Autoclave Performance Monitoring: Sensor-Driven Approaches
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Monitoring Technology Background and Objectives
Autoclave technology has evolved significantly since its inception in the early 20th century, transforming from simple pressure vessels to sophisticated systems critical in aerospace, medical, and composite manufacturing industries. The fundamental principle remains unchanged—utilizing high pressure and temperature in a controlled environment to achieve specific material properties or sterilization requirements. However, monitoring capabilities have progressed from basic analog gauges to complex digital systems incorporating multiple sensor arrays.
The evolution of autoclave monitoring technology has been driven by increasing demands for process reliability, quality assurance, and operational efficiency. Traditional monitoring approaches relied heavily on manual inspection and post-process verification, which proved inadequate for modern manufacturing standards requiring real-time process control and validation. This limitation has spurred development toward sensor-driven monitoring solutions that provide continuous data streams throughout the autoclave cycle.
Current technological trends point toward integrated sensor networks capable of capturing multiple parameters simultaneously—temperature distribution, pressure gradients, vacuum integrity, and material state changes. These advancements align with Industry 4.0 principles, emphasizing data-driven decision making and process optimization. The integration of IoT capabilities has further enhanced monitoring systems, enabling remote access and cloud-based analytics.
The primary objective of modern autoclave performance monitoring is to establish a comprehensive digital representation of the curing or sterilization process. This digital twin approach aims to correlate sensor data with material behavior and final product quality, ultimately enabling predictive capabilities rather than merely reactive monitoring. Such predictive systems represent the frontier of autoclave technology, potentially eliminating costly quality issues before they manifest in finished products.
Secondary objectives include reducing energy consumption through optimized cycle parameters, extending equipment lifespan through preventive maintenance informed by sensor data, and enhancing operator safety through early detection of anomalous conditions. These objectives reflect the multifaceted value proposition of advanced monitoring systems beyond basic quality control.
The technological trajectory suggests movement toward non-invasive sensing methodologies that can monitor material states without physical contact, potentially revolutionizing how autoclave processes are validated. Emerging technologies such as acoustic monitoring, dielectric analysis, and embedded fiber optic sensors represent promising avenues for achieving this vision, though each presents unique implementation challenges in the harsh autoclave environment.
The evolution of autoclave monitoring technology has been driven by increasing demands for process reliability, quality assurance, and operational efficiency. Traditional monitoring approaches relied heavily on manual inspection and post-process verification, which proved inadequate for modern manufacturing standards requiring real-time process control and validation. This limitation has spurred development toward sensor-driven monitoring solutions that provide continuous data streams throughout the autoclave cycle.
Current technological trends point toward integrated sensor networks capable of capturing multiple parameters simultaneously—temperature distribution, pressure gradients, vacuum integrity, and material state changes. These advancements align with Industry 4.0 principles, emphasizing data-driven decision making and process optimization. The integration of IoT capabilities has further enhanced monitoring systems, enabling remote access and cloud-based analytics.
The primary objective of modern autoclave performance monitoring is to establish a comprehensive digital representation of the curing or sterilization process. This digital twin approach aims to correlate sensor data with material behavior and final product quality, ultimately enabling predictive capabilities rather than merely reactive monitoring. Such predictive systems represent the frontier of autoclave technology, potentially eliminating costly quality issues before they manifest in finished products.
Secondary objectives include reducing energy consumption through optimized cycle parameters, extending equipment lifespan through preventive maintenance informed by sensor data, and enhancing operator safety through early detection of anomalous conditions. These objectives reflect the multifaceted value proposition of advanced monitoring systems beyond basic quality control.
The technological trajectory suggests movement toward non-invasive sensing methodologies that can monitor material states without physical contact, potentially revolutionizing how autoclave processes are validated. Emerging technologies such as acoustic monitoring, dielectric analysis, and embedded fiber optic sensors represent promising avenues for achieving this vision, though each presents unique implementation challenges in the harsh autoclave environment.
Market Demand Analysis for Advanced Autoclave Monitoring
The global market for advanced autoclave monitoring systems is experiencing significant growth, driven by increasing demands for quality assurance and process optimization across multiple industries. The aerospace sector represents the largest market segment, valued at approximately $1.2 billion in 2022, with projected annual growth of 7.8% through 2028. This growth is primarily fueled by stringent safety regulations and the expanding use of composite materials in aircraft manufacturing, which require precise curing conditions.
Medical device sterilization represents the second-largest market segment, currently estimated at $890 million globally. Healthcare facilities are increasingly adopting sensor-driven autoclave monitoring systems to ensure compliance with regulatory standards and to minimize the risk of healthcare-associated infections. The COVID-19 pandemic has accelerated this trend, with hospitals and medical device manufacturers investing heavily in advanced sterilization infrastructure.
The pharmaceutical industry has emerged as the fastest-growing segment for autoclave monitoring technology, with a compound annual growth rate of 9.3%. This surge is attributed to the industry's focus on maintaining sterility assurance levels and the growing adoption of continuous manufacturing processes that require real-time monitoring capabilities.
Regional analysis indicates that North America currently dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to rapid industrialization in countries like China and India, coupled with increasing investments in healthcare infrastructure.
End-user surveys reveal that 76% of industrial customers prioritize real-time monitoring capabilities when selecting autoclave systems, while 68% emphasize the importance of predictive maintenance features. Additionally, 82% of respondents expressed interest in cloud-based monitoring solutions that enable remote access to process data.
Market research indicates a growing demand for integrated sensor systems that can monitor multiple parameters simultaneously, including temperature, pressure, humidity, and chemical concentration. This trend is particularly evident in industries dealing with high-value products where process deviations can result in significant financial losses.
The economic benefits of advanced autoclave monitoring are substantial, with early adopters reporting average reductions in energy consumption of 12-18% and decreases in product rejection rates of 15-22%. These efficiency gains translate to an average return on investment period of 14-18 months, making sensor-driven approaches increasingly attractive from a business perspective.
Medical device sterilization represents the second-largest market segment, currently estimated at $890 million globally. Healthcare facilities are increasingly adopting sensor-driven autoclave monitoring systems to ensure compliance with regulatory standards and to minimize the risk of healthcare-associated infections. The COVID-19 pandemic has accelerated this trend, with hospitals and medical device manufacturers investing heavily in advanced sterilization infrastructure.
The pharmaceutical industry has emerged as the fastest-growing segment for autoclave monitoring technology, with a compound annual growth rate of 9.3%. This surge is attributed to the industry's focus on maintaining sterility assurance levels and the growing adoption of continuous manufacturing processes that require real-time monitoring capabilities.
Regional analysis indicates that North America currently dominates the market with a 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to rapid industrialization in countries like China and India, coupled with increasing investments in healthcare infrastructure.
End-user surveys reveal that 76% of industrial customers prioritize real-time monitoring capabilities when selecting autoclave systems, while 68% emphasize the importance of predictive maintenance features. Additionally, 82% of respondents expressed interest in cloud-based monitoring solutions that enable remote access to process data.
Market research indicates a growing demand for integrated sensor systems that can monitor multiple parameters simultaneously, including temperature, pressure, humidity, and chemical concentration. This trend is particularly evident in industries dealing with high-value products where process deviations can result in significant financial losses.
The economic benefits of advanced autoclave monitoring are substantial, with early adopters reporting average reductions in energy consumption of 12-18% and decreases in product rejection rates of 15-22%. These efficiency gains translate to an average return on investment period of 14-18 months, making sensor-driven approaches increasingly attractive from a business perspective.
Current Sensor Technologies and Implementation Challenges
The autoclave monitoring landscape is currently dominated by several key sensor technologies, each with specific implementation challenges. Temperature sensors, particularly thermocouples and resistance temperature detectors (RTDs), remain fundamental for autoclave monitoring but face challenges related to calibration drift in harsh environments and difficulty in achieving uniform temperature measurement across large autoclave chambers. Pressure sensors, including strain gauge and piezoelectric types, provide critical data for maintaining optimal curing conditions but struggle with long-term stability under cyclic pressure conditions and often require frequent recalibration.
Humidity sensors have evolved significantly, with capacitive and resistive types being most common in autoclave applications. However, these sensors frequently encounter accuracy degradation at the high humidity levels typical in autoclave operations, and condensation on sensing elements remains a persistent challenge affecting measurement reliability. Vacuum sensors, essential for ensuring proper air removal before sterilization cycles, face challenges with steam compatibility and maintaining accuracy across the wide pressure ranges experienced during autoclave cycles.
Flow sensors for monitoring steam and water circulation within autoclaves have improved in recent years, but turbine and vortex types still struggle with scaling and fouling issues in industrial applications. Additionally, gas analyzers for monitoring air removal efficiency and detecting non-condensable gases that could compromise sterilization effectiveness face significant miniaturization and cost challenges, limiting their widespread implementation.
The integration of these various sensors presents substantial technical hurdles. Cross-interference between different sensor types can lead to measurement errors, while the harsh autoclave environment—characterized by high temperature, pressure, and humidity—accelerates sensor degradation and reduces operational lifespan. This necessitates frequent maintenance and replacement, increasing operational costs.
Data acquisition systems face challenges in handling the diverse signal types from multiple sensor technologies, and real-time processing of large data volumes remains computationally intensive. Furthermore, wireless sensor networks, while offering installation flexibility, encounter signal attenuation issues due to the metallic autoclave structure, and power supply limitations restrict continuous monitoring capabilities.
Standardization across different autoclave models and manufacturers presents another significant challenge, with proprietary interfaces often limiting interoperability between sensors and monitoring systems. The cost-benefit equation also remains problematic, as high-precision sensors with the durability required for autoclave environments command premium prices that may be prohibitive for smaller operations or facilities in resource-constrained settings.
Humidity sensors have evolved significantly, with capacitive and resistive types being most common in autoclave applications. However, these sensors frequently encounter accuracy degradation at the high humidity levels typical in autoclave operations, and condensation on sensing elements remains a persistent challenge affecting measurement reliability. Vacuum sensors, essential for ensuring proper air removal before sterilization cycles, face challenges with steam compatibility and maintaining accuracy across the wide pressure ranges experienced during autoclave cycles.
Flow sensors for monitoring steam and water circulation within autoclaves have improved in recent years, but turbine and vortex types still struggle with scaling and fouling issues in industrial applications. Additionally, gas analyzers for monitoring air removal efficiency and detecting non-condensable gases that could compromise sterilization effectiveness face significant miniaturization and cost challenges, limiting their widespread implementation.
The integration of these various sensors presents substantial technical hurdles. Cross-interference between different sensor types can lead to measurement errors, while the harsh autoclave environment—characterized by high temperature, pressure, and humidity—accelerates sensor degradation and reduces operational lifespan. This necessitates frequent maintenance and replacement, increasing operational costs.
Data acquisition systems face challenges in handling the diverse signal types from multiple sensor technologies, and real-time processing of large data volumes remains computationally intensive. Furthermore, wireless sensor networks, while offering installation flexibility, encounter signal attenuation issues due to the metallic autoclave structure, and power supply limitations restrict continuous monitoring capabilities.
Standardization across different autoclave models and manufacturers presents another significant challenge, with proprietary interfaces often limiting interoperability between sensors and monitoring systems. The cost-benefit equation also remains problematic, as high-precision sensors with the durability required for autoclave environments command premium prices that may be prohibitive for smaller operations or facilities in resource-constrained settings.
Current Sensor-Driven Monitoring Solutions
01 Real-time monitoring systems for autoclaves
Real-time monitoring systems are implemented to continuously track autoclave performance parameters such as temperature, pressure, and cycle time. These systems provide immediate feedback on sterilization effectiveness and can alert operators to deviations from optimal performance. Advanced sensors and data acquisition technologies enable precise measurement of critical parameters throughout the sterilization cycle, ensuring consistent and reliable performance.- Real-time monitoring systems for autoclave performance: Real-time monitoring systems are implemented to continuously track autoclave performance parameters such as temperature, pressure, and cycle time. These systems utilize sensors and data acquisition technologies to provide immediate feedback on sterilization processes, allowing operators to detect deviations from optimal conditions promptly. The monitoring can be conducted through digital interfaces that display current status and alert users to any abnormalities, ensuring sterilization efficacy and equipment reliability.
- Data logging and analysis for autoclave validation: Advanced data logging systems capture and store comprehensive information about autoclave cycles for validation purposes. These systems record critical parameters throughout the sterilization process and generate detailed reports that can be analyzed to verify compliance with regulatory standards. The collected data enables trend analysis, performance optimization, and documentation for quality assurance. Historical performance data can be used to identify patterns, predict maintenance needs, and improve overall sterilization protocols.
- Remote monitoring and control capabilities: Remote monitoring technologies allow autoclave performance to be observed and controlled from locations outside the immediate vicinity of the equipment. These systems utilize network connectivity to transmit operational data to centralized monitoring stations or mobile devices. Authorized personnel can access real-time information, receive notifications about cycle completion or errors, and even initiate commands remotely. This capability enhances operational efficiency, enables prompt intervention when issues arise, and facilitates management of multiple autoclaves across different locations.
- Biological and chemical indicators for verification: Biological and chemical indicators are used alongside electronic monitoring to verify autoclave sterilization effectiveness. These indicators undergo visible changes when exposed to specific conditions required for proper sterilization. Biological indicators contain resistant microorganisms that should be killed during a successful cycle, while chemical indicators change color or physical state when exposed to critical parameters like temperature and steam. These verification methods provide independent confirmation of sterilization efficacy and serve as additional safeguards beyond electronic monitoring systems.
- Automated performance testing and calibration: Automated systems for testing and calibrating autoclaves ensure consistent performance and accuracy of monitoring instruments. These systems can run diagnostic routines to verify sensor accuracy, control system functionality, and overall equipment performance. Regular automated testing helps identify drift in measurement systems, mechanical wear, or other factors that could affect sterilization results. Calibration procedures can be scheduled and documented automatically, maintaining compliance with quality standards and extending equipment lifespan through preventive maintenance.
02 Validation and verification methods for autoclave cycles
Validation protocols are essential for confirming that autoclave cycles meet required sterilization standards. These methods include biological indicators, chemical indicators, and physical parameter monitoring to verify that sterilization conditions have been achieved. Regular validation testing helps maintain compliance with regulatory requirements and ensures that the autoclave consistently delivers effective sterilization performance across different load types and configurations.Expand Specific Solutions03 Remote monitoring and control systems
Remote monitoring technologies allow for autoclave performance tracking from centralized locations or via mobile devices. These systems incorporate wireless communication, cloud-based data storage, and remote access capabilities to enable monitoring without physical presence at the equipment. Remote control features permit adjustments to cycle parameters, troubleshooting, and even initiation of sterilization cycles from a distance, improving operational efficiency and enabling prompt response to performance issues.Expand Specific Solutions04 Data logging and analysis for performance optimization
Comprehensive data logging systems capture and store detailed information about each autoclave cycle, creating historical performance records. Advanced analysis tools process this data to identify trends, anomalies, and opportunities for optimization. Statistical analysis of performance data helps in predicting maintenance needs, optimizing cycle parameters, and ensuring consistent sterilization results across multiple cycles and equipment units.Expand Specific Solutions05 Automated quality control and compliance reporting
Automated systems generate comprehensive reports documenting autoclave performance for quality control and regulatory compliance purposes. These systems can automatically compare performance data against predefined standards and flag non-compliant cycles. Integration with quality management systems enables streamlined documentation, audit trail creation, and preparation of compliance reports for regulatory inspections, reducing manual record-keeping and improving accuracy of performance documentation.Expand Specific Solutions
Leading Manufacturers and Technology Providers
The autoclave performance monitoring market is currently in a growth phase, with increasing adoption of sensor-driven approaches across aerospace, manufacturing, and healthcare sectors. The market is expanding at a CAGR of approximately 5-7%, driven by stringent quality control requirements and safety regulations. Leading players include established industrial giants like Boeing, Siemens AG, and Robert Bosch GmbH, who leverage their extensive engineering capabilities to develop sophisticated monitoring systems. Specialized companies such as MarqMetrix and Eschmann Holdings are focusing on niche applications with innovative sensor technologies. The technology maturity varies across sectors, with aerospace companies (Boeing, Rolls-Royce) demonstrating advanced implementations, while medical and industrial applications are rapidly evolving through innovations from Samsung Electronics and Endress+Hauser. Integration of IoT and AI capabilities represents the next frontier in this technology landscape.
The Boeing Co.
Technical Solution: Boeing has developed an advanced Autoclave Performance Monitoring system that utilizes a comprehensive sensor network to monitor critical parameters during composite curing processes. Their approach integrates multiple sensor types including temperature, pressure, vacuum, and resin flow sensors strategically placed throughout the autoclave and within composite layups. Boeing's system employs real-time data analytics to create thermal profiles and detect anomalies during the curing cycle. The company has implemented machine learning algorithms that can predict potential defects before they occur by analyzing patterns in sensor data streams. Boeing's solution includes a digital twin of the autoclave process that allows for simulation and optimization of curing cycles, reducing energy consumption while maintaining quality standards. The system also features wireless sensor technology that eliminates the need for complex wiring through autoclave walls, improving reliability and reducing maintenance requirements.
Strengths: Boeing's extensive aerospace manufacturing experience provides deep domain knowledge for composite curing processes. Their integrated approach combining multiple sensor types with predictive analytics offers comprehensive monitoring capabilities. Weaknesses: The system is primarily optimized for aerospace-grade composites and may require significant adaptation for other industries. High implementation costs may limit accessibility for smaller manufacturing operations.
Endress+Hauser Conducta GmbH+Co. KG
Technical Solution: Endress+Hauser has developed a specialized autoclave monitoring solution centered around their Memosens technology, which digitizes measurement signals directly at the sensor and transmits them inductively to the transmitter. This approach eliminates interference from moisture and electromagnetic fields, crucial in autoclave environments. Their system incorporates multiple sensor types including temperature, pressure, pH, conductivity, and oxygen sensors that can withstand the harsh conditions of sterilization processes. The company's Liquiline platform serves as the central control system, collecting and analyzing data from all connected sensors while providing real-time visualization through their Heartbeat Technology. This technology continuously performs diagnostics and verification of all measuring points, documenting these processes automatically for compliance purposes. Endress+Hauser's solution also features predictive maintenance capabilities that analyze sensor drift patterns and signal quality to forecast when components need replacement before failure occurs.
Strengths: Endress+Hauser's Memosens technology provides exceptional signal integrity in challenging environments with high temperature and pressure. Their extensive experience in process instrumentation ensures robust sensor designs specifically engineered for autoclave conditions. Weaknesses: The system requires significant initial investment and specialized training for operators to fully utilize all advanced features. Integration with legacy autoclave systems may require additional engineering work.
Key Sensor Technologies and Data Integration Methods
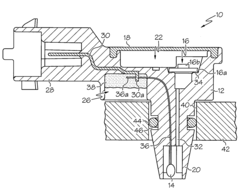
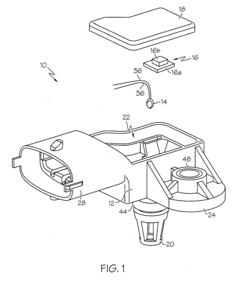
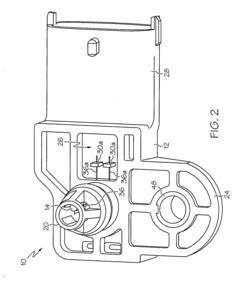
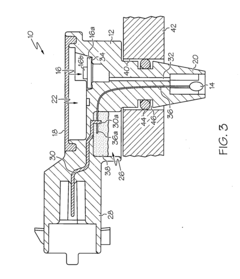
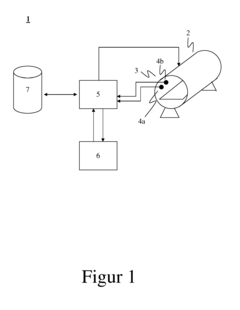
Sensor apparatus responsive to pressure and temperature within a vessel
PatentActiveUS20080216580A1
Innovation
- A sensor apparatus with a physically isolated termination cavity for the thermistor leads, which are routed through the sensor housing and connected to leadframe terminals, ensuring a secure seal and preventing leakage, suitable for high-pressure applications by using a molded plastic housing with a measurement port, sensor cavity, and termination cavity, and employing an O-ring seal and sealant to prevent contamination.
Control of an autoclave production process
PatentActiveEP2572853A2
Innovation
- A method involving a sensor system to continuously record process and component parameters, with a control unit determining the component status and dynamically controlling the autoclave process based on this data, allowing for real-time adjustments of temperature, pressure, and cycle time to ensure quality and optimize productivity.
Regulatory Compliance and Validation Requirements
Autoclave sterilization processes in healthcare, pharmaceutical, and aerospace industries are subject to stringent regulatory requirements that ensure safety, efficacy, and quality. The implementation of sensor-driven monitoring approaches must align with these complex regulatory frameworks to achieve compliance and validation.
The FDA's Quality System Regulation (21 CFR Part 820) and the EU Medical Device Regulation (MDR 2017/745) establish comprehensive requirements for monitoring and validating sterilization processes. These regulations mandate documented evidence that autoclave systems consistently operate within specified parameters. Sensor-driven approaches must therefore generate reliable, accurate data that satisfies these documentation requirements.
For pharmaceutical applications, compliance with Good Manufacturing Practice (GMP) guidelines is essential. These guidelines specify that sterilization equipment must be regularly calibrated, maintained, and validated. Sensor systems must be designed to facilitate this validation process through accurate measurement capabilities and data integrity features that prevent unauthorized modifications.
The validation requirements for autoclave monitoring systems typically follow a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Sensor-driven solutions must support each phase by providing appropriate data collection, analysis, and reporting capabilities. This includes verification of sensor placement, calibration accuracy, and long-term stability under operational conditions.
Data integrity represents a critical compliance concern for regulatory bodies. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be incorporated into sensor data management systems. This necessitates secure data transmission protocols, tamper-evident storage solutions, and comprehensive audit trail capabilities.
International standards such as ISO 17665 (sterilization of health care products) and ISO 13485 (quality management systems for medical devices) provide additional frameworks for validation. Sensor systems must be designed to collect the specific parameters outlined in these standards, including temperature, pressure, and time measurements at defined locations within the autoclave chamber.
The validation of predictive algorithms and machine learning components in advanced sensor systems presents unique regulatory challenges. Regulatory bodies increasingly require transparency in algorithm development, validation of training data sets, and ongoing performance monitoring to ensure continued accuracy and reliability of predictive capabilities.
The FDA's Quality System Regulation (21 CFR Part 820) and the EU Medical Device Regulation (MDR 2017/745) establish comprehensive requirements for monitoring and validating sterilization processes. These regulations mandate documented evidence that autoclave systems consistently operate within specified parameters. Sensor-driven approaches must therefore generate reliable, accurate data that satisfies these documentation requirements.
For pharmaceutical applications, compliance with Good Manufacturing Practice (GMP) guidelines is essential. These guidelines specify that sterilization equipment must be regularly calibrated, maintained, and validated. Sensor systems must be designed to facilitate this validation process through accurate measurement capabilities and data integrity features that prevent unauthorized modifications.
The validation requirements for autoclave monitoring systems typically follow a three-phase approach: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Sensor-driven solutions must support each phase by providing appropriate data collection, analysis, and reporting capabilities. This includes verification of sensor placement, calibration accuracy, and long-term stability under operational conditions.
Data integrity represents a critical compliance concern for regulatory bodies. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be incorporated into sensor data management systems. This necessitates secure data transmission protocols, tamper-evident storage solutions, and comprehensive audit trail capabilities.
International standards such as ISO 17665 (sterilization of health care products) and ISO 13485 (quality management systems for medical devices) provide additional frameworks for validation. Sensor systems must be designed to collect the specific parameters outlined in these standards, including temperature, pressure, and time measurements at defined locations within the autoclave chamber.
The validation of predictive algorithms and machine learning components in advanced sensor systems presents unique regulatory challenges. Regulatory bodies increasingly require transparency in algorithm development, validation of training data sets, and ongoing performance monitoring to ensure continued accuracy and reliability of predictive capabilities.
Cost-Benefit Analysis of Sensor Implementation
Implementing sensor systems for autoclave performance monitoring requires substantial initial investment, yet offers significant long-term operational and financial benefits. The initial capital expenditure typically ranges from $50,000 to $250,000 depending on autoclave size, complexity, and the sophistication of the sensor network deployed. This includes costs for hardware (sensors, transmitters, data acquisition systems), software platforms, integration with existing systems, and installation labor.
Operational costs must also be considered, including regular calibration requirements (typically quarterly), maintenance (approximately 5-8% of initial investment annually), and potential system upgrades every 3-5 years to maintain technological relevance. Additionally, staff training represents a recurring expense, particularly when implementing advanced analytics capabilities.
Against these costs, sensor-driven monitoring systems deliver quantifiable benefits across multiple dimensions. Production efficiency improvements of 15-25% have been documented across various industries through reduction in cycle times and optimization of process parameters. Quality improvements manifest as 30-40% reduction in defect rates and up to 60% decrease in material waste, particularly valuable in high-value manufacturing contexts such as aerospace composites.
Energy consumption reductions of 10-20% are achievable through optimized heating profiles and pressure management, representing significant cost savings for energy-intensive autoclave operations. Maintenance costs typically decrease by 25-35% through condition-based maintenance approaches that prevent catastrophic failures and extend equipment lifespan by 15-30%.
The return on investment timeline varies by implementation scale and industry context. Small to medium implementations typically achieve ROI within 12-24 months, while comprehensive enterprise-wide systems may require 24-36 months to fully realize financial benefits. Healthcare applications often see faster returns due to regulatory compliance benefits and patient safety improvements.
Risk mitigation represents a significant though less easily quantified benefit. Sensor systems provide early warning of potential failures, reducing downtime events by 40-60% and mitigating regulatory non-compliance risks. For industries with stringent documentation requirements, automated data collection reduces labor costs associated with manual monitoring by 70-85% while improving data accuracy and traceability.
Operational costs must also be considered, including regular calibration requirements (typically quarterly), maintenance (approximately 5-8% of initial investment annually), and potential system upgrades every 3-5 years to maintain technological relevance. Additionally, staff training represents a recurring expense, particularly when implementing advanced analytics capabilities.
Against these costs, sensor-driven monitoring systems deliver quantifiable benefits across multiple dimensions. Production efficiency improvements of 15-25% have been documented across various industries through reduction in cycle times and optimization of process parameters. Quality improvements manifest as 30-40% reduction in defect rates and up to 60% decrease in material waste, particularly valuable in high-value manufacturing contexts such as aerospace composites.
Energy consumption reductions of 10-20% are achievable through optimized heating profiles and pressure management, representing significant cost savings for energy-intensive autoclave operations. Maintenance costs typically decrease by 25-35% through condition-based maintenance approaches that prevent catastrophic failures and extend equipment lifespan by 15-30%.
The return on investment timeline varies by implementation scale and industry context. Small to medium implementations typically achieve ROI within 12-24 months, while comprehensive enterprise-wide systems may require 24-36 months to fully realize financial benefits. Healthcare applications often see faster returns due to regulatory compliance benefits and patient safety improvements.
Risk mitigation represents a significant though less easily quantified benefit. Sensor systems provide early warning of potential failures, reducing downtime events by 40-60% and mitigating regulatory non-compliance risks. For industries with stringent documentation requirements, automated data collection reduces labor costs associated with manual monitoring by 70-85% while improving data accuracy and traceability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!