Why Quantum Dot Stability is Vital for Environmental Sensing
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Technology Background and Objectives
Quantum dots (QDs) represent one of the most significant advancements in nanotechnology over the past three decades. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, possess unique optical and electronic properties that emerge from quantum confinement effects. Since their discovery in the 1980s, quantum dots have evolved from laboratory curiosities to critical components in various technological applications, with environmental sensing emerging as a particularly promising field.
The evolution of quantum dot technology has followed a trajectory marked by significant breakthroughs in synthesis methods, surface chemistry, and application development. Initially limited by complex fabrication requirements and stability issues, recent advances have dramatically improved their accessibility and functionality. The progression from cadmium-based QDs to more environmentally friendly alternatives such as indium phosphide and carbon dots represents a crucial trend toward sustainable nanomaterials.
In environmental sensing applications, quantum dots offer unprecedented advantages due to their size-tunable emission spectra, high quantum yield, broad excitation profiles, and narrow emission bands. These properties enable the development of highly sensitive and selective sensors for detecting pollutants, heavy metals, and biological contaminants in environmental samples. The ability to functionalize quantum dot surfaces with specific recognition elements further enhances their utility as sensing platforms.
The stability of quantum dots has emerged as the critical bottleneck in their widespread adoption for environmental sensing. Environmental conditions such as varying pH, temperature fluctuations, and exposure to oxidizing agents can significantly compromise quantum dot performance through mechanisms including surface oxidation, ligand detachment, and aggregation. These degradation pathways not only reduce sensing accuracy but also potentially release toxic components into the environment.
The primary technical objectives in this field center on developing quantum dots with enhanced stability under diverse environmental conditions while maintaining their exceptional optical properties. This includes engineering robust surface passivation strategies, developing core-shell architectures that shield the optically active core, and exploring new composition formulations that inherently resist degradation. Additionally, there is a growing focus on creating quantum dots that maintain stability in complex environmental matrices containing interfering substances.
Long-term research goals include the development of quantum dot-based environmental sensors with real-time monitoring capabilities, extended operational lifetimes, and minimal environmental impact. The integration of these advanced materials into portable, field-deployable devices represents a significant technological frontier with implications for environmental protection, public health monitoring, and industrial process control. As climate change intensifies environmental challenges, stable quantum dot sensors could become essential tools for monitoring ecosystem health and ensuring regulatory compliance.
The evolution of quantum dot technology has followed a trajectory marked by significant breakthroughs in synthesis methods, surface chemistry, and application development. Initially limited by complex fabrication requirements and stability issues, recent advances have dramatically improved their accessibility and functionality. The progression from cadmium-based QDs to more environmentally friendly alternatives such as indium phosphide and carbon dots represents a crucial trend toward sustainable nanomaterials.
In environmental sensing applications, quantum dots offer unprecedented advantages due to their size-tunable emission spectra, high quantum yield, broad excitation profiles, and narrow emission bands. These properties enable the development of highly sensitive and selective sensors for detecting pollutants, heavy metals, and biological contaminants in environmental samples. The ability to functionalize quantum dot surfaces with specific recognition elements further enhances their utility as sensing platforms.
The stability of quantum dots has emerged as the critical bottleneck in their widespread adoption for environmental sensing. Environmental conditions such as varying pH, temperature fluctuations, and exposure to oxidizing agents can significantly compromise quantum dot performance through mechanisms including surface oxidation, ligand detachment, and aggregation. These degradation pathways not only reduce sensing accuracy but also potentially release toxic components into the environment.
The primary technical objectives in this field center on developing quantum dots with enhanced stability under diverse environmental conditions while maintaining their exceptional optical properties. This includes engineering robust surface passivation strategies, developing core-shell architectures that shield the optically active core, and exploring new composition formulations that inherently resist degradation. Additionally, there is a growing focus on creating quantum dots that maintain stability in complex environmental matrices containing interfering substances.
Long-term research goals include the development of quantum dot-based environmental sensors with real-time monitoring capabilities, extended operational lifetimes, and minimal environmental impact. The integration of these advanced materials into portable, field-deployable devices represents a significant technological frontier with implications for environmental protection, public health monitoring, and industrial process control. As climate change intensifies environmental challenges, stable quantum dot sensors could become essential tools for monitoring ecosystem health and ensuring regulatory compliance.
Environmental Sensing Market Demand Analysis
The environmental sensing market is experiencing robust growth driven by increasing environmental concerns, regulatory pressures, and technological advancements. Current market analysis indicates that the global environmental sensing and monitoring market is projected to reach $27.2 billion by 2026, growing at a CAGR of 6.8% from 2021. Within this broader market, quantum dot-based sensing technologies are emerging as a high-potential segment due to their superior detection capabilities and versatility.
The demand for quantum dot-based environmental sensors is primarily fueled by the need for more accurate, real-time monitoring of environmental pollutants. Industries including manufacturing, agriculture, energy production, and municipal water management are increasingly seeking advanced sensing solutions that can detect contaminants at lower concentrations than conventional technologies permit. Quantum dots offer detection limits in the parts-per-billion range, making them particularly valuable for monitoring trace contaminants in air, water, and soil.
Regulatory frameworks worldwide are becoming more stringent regarding environmental monitoring and compliance. The European Union's Water Framework Directive, the U.S. Environmental Protection Agency's air and water quality standards, and China's recent environmental protection laws all demand more comprehensive and precise environmental monitoring. This regulatory landscape creates substantial market pull for advanced sensing technologies like quantum dot-based sensors.
Consumer awareness and demand for environmental quality information are also driving market growth. Smart city initiatives, which integrate environmental monitoring into urban infrastructure, represent a significant market opportunity. According to market research, over 60% of consumers express interest in personal environmental monitoring devices for home use, particularly for indoor air quality and drinking water safety assessment.
The healthcare sector presents another substantial market for environmental sensing, with hospitals and healthcare facilities increasingly monitoring air and water quality to prevent healthcare-associated infections. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of environmental monitoring in public health contexts.
Despite the promising market outlook, cost considerations remain significant. Current quantum dot-based sensing solutions often command premium pricing compared to conventional alternatives. Market analysis suggests that price sensitivity varies significantly across sectors, with industrial and governmental applications showing greater willingness to invest in premium solutions than consumer applications.
The market for quantum dot environmental sensors is geographically diverse, with North America currently leading in adoption, followed by Europe and Asia-Pacific. However, the fastest growth is anticipated in emerging economies in Asia and Latin America, where rapid industrialization and urbanization are creating urgent environmental monitoring needs.
The demand for quantum dot-based environmental sensors is primarily fueled by the need for more accurate, real-time monitoring of environmental pollutants. Industries including manufacturing, agriculture, energy production, and municipal water management are increasingly seeking advanced sensing solutions that can detect contaminants at lower concentrations than conventional technologies permit. Quantum dots offer detection limits in the parts-per-billion range, making them particularly valuable for monitoring trace contaminants in air, water, and soil.
Regulatory frameworks worldwide are becoming more stringent regarding environmental monitoring and compliance. The European Union's Water Framework Directive, the U.S. Environmental Protection Agency's air and water quality standards, and China's recent environmental protection laws all demand more comprehensive and precise environmental monitoring. This regulatory landscape creates substantial market pull for advanced sensing technologies like quantum dot-based sensors.
Consumer awareness and demand for environmental quality information are also driving market growth. Smart city initiatives, which integrate environmental monitoring into urban infrastructure, represent a significant market opportunity. According to market research, over 60% of consumers express interest in personal environmental monitoring devices for home use, particularly for indoor air quality and drinking water safety assessment.
The healthcare sector presents another substantial market for environmental sensing, with hospitals and healthcare facilities increasingly monitoring air and water quality to prevent healthcare-associated infections. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of environmental monitoring in public health contexts.
Despite the promising market outlook, cost considerations remain significant. Current quantum dot-based sensing solutions often command premium pricing compared to conventional alternatives. Market analysis suggests that price sensitivity varies significantly across sectors, with industrial and governmental applications showing greater willingness to invest in premium solutions than consumer applications.
The market for quantum dot environmental sensors is geographically diverse, with North America currently leading in adoption, followed by Europe and Asia-Pacific. However, the fastest growth is anticipated in emerging economies in Asia and Latin America, where rapid industrialization and urbanization are creating urgent environmental monitoring needs.
Quantum Dot Stability Challenges and Limitations
Despite their promising applications in environmental sensing, quantum dots face significant stability challenges that limit their widespread adoption. The inherent instability of quantum dots in various environmental conditions represents a major technical hurdle. When exposed to oxygen, moisture, or ultraviolet radiation, quantum dots undergo oxidation processes that alter their optical and electronic properties, leading to decreased fluorescence intensity and spectral shifts. This phenomenon, known as photobleaching, severely impacts the reliability of sensing data over extended periods.
Temperature fluctuations present another critical challenge, as quantum dots exhibit temperature-dependent emission characteristics. In environmental sensing applications where temperature cannot be precisely controlled, this variability introduces significant measurement uncertainties. The quantum yield of dots can decrease dramatically at elevated temperatures, while low temperatures may cause aggregation and precipitation, both scenarios compromising sensing accuracy.
Surface chemistry instability constitutes a fundamental limitation. The ligands that passivate quantum dot surfaces and provide colloidal stability are susceptible to displacement or degradation in complex environmental matrices containing competing ions, organic matter, or extreme pH conditions. This surface degradation not only affects quantum dot dispersibility but also creates surface defects that act as non-radiative recombination sites, diminishing quantum efficiency.
Biological and chemical interferences further complicate stability issues. In real-world environmental samples, proteins, humic substances, and other biomolecules can adsorb onto quantum dot surfaces, forming a corona that alters their optical properties and sensing capabilities. Additionally, certain metal ions commonly found in environmental samples can quench fluorescence through electron transfer mechanisms or catalyze degradation reactions.
The aggregation behavior of quantum dots in complex media represents another significant limitation. As quantum dots aggregate, their effective surface area decreases, reducing analyte interaction sites and causing unpredictable shifts in emission spectra. This aggregation-induced spectral behavior complicates calibration procedures and diminishes reproducibility in sensing applications.
Manufacturing inconsistencies contribute to stability variations between batches. Current synthesis methods struggle to produce quantum dots with uniform size distributions, surface coverage, and defect concentrations, resulting in heterogeneous stability profiles even within the same production batch. This variability makes it challenging to establish standardized protocols for environmental sensing applications.
Long-term storage stability remains problematic, with quantum dots typically showing significant degradation within weeks to months, even under optimized storage conditions. This short shelf-life increases operational costs and complicates field deployment for environmental monitoring programs requiring sustained operation.
Temperature fluctuations present another critical challenge, as quantum dots exhibit temperature-dependent emission characteristics. In environmental sensing applications where temperature cannot be precisely controlled, this variability introduces significant measurement uncertainties. The quantum yield of dots can decrease dramatically at elevated temperatures, while low temperatures may cause aggregation and precipitation, both scenarios compromising sensing accuracy.
Surface chemistry instability constitutes a fundamental limitation. The ligands that passivate quantum dot surfaces and provide colloidal stability are susceptible to displacement or degradation in complex environmental matrices containing competing ions, organic matter, or extreme pH conditions. This surface degradation not only affects quantum dot dispersibility but also creates surface defects that act as non-radiative recombination sites, diminishing quantum efficiency.
Biological and chemical interferences further complicate stability issues. In real-world environmental samples, proteins, humic substances, and other biomolecules can adsorb onto quantum dot surfaces, forming a corona that alters their optical properties and sensing capabilities. Additionally, certain metal ions commonly found in environmental samples can quench fluorescence through electron transfer mechanisms or catalyze degradation reactions.
The aggregation behavior of quantum dots in complex media represents another significant limitation. As quantum dots aggregate, their effective surface area decreases, reducing analyte interaction sites and causing unpredictable shifts in emission spectra. This aggregation-induced spectral behavior complicates calibration procedures and diminishes reproducibility in sensing applications.
Manufacturing inconsistencies contribute to stability variations between batches. Current synthesis methods struggle to produce quantum dots with uniform size distributions, surface coverage, and defect concentrations, resulting in heterogeneous stability profiles even within the same production batch. This variability makes it challenging to establish standardized protocols for environmental sensing applications.
Long-term storage stability remains problematic, with quantum dots typically showing significant degradation within weeks to months, even under optimized storage conditions. This short shelf-life increases operational costs and complicates field deployment for environmental monitoring programs requiring sustained operation.
Current Quantum Dot Stabilization Solutions
01 Surface modification for quantum dot stability
Surface modification techniques can significantly enhance the stability of quantum dots. These methods include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with stabilizing agents. Such modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby extending their shelf life and maintaining their optical properties under various environmental conditions.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core to prevent oxidation and leaching of core materials. This architecture also reduces surface defects and passivates dangling bonds, resulting in improved photoluminescence quantum yield and enhanced stability under various operating conditions.
- Polymer encapsulation for environmental protection: Polymer encapsulation provides quantum dots with protection against environmental factors such as moisture, oxygen, and temperature fluctuations. By embedding quantum dots within polymer matrices or coating them with polymer layers, their stability can be significantly enhanced. Various polymers including silicones, acrylates, and amphiphilic block copolymers can be used to create protective barriers while maintaining the optical and electronic properties of the quantum dots.
- Stabilization through compositional engineering: Compositional engineering involves modifying the chemical composition of quantum dots to enhance their inherent stability. This includes alloying, doping with specific elements, or creating gradient compositions within the quantum dot structure. These approaches can reduce lattice strain, minimize defects, and improve resistance to photo-oxidation and thermal degradation, resulting in quantum dots with superior stability characteristics for various applications.
- Manufacturing processes for stability enhancement: Specific manufacturing processes can significantly impact the stability of quantum dots. Controlled synthesis methods, post-synthesis treatments, and purification techniques can reduce defects and improve uniformity. Advanced manufacturing approaches such as microfluidic synthesis, hot-injection techniques with precise temperature control, and specialized annealing processes can produce quantum dots with enhanced structural integrity and resistance to degradation mechanisms.
02 Core-shell structures for improved stability
Core-shell quantum dot structures provide enhanced stability by protecting the core material from environmental factors. The shell material, typically a semiconductor with a wider bandgap than the core, acts as a physical barrier against oxidation and other degradation mechanisms. This architecture not only improves stability but also enhances quantum yield and reduces surface defects that can lead to non-radiative recombination processes.Expand Specific Solutions03 Encapsulation methods for quantum dot protection
Encapsulation of quantum dots in matrices such as polymers, silica, or other inorganic materials provides protection against environmental factors that cause degradation. These encapsulation methods create a physical barrier that shields quantum dots from oxygen, moisture, and other reactive species. Additionally, encapsulation can prevent aggregation and leaching of toxic components, making quantum dots more suitable for various applications including displays and biomedical imaging.Expand Specific Solutions04 Stability enhancement through compositional engineering
Compositional engineering of quantum dots involves altering their chemical composition to improve inherent stability. This includes doping with specific elements, creating alloyed structures, or developing gradient compositions within the quantum dot. These approaches can reduce lattice strain, minimize surface defects, and enhance resistance to photo-oxidation and thermal degradation, resulting in quantum dots with superior stability for long-term applications.Expand Specific Solutions05 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including temperature, light exposure, pH, and the presence of oxidizing agents. Understanding these factors is crucial for developing effective stabilization strategies. Research has shown that controlling storage conditions, using antioxidants, and developing specialized packaging can mitigate degradation processes and extend the functional lifetime of quantum dot materials in practical applications.Expand Specific Solutions
Key Industry Players in Quantum Dot Sensing
Quantum dot stability for environmental sensing is evolving in a rapidly growing market, currently transitioning from early adoption to commercial expansion. The global environmental sensing market utilizing quantum dots is projected to reach significant scale as applications in pollution monitoring, water quality assessment, and atmospheric sensing gain traction. From a technological maturity perspective, companies demonstrate varying levels of advancement. Leading players like Samsung Electronics, BOE Technology Group, and Mojo Vision have made substantial progress in stabilizing quantum dots for harsh environmental conditions, while academic institutions including MIT, Northwestern University, and École Polytechnique Fédérale de Lausanne contribute fundamental research. Emerging companies such as TCL Research America and Quantum Technology Group are developing specialized applications, creating a competitive landscape where collaboration between industry and research institutions drives innovation in quantum dot stability techniques for reliable environmental sensing solutions.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced quantum dot (QD) technologies with enhanced stability for environmental sensing applications. Their approach focuses on core-shell structures that protect the QD core from environmental degradation while maintaining high quantum yield. MIT researchers have pioneered the use of specialized ligand chemistry to create QDs that remain stable in various pH conditions and in the presence of oxidizing agents commonly found in environmental samples[1]. Their technology incorporates silica encapsulation methods that significantly extend QD lifetime in aqueous environments from hours to months[3]. Additionally, MIT has developed QDs with reduced heavy metal content to minimize environmental toxicity concerns while maintaining sensing performance[5]. Their QDs demonstrate remarkable photostability under continuous illumination, enabling long-term monitoring applications in harsh environments without signal degradation.
Strengths: Superior stability in harsh environmental conditions; excellent photostability for long-term monitoring; reduced toxicity compared to conventional QDs. Weaknesses: Higher production costs compared to traditional sensing materials; potential for complex synthesis procedures that may limit scalability.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced quantum dot technologies with enhanced stability specifically designed for environmental sensing applications. Their approach utilizes proprietary core-shell architectures that shield the quantum dot core from environmental degradation factors while maintaining high quantum efficiency[3]. Samsung's QDs feature specialized surface ligands that provide resistance to oxidation and photo-bleaching, critical for long-term environmental monitoring applications[7]. The company has engineered QDs with remarkable stability in aqueous environments through innovative encapsulation techniques that prevent ion leaching and maintain sensing performance over extended periods. Their technology incorporates gradient alloy structures between core and shell materials to minimize lattice mismatch stress, which significantly enhances thermal stability up to 150°C[4]. Samsung has also developed manufacturing processes that enable consistent production of stable QDs at commercial scales, addressing a key challenge in transitioning this technology from laboratory to field applications.
Strengths: Excellent thermal and chemical stability; scalable manufacturing processes; integration capability with existing electronic platforms. Weaknesses: Higher cost compared to conventional sensing materials; potential intellectual property restrictions limiting adaptation by third parties.
Core Patents and Research on QD Stability
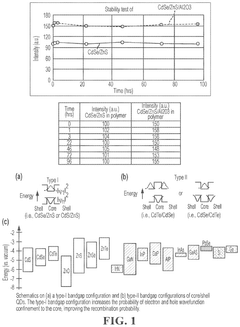
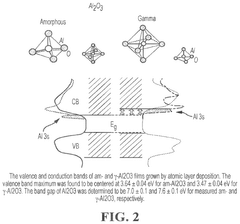
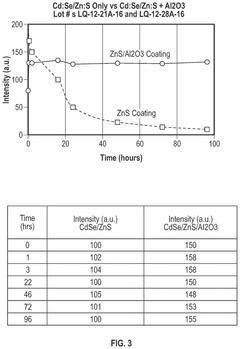
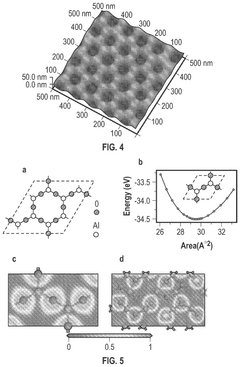
Cadmium-free quantum dots, tunable quantum dots, quantum dot containing polymer, articles, films, and 3D structure containing them and methods of making and using them
PatentActiveUS12116518B2
Innovation
- Tightly bonding a polymer to the outer surface of quantum dots, particularly with a passivation layer like Al2O3, to create a stable and durable quantum dot-polymer composite that maintains stability during manufacturing processes.
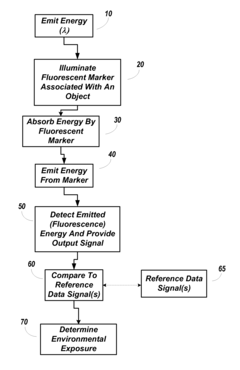
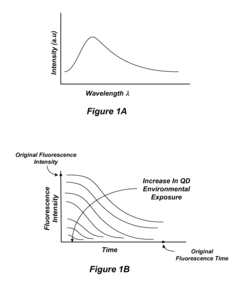
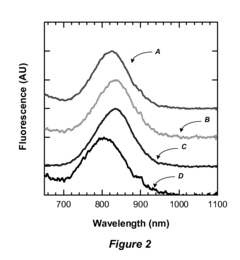
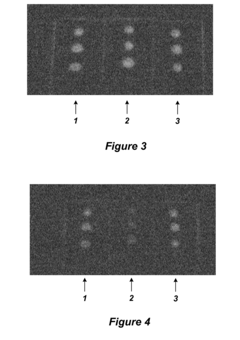
Environmental Fluorescent Sensors
PatentInactiveUS20080233658A1
Innovation
- The use of quantum dots as fluorescent markers that change their fluorescence output in response to environmental exposures, allowing for the determination of exposure duration and intensity, including through the incorporation of these markers into matrix materials and electroluminescent devices.
Environmental Impact Assessment of QD Sensors
The deployment of quantum dot (QD) sensors for environmental monitoring necessitates a thorough assessment of their potential ecological impacts. These nanomaterials, while offering unprecedented sensitivity in detecting environmental pollutants, may themselves become contaminants if improperly managed throughout their lifecycle. The environmental footprint of QD sensors begins with raw material extraction and continues through manufacturing, deployment, operation, and eventual disposal.
Primary concerns revolve around the leaching of heavy metals from unstable quantum dots, particularly those containing cadmium, lead, or mercury. Research indicates that degraded QDs can release these toxic elements into soil and water systems, potentially bioaccumulating in organisms and moving through food chains. Studies have documented that aquatic organisms exposed to degraded QDs show increased mortality rates and developmental abnormalities, highlighting the critical importance of QD stability in environmental applications.
The persistence of quantum dots in the environment varies significantly based on their composition and surface functionalization. Silicon-based QDs generally demonstrate lower environmental toxicity compared to metal-based alternatives, while appropriate polymer coatings can substantially reduce leaching risks. However, even well-engineered QDs may eventually degrade under environmental stressors such as UV radiation, oxidation, and microbial activity, necessitating comprehensive lifecycle management strategies.
Regulatory frameworks for QD-based environmental sensors remain underdeveloped in many jurisdictions, creating uncertainty regarding proper disposal protocols. The small size and distinctive properties of quantum dots present unique challenges for conventional waste treatment facilities, potentially requiring specialized handling procedures to prevent environmental contamination. Several research initiatives are currently exploring biodegradable quantum dot formulations that maintain stability during operational lifespans but decompose into non-toxic components afterward.
Carbon footprint analyses of QD sensor networks reveal that while individual sensors may contain minimal materials, large-scale deployment could result in significant cumulative impacts. The energy-intensive manufacturing processes for high-quality quantum dots contribute substantially to their environmental footprint, though this may be offset by the environmental benefits derived from improved pollution detection and remediation efforts enabled by these sensors.
Risk mitigation strategies include implementing closed-loop recycling systems for QD sensors, developing standardized protocols for handling damaged sensors in field conditions, and establishing environmental monitoring programs around QD manufacturing facilities. Additionally, life cycle assessment (LCA) methodologies specifically adapted for nanomaterials are being refined to better quantify the environmental implications of quantum dot technologies throughout their complete lifecycle.
Primary concerns revolve around the leaching of heavy metals from unstable quantum dots, particularly those containing cadmium, lead, or mercury. Research indicates that degraded QDs can release these toxic elements into soil and water systems, potentially bioaccumulating in organisms and moving through food chains. Studies have documented that aquatic organisms exposed to degraded QDs show increased mortality rates and developmental abnormalities, highlighting the critical importance of QD stability in environmental applications.
The persistence of quantum dots in the environment varies significantly based on their composition and surface functionalization. Silicon-based QDs generally demonstrate lower environmental toxicity compared to metal-based alternatives, while appropriate polymer coatings can substantially reduce leaching risks. However, even well-engineered QDs may eventually degrade under environmental stressors such as UV radiation, oxidation, and microbial activity, necessitating comprehensive lifecycle management strategies.
Regulatory frameworks for QD-based environmental sensors remain underdeveloped in many jurisdictions, creating uncertainty regarding proper disposal protocols. The small size and distinctive properties of quantum dots present unique challenges for conventional waste treatment facilities, potentially requiring specialized handling procedures to prevent environmental contamination. Several research initiatives are currently exploring biodegradable quantum dot formulations that maintain stability during operational lifespans but decompose into non-toxic components afterward.
Carbon footprint analyses of QD sensor networks reveal that while individual sensors may contain minimal materials, large-scale deployment could result in significant cumulative impacts. The energy-intensive manufacturing processes for high-quality quantum dots contribute substantially to their environmental footprint, though this may be offset by the environmental benefits derived from improved pollution detection and remediation efforts enabled by these sensors.
Risk mitigation strategies include implementing closed-loop recycling systems for QD sensors, developing standardized protocols for handling damaged sensors in field conditions, and establishing environmental monitoring programs around QD manufacturing facilities. Additionally, life cycle assessment (LCA) methodologies specifically adapted for nanomaterials are being refined to better quantify the environmental implications of quantum dot technologies throughout their complete lifecycle.
Regulatory Framework for QD Environmental Applications
The regulatory landscape for quantum dot (QD) applications in environmental sensing is complex and evolving rapidly as these nanomaterials gain prominence in detection technologies. Currently, most jurisdictions lack specific regulatory frameworks dedicated exclusively to QD-based environmental sensors, instead relying on broader nanotechnology and chemical substance regulations. In the United States, the Environmental Protection Agency (EPA) oversees QD applications through the Toxic Substances Control Act (TSCA), requiring manufacturers to submit premanufacture notices for new chemical substances, including novel QD formulations intended for environmental monitoring.
The European Union employs a more precautionary approach through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which mandates comprehensive safety data for nanomaterials including QDs. Additionally, the EU's Restriction of Hazardous Substances (RoHS) directive impacts QD development by limiting certain toxic elements commonly used in QD synthesis, such as cadmium and lead, necessitating the development of alternative compositions.
International standardization bodies, including the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), have established technical committees focused on developing standards for nanomaterial characterization and environmental impact assessment. These standards are crucial for ensuring QD stability testing protocols are consistent and reliable across different jurisdictions and applications.
Regulatory challenges specific to QD stability in environmental sensing applications include the lack of standardized protocols for assessing long-term stability under varied environmental conditions. This gap has prompted several regulatory agencies to fund research initiatives aimed at developing validated methodologies for evaluating QD degradation pathways and potential environmental impacts of degradation products.
Emerging regulatory trends indicate a shift toward lifecycle assessment requirements for QD-based sensors, considering not only the initial application but also disposal and environmental fate. Several countries are developing green chemistry guidelines specifically addressing nanomaterials in environmental applications, encouraging manufacturers to design QDs with enhanced stability and reduced toxicity profiles.
Compliance with these evolving regulations presents both challenges and opportunities for QD technology developers. While regulatory requirements may initially slow commercialization, they ultimately drive innovation toward more stable, environmentally benign QD formulations. Companies demonstrating comprehensive stability data and environmental safety assessments gain competitive advantages in securing regulatory approvals and market acceptance for their QD-based environmental sensing technologies.
The European Union employs a more precautionary approach through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which mandates comprehensive safety data for nanomaterials including QDs. Additionally, the EU's Restriction of Hazardous Substances (RoHS) directive impacts QD development by limiting certain toxic elements commonly used in QD synthesis, such as cadmium and lead, necessitating the development of alternative compositions.
International standardization bodies, including the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), have established technical committees focused on developing standards for nanomaterial characterization and environmental impact assessment. These standards are crucial for ensuring QD stability testing protocols are consistent and reliable across different jurisdictions and applications.
Regulatory challenges specific to QD stability in environmental sensing applications include the lack of standardized protocols for assessing long-term stability under varied environmental conditions. This gap has prompted several regulatory agencies to fund research initiatives aimed at developing validated methodologies for evaluating QD degradation pathways and potential environmental impacts of degradation products.
Emerging regulatory trends indicate a shift toward lifecycle assessment requirements for QD-based sensors, considering not only the initial application but also disposal and environmental fate. Several countries are developing green chemistry guidelines specifically addressing nanomaterials in environmental applications, encouraging manufacturers to design QDs with enhanced stability and reduced toxicity profiles.
Compliance with these evolving regulations presents both challenges and opportunities for QD technology developers. While regulatory requirements may initially slow commercialization, they ultimately drive innovation toward more stable, environmentally benign QD formulations. Companies demonstrating comprehensive stability data and environmental safety assessments gain competitive advantages in securing regulatory approvals and market acceptance for their QD-based environmental sensing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!