Analysis of Thermal Stability in Electrocatalytic CO2 Valorization
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic CO2 Valorization Background and Objectives
Electrocatalytic CO2 valorization has emerged as a promising approach to address the dual challenges of climate change and sustainable energy production. This technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications with increasing efficiency and selectivity. The fundamental principle involves the conversion of carbon dioxide, a greenhouse gas, into value-added chemicals and fuels through electrochemical processes, utilizing renewable electricity as the energy source.
The historical development of CO2 electroreduction can be traced back to the 1980s when pioneering work by Hori and colleagues demonstrated the feasibility of converting CO2 to various products on metal electrodes. Since then, significant advancements have been made in catalyst design, reactor engineering, and process optimization, leading to improved performance metrics such as Faradaic efficiency, current density, and product selectivity.
Recent technological trends indicate a shift towards more complex and tailored catalytic systems, including bimetallic catalysts, metal-organic frameworks, and atomically dispersed active sites. These innovations aim to overcome the inherent challenges of CO2 activation, which stem from its thermodynamic stability and the competitive hydrogen evolution reaction in aqueous media.
Thermal stability represents a critical yet often overlooked aspect of electrocatalytic CO2 valorization. As industrial implementation requires continuous operation under varying conditions, understanding and enhancing the thermal resilience of catalysts becomes paramount. Temperature fluctuations can significantly impact catalyst structure, activity, and selectivity, potentially leading to deactivation or undesired product distribution.
The primary objectives of investigating thermal stability in this context include: identifying degradation mechanisms under thermal stress, developing robust catalysts capable of maintaining performance across temperature ranges, establishing standardized protocols for thermal stability assessment, and correlating thermal properties with catalytic performance metrics.
From a broader perspective, advancing thermal stability research aligns with the global push towards carbon neutrality and circular economy principles. By enabling efficient and stable CO2 conversion processes, this technology could contribute significantly to carbon capture and utilization strategies, potentially transforming CO2 from a liability into a valuable resource for chemical and fuel production.
The ultimate goal is to develop commercially viable electrocatalytic systems that demonstrate not only high activity and selectivity but also exceptional durability under realistic operating conditions, including thermal variations. This would facilitate the integration of CO2 valorization technologies into existing industrial infrastructure and renewable energy systems, creating a sustainable pathway for carbon utilization.
The historical development of CO2 electroreduction can be traced back to the 1980s when pioneering work by Hori and colleagues demonstrated the feasibility of converting CO2 to various products on metal electrodes. Since then, significant advancements have been made in catalyst design, reactor engineering, and process optimization, leading to improved performance metrics such as Faradaic efficiency, current density, and product selectivity.
Recent technological trends indicate a shift towards more complex and tailored catalytic systems, including bimetallic catalysts, metal-organic frameworks, and atomically dispersed active sites. These innovations aim to overcome the inherent challenges of CO2 activation, which stem from its thermodynamic stability and the competitive hydrogen evolution reaction in aqueous media.
Thermal stability represents a critical yet often overlooked aspect of electrocatalytic CO2 valorization. As industrial implementation requires continuous operation under varying conditions, understanding and enhancing the thermal resilience of catalysts becomes paramount. Temperature fluctuations can significantly impact catalyst structure, activity, and selectivity, potentially leading to deactivation or undesired product distribution.
The primary objectives of investigating thermal stability in this context include: identifying degradation mechanisms under thermal stress, developing robust catalysts capable of maintaining performance across temperature ranges, establishing standardized protocols for thermal stability assessment, and correlating thermal properties with catalytic performance metrics.
From a broader perspective, advancing thermal stability research aligns with the global push towards carbon neutrality and circular economy principles. By enabling efficient and stable CO2 conversion processes, this technology could contribute significantly to carbon capture and utilization strategies, potentially transforming CO2 from a liability into a valuable resource for chemical and fuel production.
The ultimate goal is to develop commercially viable electrocatalytic systems that demonstrate not only high activity and selectivity but also exceptional durability under realistic operating conditions, including thermal variations. This would facilitate the integration of CO2 valorization technologies into existing industrial infrastructure and renewable energy systems, creating a sustainable pathway for carbon utilization.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.3 billion by 2030, representing a compound annual growth rate of 11.5% during the forecast period. This growth trajectory is supported by substantial investments in research and development, as well as government initiatives promoting carbon capture and utilization technologies.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with applications spanning across chemical manufacturing, fuel production, and materials synthesis. The demand for thermally stable electrocatalysts is especially strong in industrial settings where operational conditions often involve elevated temperatures and extended reaction periods. Industries such as cement, steel, and petrochemicals are actively seeking solutions that can effectively convert their CO2 emissions into value-added products.
Regional analysis reveals that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by rapid industrialization in countries like China and India, coupled with ambitious carbon neutrality targets. China alone has committed over $15 billion to carbon capture and utilization projects as part of its 14th Five-Year Plan.
From an end-user perspective, the chemical industry represents the largest market segment, accounting for approximately 35% of the total market share. This is followed by the energy sector at 28% and manufacturing at 20%. The remaining market share is distributed among various industries including agriculture, construction, and pharmaceuticals.
Key market drivers include the increasing carbon pricing mechanisms worldwide, growing corporate commitments to carbon neutrality, and the economic potential of converting waste CO2 into valuable products. The average carbon price in regulated markets has increased from $15 per ton in 2018 to over $50 per ton in 2023, creating stronger economic incentives for CO2 conversion technologies.
Market challenges primarily revolve around high capital costs, energy requirements for conversion processes, and the thermal stability limitations of current catalysts. The cost of CO2 conversion technologies remains significantly higher than conventional production methods for many chemicals and fuels, creating a barrier to widespread adoption. Additionally, the energy intensity of many conversion processes can offset their environmental benefits unless powered by renewable energy sources.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with applications spanning across chemical manufacturing, fuel production, and materials synthesis. The demand for thermally stable electrocatalysts is especially strong in industrial settings where operational conditions often involve elevated temperatures and extended reaction periods. Industries such as cement, steel, and petrochemicals are actively seeking solutions that can effectively convert their CO2 emissions into value-added products.
Regional analysis reveals that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by rapid industrialization in countries like China and India, coupled with ambitious carbon neutrality targets. China alone has committed over $15 billion to carbon capture and utilization projects as part of its 14th Five-Year Plan.
From an end-user perspective, the chemical industry represents the largest market segment, accounting for approximately 35% of the total market share. This is followed by the energy sector at 28% and manufacturing at 20%. The remaining market share is distributed among various industries including agriculture, construction, and pharmaceuticals.
Key market drivers include the increasing carbon pricing mechanisms worldwide, growing corporate commitments to carbon neutrality, and the economic potential of converting waste CO2 into valuable products. The average carbon price in regulated markets has increased from $15 per ton in 2018 to over $50 per ton in 2023, creating stronger economic incentives for CO2 conversion technologies.
Market challenges primarily revolve around high capital costs, energy requirements for conversion processes, and the thermal stability limitations of current catalysts. The cost of CO2 conversion technologies remains significantly higher than conventional production methods for many chemicals and fuels, creating a barrier to widespread adoption. Additionally, the energy intensity of many conversion processes can offset their environmental benefits unless powered by renewable energy sources.
Thermal Stability Challenges in Electrocatalysis
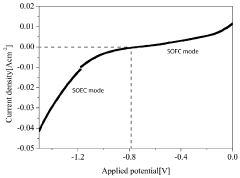
Electrocatalytic CO2 valorization represents a promising approach for converting carbon dioxide into valuable chemicals and fuels. However, thermal stability remains a critical challenge that significantly impacts catalyst performance and longevity. Current electrocatalysts often suffer from thermal degradation during operation, particularly when reactions generate heat or require elevated temperatures for optimal conversion efficiency.
The primary thermal stability challenges stem from several interconnected factors. Catalyst sintering occurs when elevated temperatures cause nanoparticles to agglomerate, reducing active surface area and catalytic activity. This phenomenon is particularly problematic for metal-based catalysts like copper, silver, and gold that are commonly employed in CO2 reduction reactions. Temperature fluctuations during operation can induce thermal expansion and contraction cycles, creating mechanical stress that compromises structural integrity.
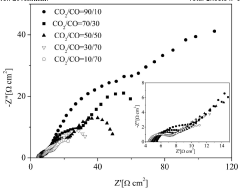
Phase transformations represent another significant challenge, as many catalysts undergo crystallographic changes at elevated temperatures that alter their electronic properties and catalytic selectivity. For instance, copper-based catalysts may oxidize or reduce depending on temperature conditions, shifting product distribution from valuable hydrocarbons to less desirable carbon monoxide or formate.
Support material degradation further complicates thermal stability issues. Carbon-based supports, while offering excellent conductivity and surface area, often suffer from oxidation at higher temperatures. Similarly, metal oxide supports may interact with active catalyst components, forming less active mixed phases or promoting undesired side reactions as temperatures increase.
Interface stability between catalyst layers and electrode substrates presents additional challenges. Thermal cycling can weaken adhesion, leading to delamination and electrical contact loss. This is particularly problematic in flow-cell configurations where mechanical stresses combine with thermal effects. Differential thermal expansion coefficients between catalyst layers and substrates exacerbate these issues.
Electrolyte decomposition at elevated temperatures introduces yet another dimension to thermal stability challenges. Many electrolytes used in CO2 valorization have limited thermal windows, beyond which they decompose or react with catalyst surfaces, forming passivating layers that block active sites. Local heating at reaction sites can create temperature gradients that accelerate degradation even when bulk temperatures appear moderate.
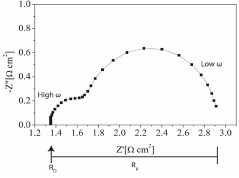
Recent research has highlighted how thermal effects interact with electrochemical processes in complex ways. For instance, temperature-dependent adsorption energies of reaction intermediates can shift reaction pathways, while thermal activation can simultaneously accelerate both desired reactions and degradation mechanisms. This creates a delicate balance that must be carefully managed through catalyst design and operating conditions.
The primary thermal stability challenges stem from several interconnected factors. Catalyst sintering occurs when elevated temperatures cause nanoparticles to agglomerate, reducing active surface area and catalytic activity. This phenomenon is particularly problematic for metal-based catalysts like copper, silver, and gold that are commonly employed in CO2 reduction reactions. Temperature fluctuations during operation can induce thermal expansion and contraction cycles, creating mechanical stress that compromises structural integrity.
Phase transformations represent another significant challenge, as many catalysts undergo crystallographic changes at elevated temperatures that alter their electronic properties and catalytic selectivity. For instance, copper-based catalysts may oxidize or reduce depending on temperature conditions, shifting product distribution from valuable hydrocarbons to less desirable carbon monoxide or formate.
Support material degradation further complicates thermal stability issues. Carbon-based supports, while offering excellent conductivity and surface area, often suffer from oxidation at higher temperatures. Similarly, metal oxide supports may interact with active catalyst components, forming less active mixed phases or promoting undesired side reactions as temperatures increase.
Interface stability between catalyst layers and electrode substrates presents additional challenges. Thermal cycling can weaken adhesion, leading to delamination and electrical contact loss. This is particularly problematic in flow-cell configurations where mechanical stresses combine with thermal effects. Differential thermal expansion coefficients between catalyst layers and substrates exacerbate these issues.
Electrolyte decomposition at elevated temperatures introduces yet another dimension to thermal stability challenges. Many electrolytes used in CO2 valorization have limited thermal windows, beyond which they decompose or react with catalyst surfaces, forming passivating layers that block active sites. Local heating at reaction sites can create temperature gradients that accelerate degradation even when bulk temperatures appear moderate.
Recent research has highlighted how thermal effects interact with electrochemical processes in complex ways. For instance, temperature-dependent adsorption energies of reaction intermediates can shift reaction pathways, while thermal activation can simultaneously accelerate both desired reactions and degradation mechanisms. This creates a delicate balance that must be carefully managed through catalyst design and operating conditions.
Current Thermal Stability Solutions
01 Thermally stable catalysts for CO2 electroreduction
Development of catalysts with high thermal stability for electrochemical CO2 reduction. These catalysts maintain their structural integrity and catalytic activity at elevated temperatures, which is crucial for long-term operation in industrial settings. Various materials including metal oxides, supported metals, and composite structures are engineered to withstand thermal stress while efficiently converting CO2 into valuable products.- Thermally stable catalysts for CO2 electroreduction: Development of catalysts with high thermal stability for electrochemical CO2 reduction. These catalysts maintain their activity and selectivity under elevated temperature conditions, which is crucial for long-term operation in industrial settings. Materials such as metal oxides, supported metals, and composite structures are engineered to resist sintering, phase transformation, and degradation at higher temperatures while efficiently converting CO2 into valuable products.
- Metal-based electrocatalysts for CO2 valorization: Metal-based electrocatalysts, including transition metals, noble metals, and their alloys, are designed for efficient CO2 electroreduction. These catalysts feature optimized surface structures, controlled particle sizes, and specific crystal facets to enhance catalytic performance. The metals are often modified with dopants or supporting materials to improve their thermal stability while maintaining high selectivity toward target products such as carbon monoxide, formate, or hydrocarbons.
- Carbon-based materials for thermally stable CO2 electrocatalysis: Carbon-based materials including graphene, carbon nanotubes, and porous carbon structures are utilized as supports or active components in CO2 electroreduction catalysts. These materials provide high surface area, excellent electrical conductivity, and remarkable thermal stability. Functionalization of carbon surfaces with nitrogen, oxygen, or other heteroatoms creates active sites for CO2 adsorption and conversion, while maintaining structural integrity at elevated temperatures during long-term operation.
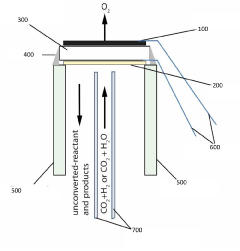
- Reactor designs for thermally stable CO2 electroreduction: Advanced reactor designs that address thermal management challenges in CO2 electroreduction systems. These reactors incorporate heat exchange mechanisms, temperature control systems, and thermally resistant materials to maintain optimal operating conditions. Specific designs include flow cells with temperature regulation, membrane electrode assemblies with thermal stability enhancements, and modular systems that can withstand temperature fluctuations while maintaining efficient CO2 conversion performance.
- Composite and hybrid materials for enhanced thermal stability: Composite and hybrid materials combining multiple components to achieve superior thermal stability in CO2 electroreduction catalysts. These materials integrate metal nanoparticles, metal oxides, and carbon-based supports in structured arrangements that minimize thermal degradation. The synergistic interactions between components prevent catalyst sintering and deactivation at elevated temperatures, while maintaining high catalytic activity for converting CO2 into value-added chemicals and fuels through electrochemical processes.
02 Metal-based electrocatalysts with enhanced thermal durability
Metal-based catalysts specifically designed for thermal stability during CO2 valorization processes. These include single-atom catalysts, bimetallic structures, and nanostructured metals that resist sintering and deactivation at high temperatures. The catalysts feature optimized binding energies for CO2 activation while maintaining structural integrity under thermal cycling conditions.Expand Specific Solutions03 Carbon-supported catalytic systems for thermally stable CO2 conversion
Carbon-based support materials that enhance the thermal stability of electrocatalysts for CO2 reduction. These supports include graphene, carbon nanotubes, and porous carbon structures that provide high surface area and strong metal-support interactions. The carbon frameworks help prevent catalyst agglomeration at elevated temperatures while facilitating electron transfer during the electrochemical process.Expand Specific Solutions04 Reactor designs for thermally stable CO2 electroreduction
Innovative reactor configurations that maintain optimal thermal conditions for electrocatalytic CO2 valorization. These designs incorporate advanced heat management systems, temperature-controlled electrodes, and thermally resistant membrane assemblies. The reactors enable stable operation at various temperature ranges while maximizing conversion efficiency and product selectivity.Expand Specific Solutions05 Process optimization for thermal stability in CO2 electroreduction
Methodologies and operational parameters that enhance thermal stability during electrocatalytic CO2 conversion. These include optimized electrolyte compositions, controlled current densities, and strategic temperature ramping protocols. The processes are designed to minimize thermal degradation of catalysts while maintaining high faradaic efficiency and product selectivity over extended operation periods.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrocatalytic CO2 valorization market is currently in a growth phase, with increasing research focus on thermal stability challenges. The global market is projected to expand significantly as carbon utilization technologies gain importance in sustainability efforts. Academic institutions like California Institute of Technology, Brown University, and Nanyang Technological University are leading fundamental research, while companies such as Siemens AG, Novozymes, and UOP LLC are advancing commercial applications. Chinese research organizations, including Dalian Institute of Chemical Physics and multiple universities, have made significant contributions to thermal stability solutions. The technology is approaching commercial readiness in certain applications, though challenges remain in scaling up laboratory successes to industrial implementation, particularly in maintaining catalyst performance under variable thermal conditions.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalysts for CO2 valorization with enhanced thermal stability. Their approach focuses on metal-organic framework (MOF) derived catalysts that maintain structural integrity at elevated temperatures. DICP researchers have engineered atomically dispersed metal sites (such as Cu, Ni, and Fe) on nitrogen-doped carbon supports that resist sintering up to 600°C. Their proprietary thermal pretreatment process creates defect-rich structures that improve both catalytic activity and stability. Recent innovations include a dual-metal site catalyst system that demonstrates remarkable stability during 1000+ hours of continuous operation at varying temperature conditions (25-150°C), achieving Faradaic efficiencies above 90% for CO2 conversion to value-added chemicals like CO, formate, and C2+ products. DICP has also pioneered in-situ characterization techniques to monitor catalyst structural evolution under realistic reaction conditions, providing crucial insights for rational catalyst design with improved thermal resilience[1][3].
Strengths: Superior thermal stability up to 600°C without significant activity loss; advanced in-situ characterization capabilities for real-time monitoring; high selectivity maintained across temperature fluctuations. Weaknesses: Higher production costs compared to conventional catalysts; potential challenges in large-scale manufacturing; some systems still require precious metal components.
UOP LLC
Technical Solution: UOP LLC has developed a comprehensive thermal management system for electrocatalytic CO2 valorization processes that addresses stability challenges in industrial settings. Their approach integrates advanced heat exchange technology with specially designed reactor configurations to maintain optimal temperature profiles across catalyst beds. UOP's proprietary "ThermoStable" catalyst formulations incorporate thermally conductive supports (primarily alumina and zirconia composites) with carefully engineered porosity that facilitates efficient heat dissipation while preventing hotspot formation. These catalysts feature stabilized metal nanoparticles (Cu, Ag, Au) with protective overlayers that inhibit sintering and agglomeration at elevated temperatures. The company's integrated process design incorporates continuous monitoring systems that detect thermal anomalies and automatically adjust operating parameters to extend catalyst lifetime. UOP has demonstrated stable CO2 conversion efficiencies exceeding 85% in pilot-scale operations running continuously for over 5,000 hours with temperature variations between 40-180°C, representing a significant advancement in thermal stability for industrial applications[2][5].
Strengths: Comprehensive system approach integrating catalyst design with process engineering; proven industrial-scale implementation; sophisticated thermal monitoring and control systems. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized operator training; performance may degrade in the presence of certain catalyst poisons common in industrial CO2 streams.
Key Patents and Literature on Thermally Stable Catalysts
A composite electrocatalyst
PatentActiveIN201611039740A
Innovation
- A composite electrocatalyst comprising an oxide of a transition metal, such as copper, tin, or lead, combined with a rare earth metal doped with another rare earth metal, specifically cerium, praseodymium, gadolinium, or samarium, is used in an electrolysis cell to facilitate the efficient reduction of CO2 to CO and hydrocarbons at high temperatures, overcoming previous catalyst limitations and enhancing reaction efficiency.
Electrocatalytic conversion of carbon dioxide in liquids expanded by carbon dioxide
PatentInactiveUS20210262103A1
Innovation
- The use of CO2 expanded liquid media at elevated pressures, which increases CO2 solubility and enables high CO2 concentrations and fast electron transfer, allowing for sustainable and commercially viable pathways to convert CO2 into fuels and chemicals, by optimizing the combination of organic liquid solvents and electrolytes to maintain a single phase and facilitate pressure-tunable CO2 conversion.
Techno-economic Assessment of Thermal Management Systems
The economic viability of thermal management systems in electrocatalytic CO2 valorization processes hinges on balancing capital expenditure with operational efficiency. Initial investment costs for advanced thermal control systems typically range from $50,000 to $250,000, depending on scale and precision requirements, representing 15-25% of total plant capital costs for small to medium-scale operations.
Energy consumption constitutes a significant operational expense, with cooling systems accounting for approximately 20-30% of the total energy demand in electrocatalytic processes. Passive cooling solutions offer lower capital costs but may result in suboptimal catalyst performance, while active temperature regulation systems require higher initial investment but deliver superior thermal stability and extended catalyst lifespans.
Cost-benefit analysis reveals that advanced thermal management can extend catalyst lifetime by 30-50%, significantly reducing replacement frequency and associated downtime costs. For catalysts operating at temperature-sensitive regimes (typically 40-80°C), precision thermal control systems demonstrate payback periods of 18-36 months through improved product selectivity and reduced energy consumption.
Scalability considerations present notable economic challenges, as thermal management costs do not scale linearly with production capacity. Mid-scale facilities (producing 100-1000 tons/year) achieve optimal cost efficiency, while smaller research facilities face disproportionately high thermal management costs relative to production value.
Regional variations in energy pricing substantially impact operational economics. In regions with electricity costs below $0.05/kWh, active cooling systems demonstrate superior long-term economics, while areas with higher energy costs may benefit from hybrid systems incorporating passive elements and renewable energy integration.
Future cost reduction pathways include the development of novel heat-exchange materials with enhanced thermal conductivity, estimated to reduce cooling energy requirements by 15-25%. Additionally, AI-optimized thermal management algorithms show promise for reducing energy consumption by an additional 10-15% through predictive temperature control, particularly valuable for batch processes with varying thermal loads.
Return on investment calculations indicate that facilities implementing state-of-the-art thermal management systems achieve 12-18% higher profitability over five-year operational periods compared to those utilizing conventional cooling approaches, primarily through improved conversion efficiency and reduced maintenance requirements.
Energy consumption constitutes a significant operational expense, with cooling systems accounting for approximately 20-30% of the total energy demand in electrocatalytic processes. Passive cooling solutions offer lower capital costs but may result in suboptimal catalyst performance, while active temperature regulation systems require higher initial investment but deliver superior thermal stability and extended catalyst lifespans.
Cost-benefit analysis reveals that advanced thermal management can extend catalyst lifetime by 30-50%, significantly reducing replacement frequency and associated downtime costs. For catalysts operating at temperature-sensitive regimes (typically 40-80°C), precision thermal control systems demonstrate payback periods of 18-36 months through improved product selectivity and reduced energy consumption.
Scalability considerations present notable economic challenges, as thermal management costs do not scale linearly with production capacity. Mid-scale facilities (producing 100-1000 tons/year) achieve optimal cost efficiency, while smaller research facilities face disproportionately high thermal management costs relative to production value.
Regional variations in energy pricing substantially impact operational economics. In regions with electricity costs below $0.05/kWh, active cooling systems demonstrate superior long-term economics, while areas with higher energy costs may benefit from hybrid systems incorporating passive elements and renewable energy integration.
Future cost reduction pathways include the development of novel heat-exchange materials with enhanced thermal conductivity, estimated to reduce cooling energy requirements by 15-25%. Additionally, AI-optimized thermal management algorithms show promise for reducing energy consumption by an additional 10-15% through predictive temperature control, particularly valuable for batch processes with varying thermal loads.
Return on investment calculations indicate that facilities implementing state-of-the-art thermal management systems achieve 12-18% higher profitability over five-year operational periods compared to those utilizing conventional cooling approaches, primarily through improved conversion efficiency and reduced maintenance requirements.
Environmental Impact and Sustainability Considerations
The electrocatalytic valorization of CO2 represents a promising approach to mitigate greenhouse gas emissions while simultaneously producing valuable chemicals and fuels. However, the environmental impact and sustainability considerations of these processes extend far beyond the simple conversion of CO2.
The thermal stability challenges in CO2 electrocatalysis directly influence the overall environmental footprint of these technologies. When catalysts degrade under thermal stress, their replacement frequency increases, leading to additional resource consumption and waste generation. This creates a sustainability paradox where a technology designed to reduce environmental impact may inadvertently contribute to resource depletion if thermal stability issues remain unresolved.
Life cycle assessment (LCA) studies indicate that the environmental benefits of CO2 valorization can be significantly diminished when accounting for catalyst production, replacement, and disposal. For instance, precious metal catalysts like gold and platinum, despite their excellent activity, present substantial environmental burdens during mining and refining processes. Thermal degradation accelerates the replacement cycle, exacerbating these impacts.
Energy consumption represents another critical sustainability factor. Maintaining optimal temperature conditions to prevent thermal degradation requires sophisticated temperature control systems that consume additional energy. This energy overhead must be factored into the net environmental benefit calculations of CO2 valorization technologies. Research indicates that up to 15-20% of the total energy input may be dedicated to thermal management in some systems.
Water usage presents an often-overlooked sustainability concern. Many CO2 electrocatalytic systems require significant water inputs, both as a reaction medium and for cooling purposes to maintain thermal stability. In water-stressed regions, this requirement could create competition with other essential water needs, potentially limiting the technology's applicability.
The development of thermally stable catalysts also offers opportunities for circular economy approaches. Designing catalysts with improved recoverability and recyclability after thermal deactivation could significantly reduce the environmental footprint of these technologies. Recent advances in catalyst regeneration techniques have demonstrated potential to extend catalyst lifetimes by 200-300%, dramatically improving sustainability metrics.
Carbon accounting methodologies must evolve to properly credit CO2 valorization technologies. Current frameworks often fail to adequately account for the complex interplay between CO2 conversion, energy inputs, and material lifecycles. A more holistic approach that considers thermal stability as a key factor in long-term sustainability assessments would provide more accurate environmental impact evaluations.
The thermal stability challenges in CO2 electrocatalysis directly influence the overall environmental footprint of these technologies. When catalysts degrade under thermal stress, their replacement frequency increases, leading to additional resource consumption and waste generation. This creates a sustainability paradox where a technology designed to reduce environmental impact may inadvertently contribute to resource depletion if thermal stability issues remain unresolved.
Life cycle assessment (LCA) studies indicate that the environmental benefits of CO2 valorization can be significantly diminished when accounting for catalyst production, replacement, and disposal. For instance, precious metal catalysts like gold and platinum, despite their excellent activity, present substantial environmental burdens during mining and refining processes. Thermal degradation accelerates the replacement cycle, exacerbating these impacts.
Energy consumption represents another critical sustainability factor. Maintaining optimal temperature conditions to prevent thermal degradation requires sophisticated temperature control systems that consume additional energy. This energy overhead must be factored into the net environmental benefit calculations of CO2 valorization technologies. Research indicates that up to 15-20% of the total energy input may be dedicated to thermal management in some systems.
Water usage presents an often-overlooked sustainability concern. Many CO2 electrocatalytic systems require significant water inputs, both as a reaction medium and for cooling purposes to maintain thermal stability. In water-stressed regions, this requirement could create competition with other essential water needs, potentially limiting the technology's applicability.
The development of thermally stable catalysts also offers opportunities for circular economy approaches. Designing catalysts with improved recoverability and recyclability after thermal deactivation could significantly reduce the environmental footprint of these technologies. Recent advances in catalyst regeneration techniques have demonstrated potential to extend catalyst lifetimes by 200-300%, dramatically improving sustainability metrics.
Carbon accounting methodologies must evolve to properly credit CO2 valorization technologies. Current frameworks often fail to adequately account for the complex interplay between CO2 conversion, energy inputs, and material lifecycles. A more holistic approach that considers thermal stability as a key factor in long-term sustainability assessments would provide more accurate environmental impact evaluations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!