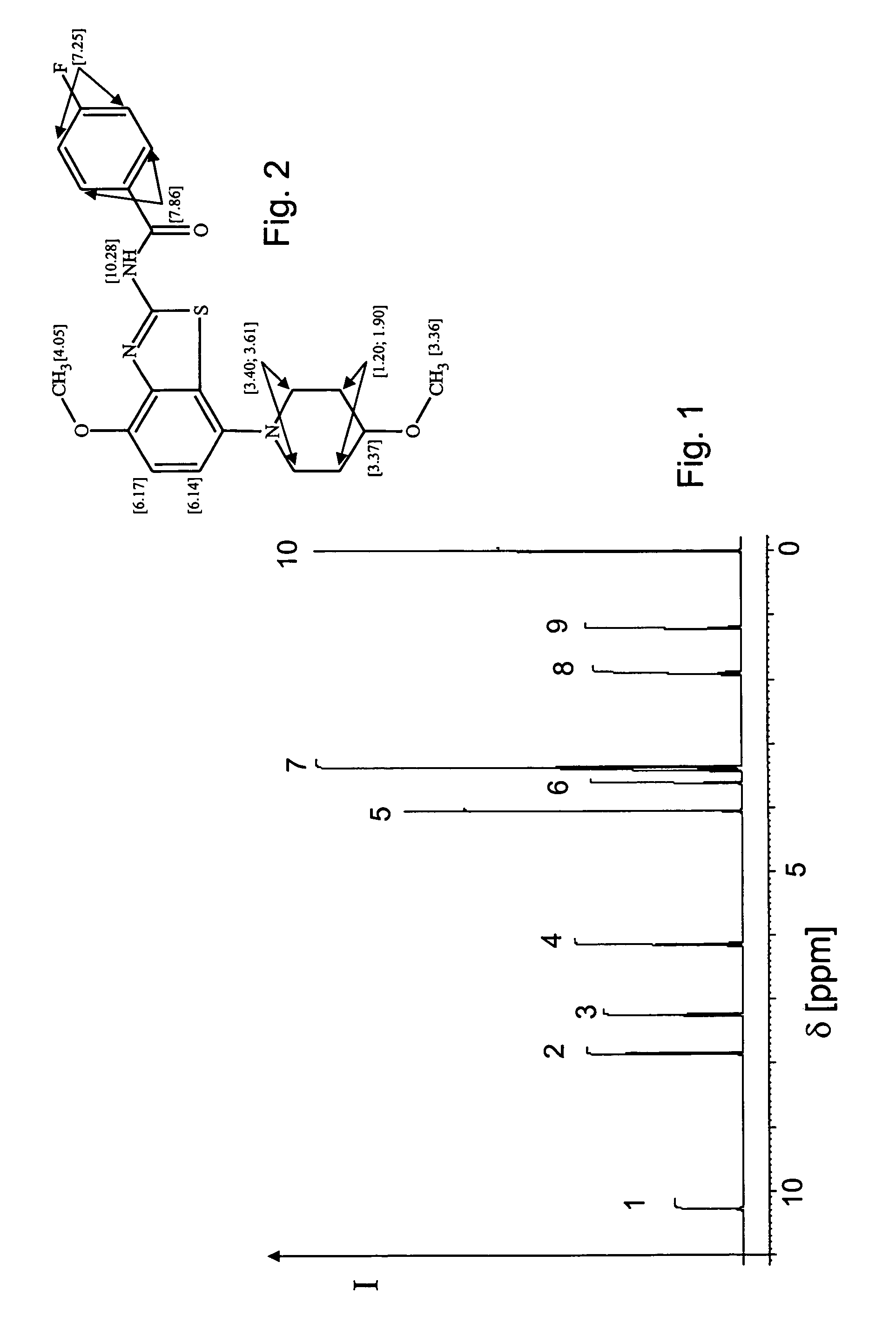
Various mathematical and computational methods are employed to separate and analyze overlapping peaks in NMR spectra. These techniques include deconvolution algorithms, Fourier transformation modifications, and specialized software tools that can distinguish between closely positioned signals. These approaches enhance spectral resolution and allow for more accurate interpretation of complex NMR data where peak overlap occurs due to similar chemical environments.- Advanced signal processing techniques for resolving overlapping peaks: Various signal processing algorithms and mathematical methods can be applied to NMR spectroscopy data to separate and resolve overlapping peaks. These techniques include deconvolution, Fourier transformation optimization, and specialized computational approaches that can distinguish between closely positioned or partially overlapping resonance signals. These methods enhance the accuracy of compound identification and quantification in complex mixtures where peak overlap is common.
- Multi-dimensional NMR techniques for peak separation: Two-dimensional and multi-dimensional NMR spectroscopy techniques provide additional spectral dimensions that help separate overlapping peaks by distributing the signals across multiple axes. These approaches include COSY, TOCSY, HSQC, and HMBC experiments that correlate different nuclei or interactions, effectively spreading the spectral information to reduce overlap and increase resolution. This enables more accurate analysis of complex molecular structures and mixtures.
- Hardware modifications and pulse sequence optimization: Specialized hardware configurations and optimized pulse sequences can significantly improve the resolution of NMR spectra and help distinguish overlapping peaks. These include high-field magnets, cryogenic probes, gradient-enhanced techniques, and selective excitation methods. By manipulating the magnetic field gradients and radiofrequency pulses, these approaches can selectively enhance certain signals while suppressing others, leading to better separation of overlapping resonances.
- Chemometric and statistical methods for peak analysis: Chemometric approaches and statistical analysis methods can be applied to extract meaningful information from NMR spectra with overlapping peaks. These include principal component analysis (PCA), partial least squares (PLS), and various clustering algorithms that can identify patterns and separate contributions from different components in a mixture. These methods are particularly useful in metabolomics, pharmaceutical analysis, and quality control applications where complex mixtures are common.
- Sample preparation and experimental condition optimization: Optimizing sample preparation methods and experimental conditions can significantly reduce peak overlap in NMR spectroscopy. Techniques include solvent selection, pH adjustment, temperature control, and addition of shift reagents that can alter the chemical environment of specific nuclei. Additionally, isotopic labeling and selective deuteration can simplify spectra by highlighting specific molecular components or reducing the number of observable signals, thereby minimizing overlap issues.
02 Multi-dimensional NMR methods for peak separation
Two-dimensional and multi-dimensional NMR techniques provide additional spectral dimensions to separate overlapping peaks that cannot be resolved in one-dimensional spectra. These methods correlate different types of nuclear interactions and spread the spectral information across multiple frequency axes, effectively reducing peak overlap and increasing the ability to distinguish between similar chemical structures or conformations.Expand Specific Solutions03 Hardware modifications to improve spectral resolution
Specialized hardware configurations and modifications to NMR instruments can significantly improve the resolution of overlapping peaks. These include higher magnetic field strengths, advanced probe designs, gradient coils with improved linearity, and optimized radio frequency circuits. Such hardware enhancements increase the frequency separation between similar chemical environments, making it easier to distinguish between overlapping signals.Expand Specific Solutions04 Pulse sequence optimization for overlapping peak resolution
Customized pulse sequences can be designed to selectively enhance or suppress specific signals in NMR spectra, helping to resolve overlapping peaks. These specialized sequences manipulate spin systems through precisely timed radiofrequency pulses and delays, allowing for selective excitation, improved coherence transfer pathways, or spectral editing that can separate otherwise overlapping resonances based on their different relaxation properties or coupling patterns.Expand Specific Solutions05 Sample preparation and experimental condition optimization
Careful sample preparation and optimization of experimental conditions can significantly reduce peak overlap in NMR spectra. Techniques include adjusting sample concentration, pH, temperature, and solvent composition to minimize line broadening and maximize spectral dispersion. Additionally, the use of deuterated solvents, paramagnetic relaxation agents, or chemical shift reagents can alter the chemical shift distribution and help resolve overlapping signals.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The NMR multidimensional approaches for solving overlapping peaks are in a growth phase, with the market expanding as analytical demands increase across pharmaceutical and research sectors. The technology has reached moderate maturity, with established players like Bruker BioSpin, Shimadzu, and F. Hoffmann-La Roche leading commercial applications. Academic institutions including Oxford University, Harvard College, and Xiamen University contribute significant research advancements. The competitive landscape features specialized instrumentation companies (Bruker, Aspect Imaging) alongside pharmaceutical entities leveraging the technology for drug development. Research foundations like Wisconsin Alumni Research Foundation and Yeda Research & Development play crucial roles in technology transfer, bridging academic innovation with industrial applications in this evolving field.
Wisconsin Alumni Research Foundation
Technical Solution: Wisconsin Alumni Research Foundation (WARF) has developed innovative approaches to resolving overlapping peaks in NMR through advanced multidimensional techniques. Their technology centers on novel pulse sequence designs that efficiently spread spectral information across multiple dimensions while minimizing experimental time. WARF's approach incorporates optimized coherence pathway selection and gradient encoding strategies that enhance spectral resolution in crowded regions. Their proprietary "Spectral Unfolding Technology" employs mathematical transformations to separate overlapping resonances based on subtle differences in relaxation properties and coupling patterns. The foundation has also pioneered hybrid acquisition schemes that combine aspects of traditional multidimensional experiments with pure-shift methodologies, effectively decoupling interactions that contribute to spectral congestion. Recent developments include machine learning algorithms that can predict optimal experiment parameters based on preliminary sample data, significantly reducing the trial-and-error process typically required for complex samples with extensive peak overlap.
Strengths: Strong theoretical foundation based on academic research excellence; innovative approaches not found in commercial systems; excellent performance for specialized applications in structural biology. Weaknesses: Less comprehensive support infrastructure compared to commercial vendors; higher technical expertise required for implementation; more limited integration with standard laboratory workflows.
Bruker Switzerland AG
Technical Solution: Bruker Switzerland AG has developed advanced multidimensional NMR spectroscopy solutions to address overlapping peaks challenges. Their technology employs sophisticated pulse sequences and gradient-enhanced methods that spread spectral information across multiple dimensions, effectively separating signals that would overlap in conventional 1D spectra. Their NMR systems incorporate proprietary TopSpin software with specialized algorithms for peak deconvolution and analysis of complex spectra. Bruker's recent innovations include non-uniform sampling (NUS) techniques that significantly reduce acquisition times for multidimensional experiments while maintaining resolution. Their hardware advancements feature high-field magnets (up to 1.2 GHz) and cryoprobe technology that enhances sensitivity by factors of 4-5 compared to conventional probes, enabling detection of lower concentration samples where peak overlap is particularly problematic.
Strengths: Industry-leading hardware sensitivity and resolution capabilities; comprehensive software ecosystem for data processing; extensive experience in multidimensional NMR implementation. Weaknesses: High cost of advanced systems limits accessibility; complex workflows require significant expertise; proprietary software ecosystem can create vendor lock-in for laboratories.
Breakthrough Algorithms for Spectral Deconvolution
Nuclear spin singlet states as a contrast mechanism for NMR spectroscopy
PatentWO2013130756A1
Innovation
- The method involves creating a nuclear spin singlet state in a target molecule using optimized RF pulse sequences, preserving its spin polarization while saturating background molecules' magnetizations, thereby enhancing spectroscopic contrast between the target and background molecules.
Method for estimating the number of nuclei of a preselected isotope in a molecular species from and NMR spectrum
PatentInactiveUS7009394B2
Innovation
- A method that selects and orders signal peaks from an NMR spectrum, applies scaling factors, and iteratively refines integral values to produce a set of integerized scaled integrals, which are then used to estimate the total number of nuclei, incorporating a calibration signal to ensure accuracy and handle deviations.
Interdisciplinary Applications of Advanced NMR
The integration of Nuclear Magnetic Resonance (NMR) spectroscopy into diverse scientific disciplines has revolutionized research methodologies across multiple fields. Beyond its traditional applications in chemistry, advanced NMR techniques for resolving overlapping peaks have found significant utility in pharmaceutical development, where they enable precise characterization of complex drug formulations and metabolites. These multidimensional approaches allow researchers to distinguish between closely related molecular structures that would otherwise appear as indistinguishable signals in conventional one-dimensional spectra.
In materials science, the application of these advanced NMR techniques has facilitated the analysis of polymer compositions and nanostructured materials with unprecedented detail. By resolving overlapping resonances, scientists can now accurately determine the spatial arrangement and interactions between different components in composite materials, leading to improved design and performance characteristics.
The medical field has particularly benefited from these developments, with multidimensional NMR approaches enabling more accurate metabolomic profiling in biological samples. This capability has proven invaluable for disease biomarker discovery, where subtle changes in metabolite concentrations can be detected despite spectral crowding. The ability to deconvolute overlapping signals has enhanced diagnostic accuracy and opened new avenues for personalized medicine approaches.
Environmental science applications have expanded significantly, with researchers employing these techniques to identify and quantify complex mixtures of pollutants in soil and water samples. The superior resolution offered by multidimensional methods allows for reliable monitoring of environmental contaminants even at low concentrations or in the presence of interfering compounds.
Food science and agriculture have adopted these advanced NMR approaches for quality control and authentication purposes. The ability to resolve overlapping peaks enables more accurate profiling of food components, detection of adulterants, and verification of geographical origin claims for products like wine, olive oil, and honey.
In structural biology, these techniques have transformed protein structure determination by allowing scientists to resolve overlapping resonances from large biomolecules. This capability has accelerated the elucidation of protein-ligand interactions and conformational dynamics, contributing significantly to drug discovery efforts and fundamental understanding of biological processes.
The interdisciplinary nature of these applications has fostered collaborative research environments where chemists, biologists, physicians, and materials scientists work together to leverage the full potential of advanced NMR methodologies, creating a rich ecosystem of innovation across scientific boundaries.
In materials science, the application of these advanced NMR techniques has facilitated the analysis of polymer compositions and nanostructured materials with unprecedented detail. By resolving overlapping resonances, scientists can now accurately determine the spatial arrangement and interactions between different components in composite materials, leading to improved design and performance characteristics.
The medical field has particularly benefited from these developments, with multidimensional NMR approaches enabling more accurate metabolomic profiling in biological samples. This capability has proven invaluable for disease biomarker discovery, where subtle changes in metabolite concentrations can be detected despite spectral crowding. The ability to deconvolute overlapping signals has enhanced diagnostic accuracy and opened new avenues for personalized medicine approaches.
Environmental science applications have expanded significantly, with researchers employing these techniques to identify and quantify complex mixtures of pollutants in soil and water samples. The superior resolution offered by multidimensional methods allows for reliable monitoring of environmental contaminants even at low concentrations or in the presence of interfering compounds.
Food science and agriculture have adopted these advanced NMR approaches for quality control and authentication purposes. The ability to resolve overlapping peaks enables more accurate profiling of food components, detection of adulterants, and verification of geographical origin claims for products like wine, olive oil, and honey.
In structural biology, these techniques have transformed protein structure determination by allowing scientists to resolve overlapping resonances from large biomolecules. This capability has accelerated the elucidation of protein-ligand interactions and conformational dynamics, contributing significantly to drug discovery efforts and fundamental understanding of biological processes.
The interdisciplinary nature of these applications has fostered collaborative research environments where chemists, biologists, physicians, and materials scientists work together to leverage the full potential of advanced NMR methodologies, creating a rich ecosystem of innovation across scientific boundaries.
Standardization and Validation Protocols
The standardization and validation of multidimensional NMR methods for resolving overlapping peaks requires rigorous protocols to ensure reproducibility and reliability across different laboratories and experimental conditions. These protocols must address the inherent variability in NMR spectroscopy while maintaining scientific integrity and analytical precision.
Standardization begins with sample preparation guidelines that specify concentration ranges, solvent selection criteria, and internal reference standards. For multidimensional approaches, these standards must account for the increased complexity of experimental parameters and the potential for artifacts. Detailed procedures for calibration of pulse sequences, optimization of delay times, and adjustment of acquisition parameters form the foundation of standardized methodologies.
Validation protocols for multidimensional NMR techniques involve systematic assessment of method performance characteristics including specificity, accuracy, precision, detection limits, and robustness. Statistical approaches such as Design of Experiments (DoE) provide frameworks for evaluating these characteristics while minimizing experimental runs. Validation studies should incorporate samples with known overlapping resonances of varying degrees of complexity to challenge the resolving power of the methods.
Inter-laboratory comparison studies represent a critical component of validation, requiring multiple facilities to analyze identical samples using standardized protocols. These studies reveal systematic biases and variability sources that might otherwise remain undetected in single-laboratory validations. The results inform refinements to experimental procedures and data processing algorithms.
Quality control measures must be integrated throughout the analytical workflow, from instrument performance verification to final data interpretation. Regular testing with certified reference materials helps maintain long-term consistency and enables meaningful comparison of results obtained at different times or locations. For multidimensional NMR specifically, quality metrics should address resolution enhancement, signal-to-noise improvements, and quantitative accuracy in peak deconvolution.
Documentation requirements constitute another essential aspect of standardization, encompassing detailed records of experimental conditions, processing parameters, and interpretation criteria. Standardized reporting formats facilitate data sharing and meta-analysis across the scientific community, accelerating method refinement and adoption. These documentation standards should evolve alongside technological advancements in NMR instrumentation and data processing capabilities.
Regulatory considerations vary by application domain, with particularly stringent requirements in pharmaceutical analysis and clinical diagnostics. Compliance with relevant guidelines from organizations such as ICH, FDA, and USP may be necessary depending on the intended use of the analytical results. Validation protocols should be designed with these regulatory frameworks in mind to ensure acceptance of the resulting data.
Standardization begins with sample preparation guidelines that specify concentration ranges, solvent selection criteria, and internal reference standards. For multidimensional approaches, these standards must account for the increased complexity of experimental parameters and the potential for artifacts. Detailed procedures for calibration of pulse sequences, optimization of delay times, and adjustment of acquisition parameters form the foundation of standardized methodologies.
Validation protocols for multidimensional NMR techniques involve systematic assessment of method performance characteristics including specificity, accuracy, precision, detection limits, and robustness. Statistical approaches such as Design of Experiments (DoE) provide frameworks for evaluating these characteristics while minimizing experimental runs. Validation studies should incorporate samples with known overlapping resonances of varying degrees of complexity to challenge the resolving power of the methods.
Inter-laboratory comparison studies represent a critical component of validation, requiring multiple facilities to analyze identical samples using standardized protocols. These studies reveal systematic biases and variability sources that might otherwise remain undetected in single-laboratory validations. The results inform refinements to experimental procedures and data processing algorithms.
Quality control measures must be integrated throughout the analytical workflow, from instrument performance verification to final data interpretation. Regular testing with certified reference materials helps maintain long-term consistency and enables meaningful comparison of results obtained at different times or locations. For multidimensional NMR specifically, quality metrics should address resolution enhancement, signal-to-noise improvements, and quantitative accuracy in peak deconvolution.
Documentation requirements constitute another essential aspect of standardization, encompassing detailed records of experimental conditions, processing parameters, and interpretation criteria. Standardized reporting formats facilitate data sharing and meta-analysis across the scientific community, accelerating method refinement and adoption. These documentation standards should evolve alongside technological advancements in NMR instrumentation and data processing capabilities.
Regulatory considerations vary by application domain, with particularly stringent requirements in pharmaceutical analysis and clinical diagnostics. Compliance with relevant guidelines from organizations such as ICH, FDA, and USP may be necessary depending on the intended use of the analytical results. Validation protocols should be designed with these regulatory frameworks in mind to ensure acceptance of the resulting data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!