Autoclave Load Specifics: Achieving Optimal Sterilization Metrics
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Sterilization Technology Evolution and Objectives
Sterilization technology has evolved significantly since the invention of the first pressure steam sterilizer by Charles Chamberland in 1879. This pioneering autoclave established the fundamental principles of using pressurized steam for microbial decontamination, which remain central to modern sterilization practices. The subsequent century witnessed gradual refinements in design, materials, and control systems, transforming autoclaves from simple pressure vessels to sophisticated medical devices with precise parameter control capabilities.
The 1950s-1970s marked a pivotal era with the introduction of automated cycle controls and recording systems, enhancing process reliability and documentation. By the 1980s-1990s, microprocessor-controlled systems revolutionized autoclave technology, enabling precise monitoring and validation of critical parameters including temperature, pressure, and exposure time. This technological progression aligned with evolving regulatory frameworks and standardization efforts by organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO).
Recent technological advancements have focused on optimizing load-specific sterilization parameters. Traditional approaches relied on generalized cycles, often resulting in either inadequate sterilization or excessive processing that damaged sensitive instruments. Contemporary research emphasizes tailored sterilization protocols based on specific load characteristics, including density, material composition, and geometric configuration. This paradigm shift from standardized to customized sterilization represents a significant evolution in the field.
The primary objective of modern autoclave technology development is achieving consistent and validated sterility assurance levels (SAL) while optimizing resource utilization and minimizing cycle times. This involves developing sophisticated algorithms that can dynamically adjust sterilization parameters based on real-time load assessment. Secondary objectives include reducing energy consumption, minimizing water usage, and extending the lifespan of sterilized instruments through gentler yet effective processing conditions.
Future technological trajectories point toward intelligent sterilization systems incorporating machine learning algorithms capable of predicting optimal sterilization parameters for novel load configurations. These systems aim to achieve perfect sterilization efficacy while minimizing resource consumption and processing time. Additional development goals include enhanced connectivity for integration with hospital information systems, improved sustainability metrics, and advanced validation methodologies that can provide real-time sterility assurance without relying solely on biological indicators or parametric release criteria.
The 1950s-1970s marked a pivotal era with the introduction of automated cycle controls and recording systems, enhancing process reliability and documentation. By the 1980s-1990s, microprocessor-controlled systems revolutionized autoclave technology, enabling precise monitoring and validation of critical parameters including temperature, pressure, and exposure time. This technological progression aligned with evolving regulatory frameworks and standardization efforts by organizations such as the Association for the Advancement of Medical Instrumentation (AAMI) and the International Organization for Standardization (ISO).
Recent technological advancements have focused on optimizing load-specific sterilization parameters. Traditional approaches relied on generalized cycles, often resulting in either inadequate sterilization or excessive processing that damaged sensitive instruments. Contemporary research emphasizes tailored sterilization protocols based on specific load characteristics, including density, material composition, and geometric configuration. This paradigm shift from standardized to customized sterilization represents a significant evolution in the field.
The primary objective of modern autoclave technology development is achieving consistent and validated sterility assurance levels (SAL) while optimizing resource utilization and minimizing cycle times. This involves developing sophisticated algorithms that can dynamically adjust sterilization parameters based on real-time load assessment. Secondary objectives include reducing energy consumption, minimizing water usage, and extending the lifespan of sterilized instruments through gentler yet effective processing conditions.
Future technological trajectories point toward intelligent sterilization systems incorporating machine learning algorithms capable of predicting optimal sterilization parameters for novel load configurations. These systems aim to achieve perfect sterilization efficacy while minimizing resource consumption and processing time. Additional development goals include enhanced connectivity for integration with hospital information systems, improved sustainability metrics, and advanced validation methodologies that can provide real-time sterility assurance without relying solely on biological indicators or parametric release criteria.
Market Demand Analysis for Advanced Sterilization Solutions
The global sterilization market is experiencing robust growth, driven by increasing healthcare expenditures, rising surgical procedures, and growing awareness of infection control protocols. The market for advanced autoclave sterilization solutions was valued at approximately 2.8 billion USD in 2022 and is projected to reach 4.1 billion USD by 2028, representing a compound annual growth rate of 6.7%. This growth trajectory underscores the significant demand for more sophisticated sterilization technologies that can deliver optimal metrics while maintaining efficiency.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users, accounting for nearly 65% of the total market share. These institutions are increasingly seeking sterilization solutions that offer precise load-specific parameters to ensure complete sterilization while preserving instrument integrity and extending equipment lifespan.
The pharmaceutical and biotechnology sectors represent another rapidly expanding market segment, with an estimated growth rate of 8.2% annually. These industries require highly specialized sterilization protocols for sensitive materials and complex equipment, creating demand for autoclave systems with advanced monitoring capabilities and customizable cycle parameters.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging economies across Asia-Pacific and Latin America, where healthcare infrastructure development and increasing adoption of stringent sterilization standards are creating new market opportunities.
A significant market trend is the growing demand for sterilization validation services, which has expanded at 9.3% annually over the past five years. This reflects the increasing regulatory requirements and the need for documented evidence of sterilization efficacy across different load configurations.
Consumer preferences are shifting toward autoclave systems that offer comprehensive data logging, remote monitoring capabilities, and integration with facility management systems. According to recent industry surveys, 78% of healthcare facilities prioritize these features when making purchasing decisions for new sterilization equipment.
The COVID-19 pandemic has accelerated market demand, with 67% of healthcare facilities reporting increased sterilization requirements and 54% indicating plans to upgrade their existing sterilization infrastructure within the next two years. This has created a significant opportunity for technologies that can optimize sterilization metrics across diverse load specifications while maintaining operational efficiency.
Healthcare facilities, particularly hospitals and ambulatory surgical centers, constitute the largest segment of end-users, accounting for nearly 65% of the total market share. These institutions are increasingly seeking sterilization solutions that offer precise load-specific parameters to ensure complete sterilization while preserving instrument integrity and extending equipment lifespan.
The pharmaceutical and biotechnology sectors represent another rapidly expanding market segment, with an estimated growth rate of 8.2% annually. These industries require highly specialized sterilization protocols for sensitive materials and complex equipment, creating demand for autoclave systems with advanced monitoring capabilities and customizable cycle parameters.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging economies across Asia-Pacific and Latin America, where healthcare infrastructure development and increasing adoption of stringent sterilization standards are creating new market opportunities.
A significant market trend is the growing demand for sterilization validation services, which has expanded at 9.3% annually over the past five years. This reflects the increasing regulatory requirements and the need for documented evidence of sterilization efficacy across different load configurations.
Consumer preferences are shifting toward autoclave systems that offer comprehensive data logging, remote monitoring capabilities, and integration with facility management systems. According to recent industry surveys, 78% of healthcare facilities prioritize these features when making purchasing decisions for new sterilization equipment.
The COVID-19 pandemic has accelerated market demand, with 67% of healthcare facilities reporting increased sterilization requirements and 54% indicating plans to upgrade their existing sterilization infrastructure within the next two years. This has created a significant opportunity for technologies that can optimize sterilization metrics across diverse load specifications while maintaining operational efficiency.
Current Autoclave Technology Limitations and Challenges
Despite significant advancements in autoclave technology, several critical limitations and challenges persist in achieving optimal sterilization metrics. Current autoclave systems face substantial difficulties in load-specific optimization, particularly when processing diverse materials with varying thermal properties. The fundamental challenge lies in ensuring uniform heat distribution throughout heterogeneous loads, as dense materials and complex geometries create "cold spots" where sterilization parameters may not reach threshold values.
Temperature and pressure inconsistencies represent another significant limitation in contemporary autoclave systems. Many units struggle to maintain precise parameters throughout the entire sterilization cycle, especially during the critical exposure phase. These fluctuations can compromise sterilization efficacy, particularly for sensitive instruments or complex medical devices with narrow sterilization windows.
Validation methodologies for current autoclave systems remain problematic, with biological indicators and chemical integrators providing only retrospective confirmation of sterilization efficacy. This post-process verification approach creates inherent risks, as sterilization failures are only detected after cycle completion, potentially allowing compromised items to enter the sterile supply chain.
Energy efficiency constraints pose both operational and environmental challenges. Traditional autoclave systems consume substantial amounts of water and electricity, with thermal losses occurring throughout the sterilization process. These inefficiencies not only increase operational costs but also contribute to larger environmental footprints for healthcare facilities and industrial operations.
Load density optimization represents a persistent technical hurdle. Current systems often employ standardized cycles regardless of load composition, resulting in either excessive processing (wasting resources) or insufficient parameter exposure (compromising sterility). The absence of real-time adaptive control mechanisms prevents dynamic adjustment to specific load characteristics.
Documentation and traceability limitations further complicate autoclave operations. Many systems lack comprehensive data capture capabilities for critical parameters throughout the sterilization cycle. This deficiency creates challenges for regulatory compliance and quality assurance, particularly in highly regulated environments such as pharmaceutical manufacturing and healthcare settings.
Material compatibility issues continue to constrain autoclave applications. High-temperature steam sterilization remains incompatible with heat-sensitive materials, while alternative low-temperature methods often require longer cycle times or specialized equipment. This limitation forces facilities to maintain multiple sterilization technologies, increasing complexity and operational costs.
Human Engineering Automation
Temperature and pressure inconsistencies represent another significant limitation in contemporary autoclave systems. Many units struggle to maintain precise parameters throughout the entire sterilization cycle, especially during the critical exposure phase. These fluctuations can compromise sterilization efficacy, particularly for sensitive instruments or complex medical devices with narrow sterilization windows.
Validation methodologies for current autoclave systems remain problematic, with biological indicators and chemical integrators providing only retrospective confirmation of sterilization efficacy. This post-process verification approach creates inherent risks, as sterilization failures are only detected after cycle completion, potentially allowing compromised items to enter the sterile supply chain.
Energy efficiency constraints pose both operational and environmental challenges. Traditional autoclave systems consume substantial amounts of water and electricity, with thermal losses occurring throughout the sterilization process. These inefficiencies not only increase operational costs but also contribute to larger environmental footprints for healthcare facilities and industrial operations.
Load density optimization represents a persistent technical hurdle. Current systems often employ standardized cycles regardless of load composition, resulting in either excessive processing (wasting resources) or insufficient parameter exposure (compromising sterility). The absence of real-time adaptive control mechanisms prevents dynamic adjustment to specific load characteristics.
Documentation and traceability limitations further complicate autoclave operations. Many systems lack comprehensive data capture capabilities for critical parameters throughout the sterilization cycle. This deficiency creates challenges for regulatory compliance and quality assurance, particularly in highly regulated environments such as pharmaceutical manufacturing and healthcare settings.
Material compatibility issues continue to constrain autoclave applications. High-temperature steam sterilization remains incompatible with heat-sensitive materials, while alternative low-temperature methods often require longer cycle times or specialized equipment. This limitation forces facilities to maintain multiple sterilization technologies, increasing complexity and operational costs.
Human Engineering Automation
Current Load Configuration and Sterilization Parameter Optimization
01 Temperature and pressure monitoring in autoclave sterilization
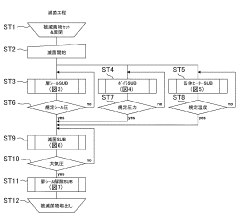
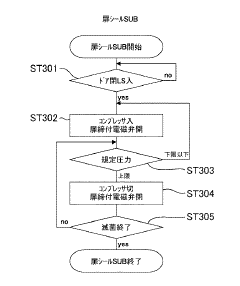
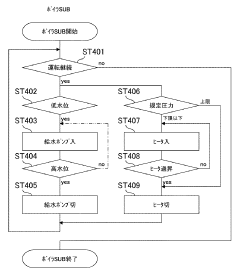
Effective autoclave sterilization requires precise monitoring of temperature and pressure parameters. Modern autoclaves incorporate sensors and monitoring systems to track these critical metrics throughout the sterilization cycle. These systems ensure that the required conditions for sterilization are maintained consistently, typically at temperatures of 121-134°C and pressures of 15-30 psi. Real-time monitoring allows for validation of the sterilization process and helps identify any deviations that might compromise sterilization efficacy.- Temperature and pressure monitoring in autoclave sterilization: Effective autoclave sterilization requires precise monitoring of temperature and pressure parameters. Modern autoclave systems incorporate sensors and monitoring devices that continuously track these critical metrics throughout the sterilization cycle. These monitoring systems ensure that the required sterilization conditions are maintained for the appropriate duration, typically 121°C at 15 psi for a specified time period. Real-time data collection allows for validation of the sterilization process and helps identify any deviations that might compromise sterilization efficacy.
- Biological indicators for sterilization validation: Biological indicators are used to verify the effectiveness of autoclave sterilization processes. These indicators contain highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than most pathogens. After the sterilization cycle, these indicators are incubated to determine if any spores survived. The absence of growth confirms successful sterilization. This method provides a reliable metric for validating autoclave performance and ensuring that sterilization parameters were sufficient to eliminate all microbial life.
- Chemical indicators and integrators for sterilization monitoring: Chemical indicators and integrators provide visual confirmation of sterilization conditions. These indicators change color or physical state when exposed to specific combinations of temperature, pressure, and time in an autoclave. They can be classified into different classes based on their complexity and parameters monitored. Single-parameter indicators verify exposure to the sterilization process, while multi-parameter indicators and integrators respond to multiple critical variables. These tools offer immediate visual feedback on whether sterilization conditions were achieved, complementing biological indicators in a comprehensive monitoring system.
- Cycle documentation and data management systems: Modern autoclave sterilization processes incorporate sophisticated data management systems that document each sterilization cycle. These systems record critical parameters including temperature profiles, pressure readings, exposure time, and operator information. The documentation provides traceability and accountability for each sterilization cycle, allowing for retrospective analysis and quality assurance. Advanced systems may include automated report generation, electronic signatures, and integration with facility-wide quality management systems to ensure compliance with regulatory requirements and sterilization standards.
- Load configuration and sterilization efficacy testing: The arrangement and composition of items within an autoclave significantly impact sterilization efficacy. Proper load configuration ensures steam penetration to all surfaces and prevents the formation of air pockets that could shield microorganisms from sterilization conditions. Sterilization efficacy testing includes methods such as Bowie-Dick tests for steam penetration, vacuum leak tests, and load-specific validation protocols. These tests verify that the autoclave can effectively sterilize specific load types and configurations, providing metrics to optimize loading patterns and cycle parameters for different materials and equipment.
02 Biological indicators for sterilization validation
Biological indicators are used to verify the effectiveness of autoclave sterilization processes. These indicators contain highly resistant bacterial spores, typically Geobacillus stearothermophilus, which are more difficult to kill than most pathogens. After sterilization, these indicators are incubated to determine if any spores survived the process. The absence of growth confirms successful sterilization. This method provides a reliable metric for validating autoclave performance and ensuring that sterilization parameters were sufficient to eliminate all microbial life.Expand Specific Solutions03 Chemical indicators and integrators for sterilization monitoring
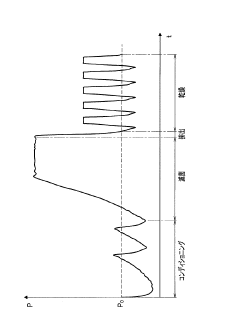
Chemical indicators and integrators provide visual confirmation of sterilization conditions. These indicators change color or physical state when exposed to specific combinations of temperature, pressure, and time in an autoclave. They can be classified into different classes based on their complexity and accuracy, from simple process indicators to multi-parameter integrators. Chemical indicators offer immediate feedback on whether sterilization conditions were achieved, complementing biological indicators and physical monitoring systems in a comprehensive sterilization assurance program.Expand Specific Solutions04 Cycle time and F0 value as sterilization metrics
The effectiveness of autoclave sterilization is often quantified using metrics such as cycle time and F0 value. Cycle time refers to the total duration of the sterilization process, including heating, sterilization, and cooling phases. The F0 value is a calculated metric that integrates time and temperature to measure the lethality of the sterilization process, typically expressed in minutes at a reference temperature of 121°C. These metrics help in standardizing sterilization protocols and ensuring that sufficient thermal energy is delivered to achieve complete sterilization across different load types and autoclave models.Expand Specific Solutions05 Automated documentation and validation systems
Modern autoclave sterilization processes incorporate automated documentation and validation systems to ensure compliance with regulatory standards. These systems record all critical parameters throughout the sterilization cycle, including temperature, pressure, time, and any deviations from set parameters. The data is stored electronically and can be used to generate sterilization certificates and validation reports. Automated systems reduce human error in documentation, provide traceability for each sterilization cycle, and facilitate quality assurance audits by maintaining comprehensive records of sterilization metrics.Expand Specific Solutions
Key Manufacturers and Competitors in Autoclave Industry
The autoclave sterilization market is currently in a mature growth phase with increasing demand driven by stringent healthcare regulations and pharmaceutical manufacturing standards. The global market size is estimated to exceed $3 billion, growing at 5-7% annually. Technologically, the field is evolving from basic sterilization systems toward sophisticated solutions with advanced monitoring capabilities. Leading players include Fedegari Autoclavi, a pioneer in sterilization systems with comprehensive bio-decontamination solutions; SCHOTT AG, contributing specialized glass components critical for autoclave integrity; and Stryker Corp., integrating sterilization technologies into broader medical device ecosystems. Emerging competitors like Remeda AB are focusing on ergonomic handling systems that complement traditional autoclave operations, while established industrial conglomerates such as Mitsubishi Electric and Parker-Hannifin provide automation components that enhance load optimization and process control.
Fedegari Autoclavi SpA
Technical Solution: Fedegari has developed advanced autoclave systems with patented Thema4 process controllers that provide real-time monitoring and adjustment of critical sterilization parameters. Their technology incorporates multi-variable control algorithms that simultaneously manage temperature, pressure, and time parameters to achieve optimal F0 values (measure of lethality) across varied load configurations. Fedegari's systems feature dynamic air removal through pulsed vacuum technology and sophisticated steam distribution systems that ensure temperature uniformity within ±0.5°C throughout the chamber. Their latest innovation includes load-specific cycle development software that creates customized sterilization protocols based on load density, material composition, and geometric arrangement to maximize efficacy while minimizing cycle times.
Strengths: Superior precision in parameter control, advanced data management systems for compliance documentation, and customizable cycle development for specific load types. Weaknesses: Higher initial investment costs compared to standard systems, and complex validation requirements for their advanced control systems that may require specialized training.
SANYO Electric Co., Ltd.
Technical Solution: SANYO has developed precision autoclave technology with their MCO-series laboratory sterilizers that incorporate advanced load sensing and cycle optimization. Their systems feature Direct Heat and Air Jacket conditioning that creates uniform temperature distribution throughout the chamber, addressing the challenge of cold spots in varied load configurations. SANYO's autoclaves utilize proprietary algorithms that calculate heat penetration rates based on load density and composition, automatically adjusting sterilization parameters to achieve validated sterility assurance levels (SAL). Their technology includes integrated monitoring systems with multiple temperature and pressure sensors that provide comprehensive data on sterilization metrics throughout the cycle. SANYO has also pioneered energy-efficient sterilization cycles that optimize resource consumption while maintaining sterilization efficacy through precise steam generation and distribution control.
Strengths: Excellent temperature uniformity even with varied loads, energy-efficient operation that reduces operational costs, and comprehensive data management for validation. Weaknesses: More focused on laboratory applications than industrial-scale sterilization, and limited customization options for specialized load requirements.
Critical Patents and Research in Autoclave Load Management
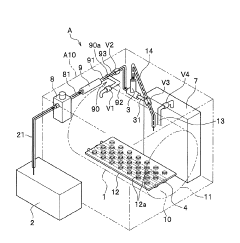
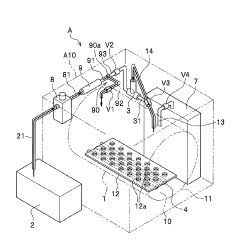
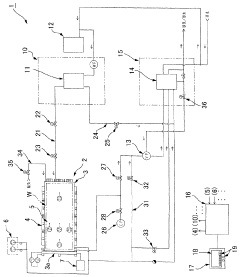
High pressure steam sterilization device
PatentActiveJP2020195539A
Innovation
- A compact high-pressure steam sterilizer incorporating a sterilization tank, driving gas supply means, ejector, steam generating means, and control means to sequentially perform sterilization, pressure reduction, and drying processes using compressed air and an ejector to enhance drying performance.
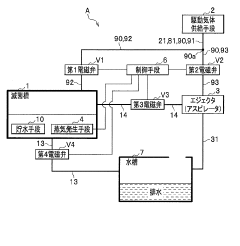
Flow-type high-pressure steam sterilization method and flow-type sterilizer by soft hydrothermal process
PatentInactiveJPWO2017010525A1
Innovation
- A flow-type high-pressure steam sterilization method using a soft hydrothermal process that involves an air evacuation step, temperature and pressure increase, high-pressure steam sterilization with highly saturated steam circulation, and a controlled drying process to minimize condensation and shorten drying times.
Regulatory Standards and Compliance Requirements
Regulatory compliance in autoclave sterilization processes is governed by stringent international and regional standards that ensure patient safety and infection control. The primary regulatory bodies include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and the International Organization for Standardization (ISO) globally. These organizations have established comprehensive frameworks that define the parameters for effective sterilization.
ISO 17665 serves as the cornerstone standard for moist heat sterilization, detailing the development, validation, and routine control requirements for sterilization processes. This standard mandates specific temperature-pressure relationships, exposure times, and documentation protocols that must be adhered to for compliant operation. Additionally, ISO 11138 and ISO 11140 provide guidelines for biological and chemical indicators respectively, which are essential for verification of sterilization efficacy.
Healthcare facilities must comply with regional regulations such as the EU Medical Device Regulation (MDR) in Europe and the Code of Federal Regulations Title 21 in the United States. These regulations require detailed documentation of sterilization cycles, including load configuration, temperature profiles, pressure readings, and exposure times. Non-compliance can result in severe penalties, product recalls, and potential legal liabilities.
Validation requirements constitute another critical aspect of regulatory compliance. The FDA's Quality System Regulation mandates that sterilization processes undergo installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). These validation procedures must be performed using worst-case scenarios to ensure that the autoclave can consistently achieve sterility assurance levels (SAL) of 10^-6 or better under all operating conditions.
Record-keeping requirements are equally stringent, with regulations mandating the maintenance of sterilization cycle records for periods ranging from two to ten years, depending on the jurisdiction and application. These records must include parametric data, biological indicator results, and any deviations from standard operating procedures, along with corrective actions taken.
Recent regulatory trends indicate an increasing focus on parametric release methodologies, which rely on physical measurements rather than biological indicators for batch release decisions. This approach requires more sophisticated monitoring systems and tighter process controls but offers advantages in terms of efficiency and reliability. Regulatory bodies are also placing greater emphasis on risk management frameworks, requiring facilities to implement comprehensive risk assessment protocols for their sterilization processes.
Compliance with these regulatory standards necessitates ongoing staff training, regular equipment calibration, and periodic revalidation of processes. Organizations must stay abreast of regulatory updates and technological advancements to ensure continued compliance in this critical aspect of healthcare delivery and medical device manufacturing.
ISO 17665 serves as the cornerstone standard for moist heat sterilization, detailing the development, validation, and routine control requirements for sterilization processes. This standard mandates specific temperature-pressure relationships, exposure times, and documentation protocols that must be adhered to for compliant operation. Additionally, ISO 11138 and ISO 11140 provide guidelines for biological and chemical indicators respectively, which are essential for verification of sterilization efficacy.
Healthcare facilities must comply with regional regulations such as the EU Medical Device Regulation (MDR) in Europe and the Code of Federal Regulations Title 21 in the United States. These regulations require detailed documentation of sterilization cycles, including load configuration, temperature profiles, pressure readings, and exposure times. Non-compliance can result in severe penalties, product recalls, and potential legal liabilities.
Validation requirements constitute another critical aspect of regulatory compliance. The FDA's Quality System Regulation mandates that sterilization processes undergo installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). These validation procedures must be performed using worst-case scenarios to ensure that the autoclave can consistently achieve sterility assurance levels (SAL) of 10^-6 or better under all operating conditions.
Record-keeping requirements are equally stringent, with regulations mandating the maintenance of sterilization cycle records for periods ranging from two to ten years, depending on the jurisdiction and application. These records must include parametric data, biological indicator results, and any deviations from standard operating procedures, along with corrective actions taken.
Recent regulatory trends indicate an increasing focus on parametric release methodologies, which rely on physical measurements rather than biological indicators for batch release decisions. This approach requires more sophisticated monitoring systems and tighter process controls but offers advantages in terms of efficiency and reliability. Regulatory bodies are also placing greater emphasis on risk management frameworks, requiring facilities to implement comprehensive risk assessment protocols for their sterilization processes.
Compliance with these regulatory standards necessitates ongoing staff training, regular equipment calibration, and periodic revalidation of processes. Organizations must stay abreast of regulatory updates and technological advancements to ensure continued compliance in this critical aspect of healthcare delivery and medical device manufacturing.
Environmental Impact and Energy Efficiency Considerations
Autoclave sterilization processes, while essential for ensuring safety in healthcare and industrial settings, carry significant environmental implications that warrant careful consideration. Traditional autoclave systems consume substantial amounts of energy, with typical cycles requiring temperatures of 121-134°C maintained for extended periods. This energy consumption translates to considerable carbon emissions, particularly in facilities operating multiple units or conducting numerous sterilization cycles daily.
Water usage represents another critical environmental concern, as conventional autoclaves can consume between 25-75 gallons per cycle depending on size and configuration. This water is often treated with chemicals before disposal, creating potential downstream environmental impacts if not properly managed. Additionally, the steam generation process itself contributes to facility heat loads, potentially increasing HVAC demands and further expanding the carbon footprint of sterilization operations.
Recent technological innovations have focused on improving autoclave energy efficiency through several approaches. Advanced insulation materials have reduced heat loss during operation, while intelligent cycle management systems optimize temperature profiles based on specific load characteristics. Some manufacturers have introduced heat recovery systems that capture and repurpose waste heat from exhaust steam, reducing overall energy requirements by 15-30% compared to conventional models.
Water recycling technologies represent another significant advancement, with closed-loop systems capable of reducing water consumption by up to 90% compared to traditional autoclaves. These systems filter and treat water for reuse across multiple sterilization cycles, substantially reducing both water consumption and wastewater generation. Complementary technologies include condensate return systems and water-efficient vacuum generation methods.
The economic implications of these environmental considerations are increasingly significant. Rising energy costs and stricter environmental regulations have shifted the total cost of ownership calculations for sterilization equipment. While energy-efficient autoclaves typically carry higher initial purchase prices, lifecycle cost analyses frequently demonstrate superior long-term value through reduced operational expenses and compliance costs.
Organizations implementing comprehensive sterilization sustainability programs have reported payback periods of 2-5 years for investments in energy-efficient autoclave technologies. These calculations become even more favorable when factoring in available incentives from utilities and government agencies promoting energy conservation. Forward-thinking facilities are now incorporating these environmental metrics into their sterilization validation protocols, recognizing that optimal sterilization increasingly means achieving both safety and sustainability objectives simultaneously.
Water usage represents another critical environmental concern, as conventional autoclaves can consume between 25-75 gallons per cycle depending on size and configuration. This water is often treated with chemicals before disposal, creating potential downstream environmental impacts if not properly managed. Additionally, the steam generation process itself contributes to facility heat loads, potentially increasing HVAC demands and further expanding the carbon footprint of sterilization operations.
Recent technological innovations have focused on improving autoclave energy efficiency through several approaches. Advanced insulation materials have reduced heat loss during operation, while intelligent cycle management systems optimize temperature profiles based on specific load characteristics. Some manufacturers have introduced heat recovery systems that capture and repurpose waste heat from exhaust steam, reducing overall energy requirements by 15-30% compared to conventional models.
Water recycling technologies represent another significant advancement, with closed-loop systems capable of reducing water consumption by up to 90% compared to traditional autoclaves. These systems filter and treat water for reuse across multiple sterilization cycles, substantially reducing both water consumption and wastewater generation. Complementary technologies include condensate return systems and water-efficient vacuum generation methods.
The economic implications of these environmental considerations are increasingly significant. Rising energy costs and stricter environmental regulations have shifted the total cost of ownership calculations for sterilization equipment. While energy-efficient autoclaves typically carry higher initial purchase prices, lifecycle cost analyses frequently demonstrate superior long-term value through reduced operational expenses and compliance costs.
Organizations implementing comprehensive sterilization sustainability programs have reported payback periods of 2-5 years for investments in energy-efficient autoclave technologies. These calculations become even more favorable when factoring in available incentives from utilities and government agencies promoting energy conservation. Forward-thinking facilities are now incorporating these environmental metrics into their sterilization validation protocols, recognizing that optimal sterilization increasingly means achieving both safety and sustainability objectives simultaneously.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!