Organic electroluminescent material containing iridium and preparation method thereof, and organic electroluminescence device
An electroluminescence and electromechanical technology, applied in the field of iridium-containing organic electroluminescence materials and their preparation, and organic electroluminescence devices, which can solve the problems of poor blue color purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] A preparation method of the above-mentioned iridium-containing organic electroluminescent material, comprising the steps of:
[0044] The following steps are carried out under anhydrous and anaerobic conditions unless otherwise specified, such as in N 2Or under an inert gas atmosphere, etc., the solvent used can also adopt other solvents that have better compatibility with the reactants except the solvents given in each step.
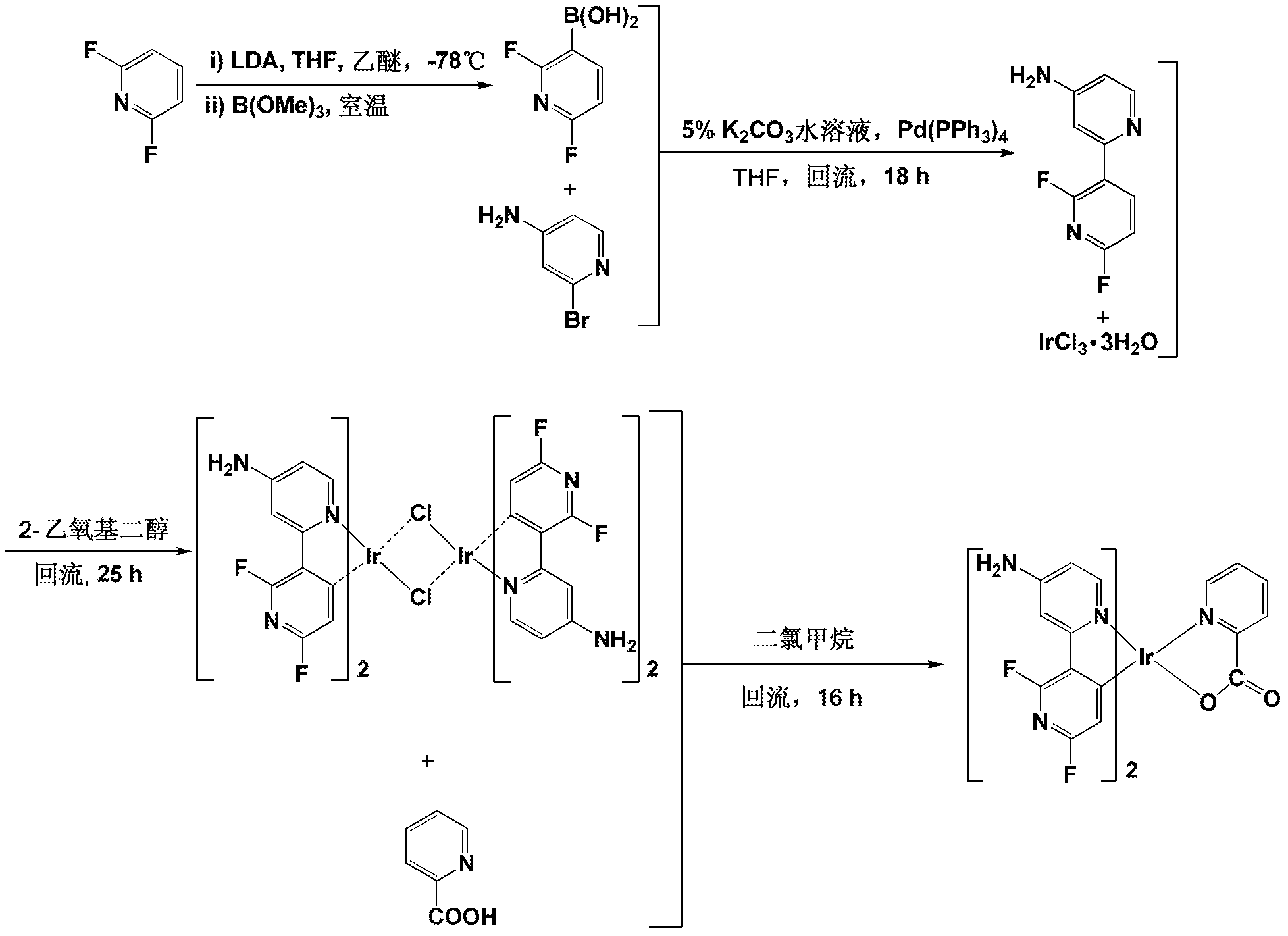
[0045] Step S1: In ether solvent, react compound D (2,6-difluoropyridine) with lithium diisopropylamide (LDA) in tetrahydrofuran (THF) at -78°C to prepare 2,6-difluoropyridine After base-3-lithium, at room temperature with trimethyl borate (B(OMe) 3 ) reaction to obtain compound G (2,6-difluoro-3-boronic acid pyridine), wherein, the molar ratio of compound D to lithium diisopropylamide is 1:1~1:1.5, and the molar ratio of trimethyl borate to compound D The ratio is 1:1~1.5:1, and the reaction formula is as follows:
[0046]
[0047] In a pr...
Embodiment 1
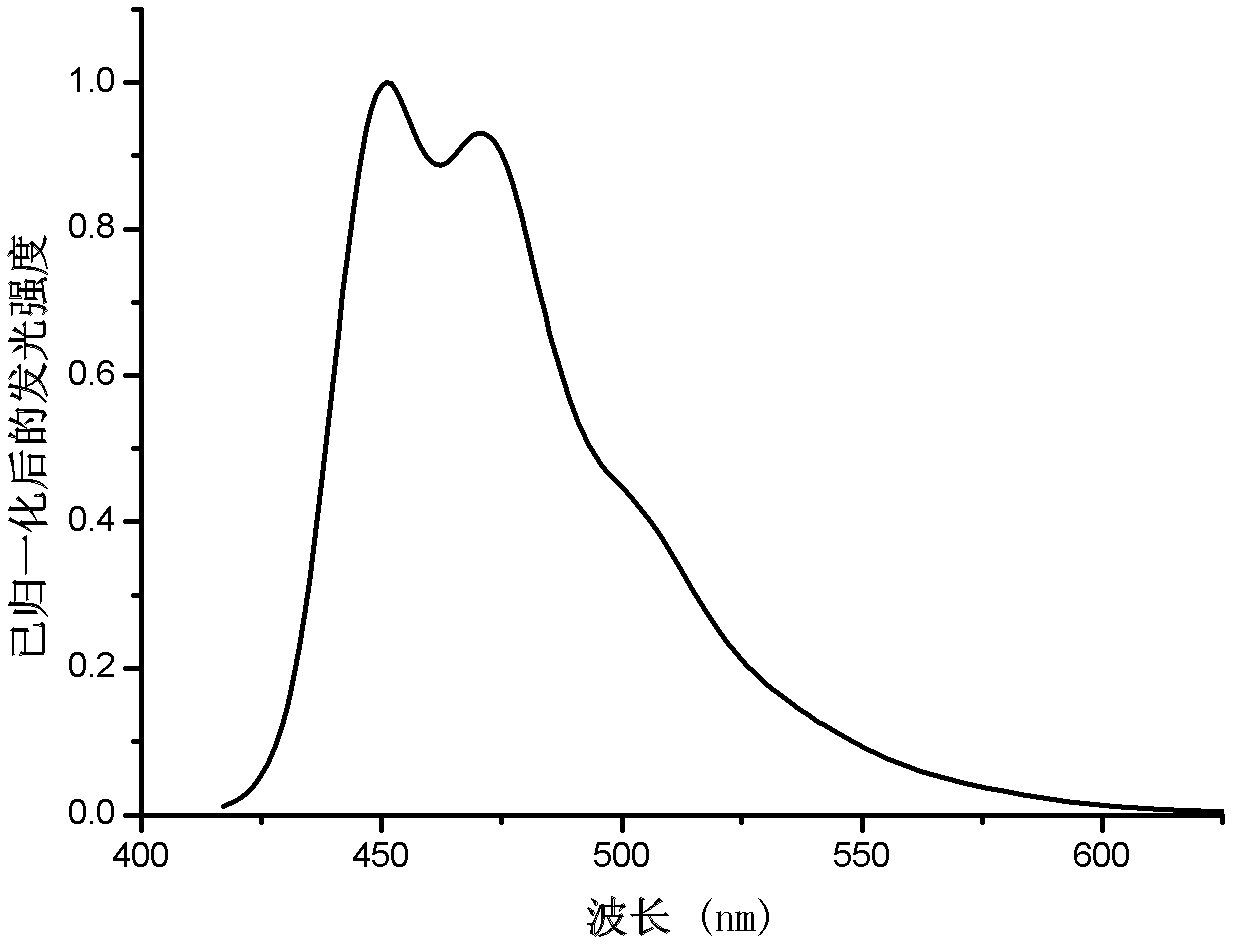
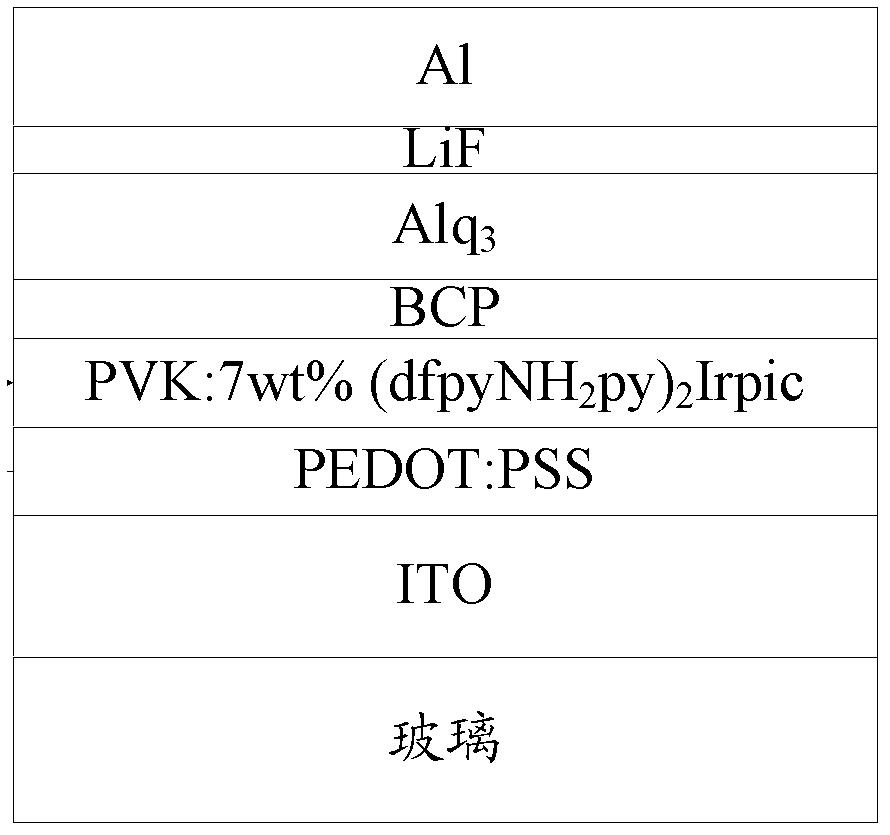
[0060] Example 1: Complex bis(2',6'-difluoro-4-amino-2,3'-bipyridine-C,N 2 ') (2-pyridinecarboxylic acid) iridium synthesis, please refer to figure 1 .
[0061] (1) Synthesis of 2,6-difluoro-3-boronic acid pyridine
[0062] Under the protection of nitrogen, slowly add 7.5mL of 1.6M lithium diisopropylamine THF solution dropwise to the mixed solution containing 0.91mL of 10mmol 2,6-difluoropyridine and 40mL of diethyl ether at -78°C, and keep stirring at -78°C 1 hour. After adding 1.40 mL of 12.5 mmol trimethyl borate to the reaction system, the temperature was naturally raised to room temperature, and the stirring reaction was continued for 1 hour. Then, 20 mL of 5% NaOH aqueous solution was slowly added to the reaction mixture to terminate the reaction. After stirring for 10 minutes, an appropriate amount of 3M aqueous HCl was added dropwise to adjust the pH to neutral. After multiple extractions with ethyl acetate, the organic phases were combined, and the solvent was re...
Embodiment 2
[0079] Example 2: Complex bis(2',6'-difluoro-4-amino-2,3'-bipyridine-C,N 2 ') Synthesis of (2-pyridinecarboxylic acid) iridium
[0080] (1) Refer to Example 1 for the synthesis steps of 2,6-difluoro-3-pyridine boronic acid;
[0081] (2) For the synthesis steps of 2′,6′-difluoro-4-amino-2,3′-bipyridine, see Example 1;
[0082] (3) Two bridge compounds (dfpyNH 2 py) 2 Ir(μ-Cl)Ir(dfpyNH 2 py) 2 The synthesis steps are referring to Example 1;
[0083] (4) Complexes (dfpyNH 2 py) 2 Synthesis of Irpic
[0084] Under nitrogen protection, 0.30g 2.4mmol picolinic acid and 0.69g 0.8mmol two-bridge compound (dfpyNH 2 py) 2 Ir(μ-Cl)Ir(dfpyNH 2 py) 2 Dissolve in 60mL 1,2-dichloroethane, stir and heat to reflux state, and react for 15 hours. After naturally cooling to room temperature, an appropriate amount of distilled water was added to the reacted mixed solution, and solids were precipitated. Filtrate to collect the crude product, and use the dichloromethane and petroleum e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com