Hematopoietic cell culture nutrient supplement
a hematopoietic cell and nutrient supplement technology, applied in the field of hematopoietic cell culture nutrient supplement, can solve the problems of toxic to cells, disadvantageous use of serum in the culture of hematopoietic cells, and inability to define the components contained in animal sera in the cell culture system, so as to increase or enhance increase or enhance the effect of the shelf life of the medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Methods
[0125]In the examples that follow, unless otherwise indicated, the methods of obtaining bone marrow cells, selecting CD34+ cells, assaying cell proliferation, and flow cytometry assays were performed as described in this Example.
Bone Marrow Cells
[0126]Bone marrow was obtained from a population of screened normal donors (courtesy of Roswell Park Cancer Institute). Marrow was aspirated from the posterior iliac crest and placed into sterile heparinized tubes. In the laboratory, approximately 25 mL of heparinized bone marrow was diluted with an equal volume of Hank's Balanced Salt Solution (without calcium or magnesium) (Life Technologies, Gaithersburg, Md.) and carefully layered over Nycoprep™ 1.077 (Life Technologies) in 50 mL tubes. Samples were then centrifuged at 800×g for 30 minutes at room temperature. After centrifugation, the band of bone marrow mononuclear cells (BMMC) at the interface was removed by pipetting. Cells were washed once with Hank's Balanced Sa...
example 2
Proliferation of CD34+-Selected Cells Under Serum-Free Culture Conditions
[0133]In order to study the ability of CD34+ cells to expand and differentiate under various culture conditions, CD34+ cells were selected from normal donor bone marrow. Initially, 1.3% of the total bone marrow cells were CD34+ (Table 4), in agreement with reported values (Cinin, C. I. et al., J. Immunol. 133:157 (1984)). The selection process enriched CD34+ cells to 64% from 1% in freshly aspirated normal bone marrow cells.
[0134]The absence of serum allows the study of the effect of early-acting or late-acting hematopoietic growth factors on cell expansion. Various combinations of human recombinant cytokines were examined for their ability to support proliferation (Table 5). Using StemPro-34™ SFM, it was possible to stimulate variable degrees of cell proliferation, in the absence of the confounding effects of serum, by altering the combinations of growth factors employed.
[0135]In preliminary studies, stem cell...
example 3
Kinetics of CD34+ Cell Expansion
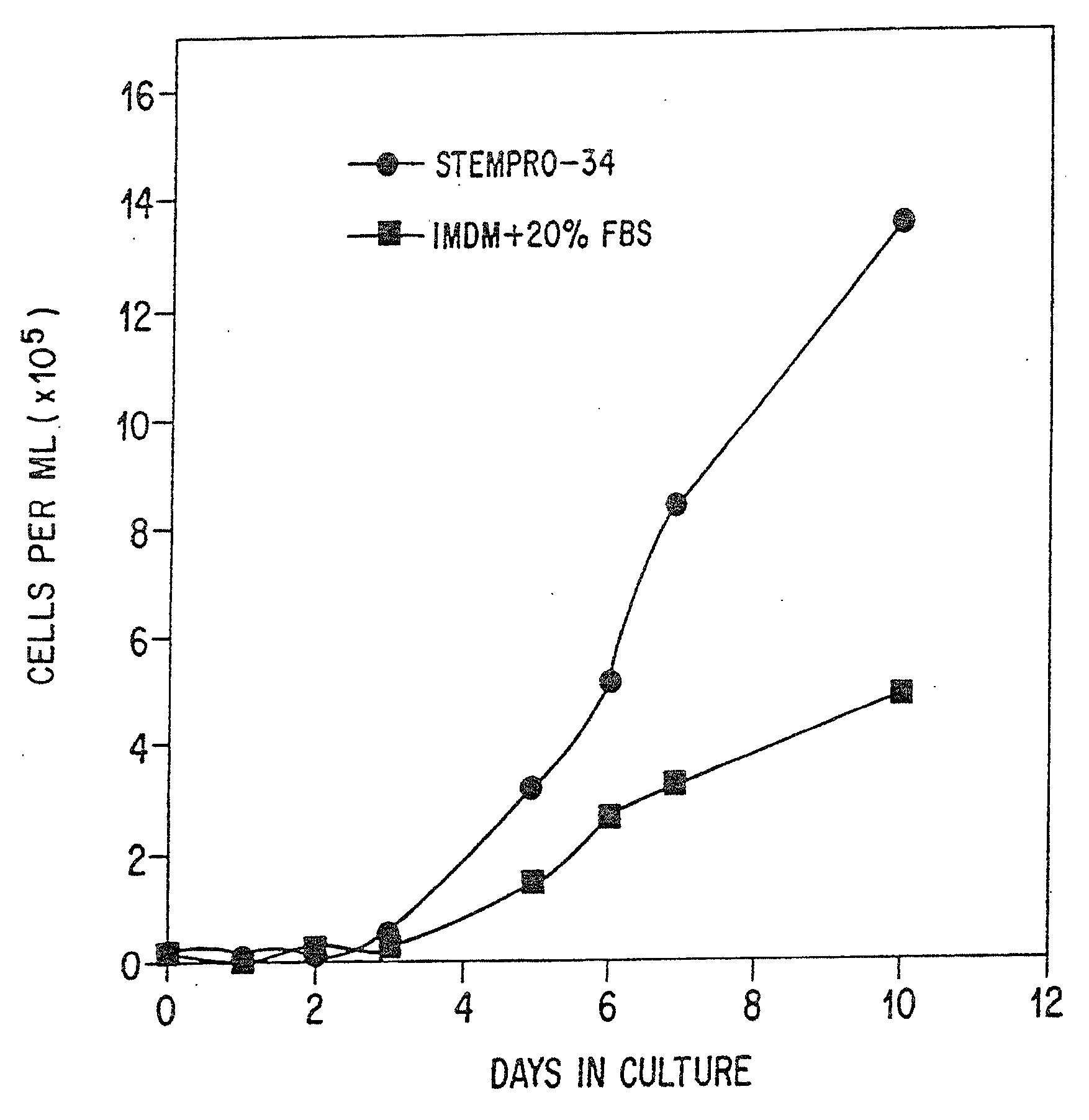
[0137]The kinetics of cell expansion in StemPro34™ SFM were compared to the kinetics of cell expansion in serum-containing medium. CD34+ bone marrow cells were seeded at an initial density of 2×104 cells / well in 24 well plates. Final concentrations of human recombinant factors SCF (100 ng / mL), IL-3 (50 ng / mL) and GM-CSF (25 ng / mL) were added to either StemPro™-34 SFM or IMDM / 20% FBS at setup. On the days indicated, wells were harvested and the cells counted using a hemocytometer and trypan blue dye exclusion. Results are depicted in FIG. 1.
[0138]In both StemPro34™ SFM and the serum-supplemented cultures, an initial lag phase (about 3 days) was observed to precede cell expansion. This initial lag phase may reflect experimental observations that the majority of hematopoietic stem cells (i.e., CD34+ / CD33− / CD38−) are in a quiescent G0 state and require stimulatory signals for expansion and subsequent differentiation (Traycoff, C. M. et al., Exp. Hematol. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage temperatures | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com