Autoclave Safety Protocols: Maintaining Compliance in Biotech
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Technology Evolution and Safety Objectives
Autoclaves have evolved significantly since their inception in the 19th century when Charles Chamberland developed the first pressure sterilizer. Initially designed as simple pressure vessels, modern autoclaves have transformed into sophisticated systems incorporating advanced control mechanisms, safety features, and compliance monitoring capabilities. This evolution has been driven by increasing regulatory requirements in the biotech industry and the growing complexity of sterilization needs for diverse biological materials and equipment.
The technological trajectory of autoclaves has seen several key advancements, including the transition from manual to automated operation, integration of microprocessor controls, development of vacuum systems for improved air removal, and implementation of real-time monitoring systems. These innovations have collectively enhanced sterilization efficacy while simultaneously improving operational safety and compliance verification capabilities.
In the biotech sector, autoclave technology objectives have become increasingly focused on maintaining stringent safety standards while ensuring complete sterilization. Current technological goals include achieving uniform temperature distribution throughout the chamber, minimizing cycle times without compromising sterilization efficacy, reducing utility consumption, and implementing comprehensive documentation systems for regulatory compliance.
Safety objectives have evolved in parallel with technological capabilities. Modern autoclave safety protocols aim to prevent both immediate hazards (such as steam burns, pressure-related accidents) and long-term risks (such as incomplete sterilization leading to biological contamination). These objectives necessitate multi-layered safety systems including mechanical pressure relief valves, electronic monitoring of critical parameters, redundant control systems, and automated fault detection.
The regulatory landscape has significantly shaped autoclave technology development, with standards from organizations such as the FDA, AAMI, and ISO establishing increasingly rigorous requirements for validation, monitoring, and documentation. These standards have driven the integration of data logging capabilities, electronic record-keeping systems, and process validation protocols into modern autoclave designs.
Looking forward, emerging trends in autoclave technology include the implementation of IoT capabilities for remote monitoring and predictive maintenance, AI-assisted cycle optimization, enhanced energy efficiency measures, and improved user interfaces designed to minimize human error. These advancements aim to further enhance safety while reducing operational costs and environmental impact.
The convergence of these technological developments and safety objectives reflects the biotech industry's dual commitment to scientific advancement and rigorous safety standards. As sterilization requirements become more complex and regulatory oversight more stringent, autoclave technology continues to evolve toward systems that provide both absolute sterilization assurance and comprehensive safety compliance.
The technological trajectory of autoclaves has seen several key advancements, including the transition from manual to automated operation, integration of microprocessor controls, development of vacuum systems for improved air removal, and implementation of real-time monitoring systems. These innovations have collectively enhanced sterilization efficacy while simultaneously improving operational safety and compliance verification capabilities.
In the biotech sector, autoclave technology objectives have become increasingly focused on maintaining stringent safety standards while ensuring complete sterilization. Current technological goals include achieving uniform temperature distribution throughout the chamber, minimizing cycle times without compromising sterilization efficacy, reducing utility consumption, and implementing comprehensive documentation systems for regulatory compliance.
Safety objectives have evolved in parallel with technological capabilities. Modern autoclave safety protocols aim to prevent both immediate hazards (such as steam burns, pressure-related accidents) and long-term risks (such as incomplete sterilization leading to biological contamination). These objectives necessitate multi-layered safety systems including mechanical pressure relief valves, electronic monitoring of critical parameters, redundant control systems, and automated fault detection.
The regulatory landscape has significantly shaped autoclave technology development, with standards from organizations such as the FDA, AAMI, and ISO establishing increasingly rigorous requirements for validation, monitoring, and documentation. These standards have driven the integration of data logging capabilities, electronic record-keeping systems, and process validation protocols into modern autoclave designs.
Looking forward, emerging trends in autoclave technology include the implementation of IoT capabilities for remote monitoring and predictive maintenance, AI-assisted cycle optimization, enhanced energy efficiency measures, and improved user interfaces designed to minimize human error. These advancements aim to further enhance safety while reducing operational costs and environmental impact.
The convergence of these technological developments and safety objectives reflects the biotech industry's dual commitment to scientific advancement and rigorous safety standards. As sterilization requirements become more complex and regulatory oversight more stringent, autoclave technology continues to evolve toward systems that provide both absolute sterilization assurance and comprehensive safety compliance.
Market Demand for Compliant Sterilization Solutions
The global market for compliant sterilization solutions in biotechnology has experienced significant growth over the past decade, driven primarily by increasing regulatory scrutiny and the expansion of the biotech and pharmaceutical industries. Current market valuations indicate that the global autoclave and sterilization equipment market exceeds $3 billion, with a compound annual growth rate of approximately 6.8% projected through 2028.
Healthcare facilities, research laboratories, and biopharmaceutical manufacturing plants represent the largest customer segments, collectively accounting for over 70% of market demand. These institutions face mounting pressure to maintain rigorous compliance with evolving regulatory frameworks, including FDA regulations in the United States, EU GMP guidelines in Europe, and similar standards across Asia-Pacific regions.
The COVID-19 pandemic has substantially accelerated market demand, as heightened awareness of contamination risks has prompted facilities to upgrade their sterilization infrastructure. This trend is particularly evident in emerging economies where biotechnology sectors are rapidly expanding, creating new demand centers for advanced autoclave systems with enhanced safety protocols.
Customer requirements have shifted noticeably toward integrated compliance solutions rather than standalone equipment. End-users increasingly demand autoclave systems with built-in validation capabilities, automated documentation features, and real-time monitoring technologies that simplify regulatory adherence. This shift represents a fundamental change in purchasing criteria, with compliance capabilities now ranking alongside traditional factors like capacity and energy efficiency.
Risk mitigation has emerged as a critical market driver, with organizations willing to invest significantly in systems that reduce human error and provide comprehensive audit trails. The average cost of non-compliance incidents in biotech facilities can reach millions of dollars when considering regulatory penalties, product recalls, and reputational damage.
Industry surveys indicate that over 85% of biotech facility managers consider automated compliance features "very important" or "essential" in new sterilization equipment purchases. This represents a marked increase from just five years ago, when only about 60% placed similar emphasis on these capabilities.
The market also shows growing demand segmentation between high-volume industrial applications and specialized research needs. While pharmaceutical manufacturing facilities typically require large-capacity systems with standardized protocols, research institutions increasingly seek flexible systems capable of handling diverse sterilization requirements while maintaining consistent compliance documentation.
Human resources constraints further drive demand for automated compliance solutions, as facilities face challenges in recruiting and retaining qualified staff with specialized knowledge of sterilization protocols and regulatory requirements. This workforce limitation pushes organizations toward systems that embed compliance expertise within their operational design.
Healthcare facilities, research laboratories, and biopharmaceutical manufacturing plants represent the largest customer segments, collectively accounting for over 70% of market demand. These institutions face mounting pressure to maintain rigorous compliance with evolving regulatory frameworks, including FDA regulations in the United States, EU GMP guidelines in Europe, and similar standards across Asia-Pacific regions.
The COVID-19 pandemic has substantially accelerated market demand, as heightened awareness of contamination risks has prompted facilities to upgrade their sterilization infrastructure. This trend is particularly evident in emerging economies where biotechnology sectors are rapidly expanding, creating new demand centers for advanced autoclave systems with enhanced safety protocols.
Customer requirements have shifted noticeably toward integrated compliance solutions rather than standalone equipment. End-users increasingly demand autoclave systems with built-in validation capabilities, automated documentation features, and real-time monitoring technologies that simplify regulatory adherence. This shift represents a fundamental change in purchasing criteria, with compliance capabilities now ranking alongside traditional factors like capacity and energy efficiency.
Risk mitigation has emerged as a critical market driver, with organizations willing to invest significantly in systems that reduce human error and provide comprehensive audit trails. The average cost of non-compliance incidents in biotech facilities can reach millions of dollars when considering regulatory penalties, product recalls, and reputational damage.
Industry surveys indicate that over 85% of biotech facility managers consider automated compliance features "very important" or "essential" in new sterilization equipment purchases. This represents a marked increase from just five years ago, when only about 60% placed similar emphasis on these capabilities.
The market also shows growing demand segmentation between high-volume industrial applications and specialized research needs. While pharmaceutical manufacturing facilities typically require large-capacity systems with standardized protocols, research institutions increasingly seek flexible systems capable of handling diverse sterilization requirements while maintaining consistent compliance documentation.
Human resources constraints further drive demand for automated compliance solutions, as facilities face challenges in recruiting and retaining qualified staff with specialized knowledge of sterilization protocols and regulatory requirements. This workforce limitation pushes organizations toward systems that embed compliance expertise within their operational design.
Current Autoclave Safety Challenges in Biotech
The biotech industry faces significant challenges in maintaining autoclave safety compliance due to evolving regulatory frameworks and technological advancements. Current autoclave operations in biotech facilities encounter several critical safety issues that demand immediate attention. The primary concern revolves around outdated validation protocols that fail to address the specific requirements of modern biotech applications, particularly when processing novel biomaterials and genetically modified organisms.
Pressure management systems in many existing autoclaves demonstrate inconsistencies during sterilization cycles, leading to potential safety hazards and compromised sterilization efficacy. These fluctuations are especially problematic when processing sensitive biological materials that require precise temperature and pressure conditions. Documentation and record-keeping practices also present significant challenges, with many facilities still relying on manual logging systems prone to human error and lacking real-time monitoring capabilities.
Cross-contamination risks have emerged as a growing concern, particularly in multi-user facilities where autoclaves process diverse biological materials. The absence of standardized cleaning protocols between cycles increases the likelihood of biological material transfer, potentially compromising research integrity and biosafety levels. Additionally, many facilities struggle with inadequate training programs for personnel, resulting in procedural inconsistencies and increased accident rates during autoclave operation.
Maintenance compliance represents another critical challenge, with approximately 40% of biotech facilities reporting delayed maintenance schedules due to operational demands and resource constraints. This deferred maintenance significantly increases the risk of mechanical failures during operation, potentially leading to catastrophic accidents and exposure to hazardous materials.
The integration of automation systems with legacy autoclave equipment has created interoperability issues, resulting in control system failures and inaccurate cycle reporting. These technical limitations hinder the implementation of advanced safety features such as remote monitoring and predictive maintenance algorithms that could enhance operational safety.
Waste management protocols for autoclave effluent and condensate present environmental compliance challenges, particularly regarding the disposal of potentially hazardous biological waste. Current filtration and neutralization systems often fail to meet increasingly stringent environmental regulations, exposing facilities to regulatory penalties and environmental contamination risks.
Emergency response procedures for autoclave failures remain inadequate in many biotech settings, with unclear protocols for containing potential biological hazards in the event of sterilization failure. This gap in emergency preparedness represents a significant vulnerability in overall facility biosafety management systems and requires comprehensive reassessment to ensure compliance with current safety standards.
Pressure management systems in many existing autoclaves demonstrate inconsistencies during sterilization cycles, leading to potential safety hazards and compromised sterilization efficacy. These fluctuations are especially problematic when processing sensitive biological materials that require precise temperature and pressure conditions. Documentation and record-keeping practices also present significant challenges, with many facilities still relying on manual logging systems prone to human error and lacking real-time monitoring capabilities.
Cross-contamination risks have emerged as a growing concern, particularly in multi-user facilities where autoclaves process diverse biological materials. The absence of standardized cleaning protocols between cycles increases the likelihood of biological material transfer, potentially compromising research integrity and biosafety levels. Additionally, many facilities struggle with inadequate training programs for personnel, resulting in procedural inconsistencies and increased accident rates during autoclave operation.
Maintenance compliance represents another critical challenge, with approximately 40% of biotech facilities reporting delayed maintenance schedules due to operational demands and resource constraints. This deferred maintenance significantly increases the risk of mechanical failures during operation, potentially leading to catastrophic accidents and exposure to hazardous materials.
The integration of automation systems with legacy autoclave equipment has created interoperability issues, resulting in control system failures and inaccurate cycle reporting. These technical limitations hinder the implementation of advanced safety features such as remote monitoring and predictive maintenance algorithms that could enhance operational safety.
Waste management protocols for autoclave effluent and condensate present environmental compliance challenges, particularly regarding the disposal of potentially hazardous biological waste. Current filtration and neutralization systems often fail to meet increasingly stringent environmental regulations, exposing facilities to regulatory penalties and environmental contamination risks.
Emergency response procedures for autoclave failures remain inadequate in many biotech settings, with unclear protocols for containing potential biological hazards in the event of sterilization failure. This gap in emergency preparedness represents a significant vulnerability in overall facility biosafety management systems and requires comprehensive reassessment to ensure compliance with current safety standards.
Established Autoclave Safety Protocol Frameworks
01 Automated monitoring and compliance systems for autoclaves
Advanced systems that automatically monitor autoclave operations to ensure compliance with safety protocols. These systems track critical parameters such as temperature, pressure, and cycle time in real-time, alerting operators to deviations from established safety standards. The automation reduces human error and provides comprehensive documentation for regulatory compliance purposes.- Automated monitoring and compliance systems for autoclaves: Advanced monitoring systems that automatically track autoclave operations, parameters, and compliance with safety protocols. These systems use sensors to collect real-time data on temperature, pressure, and cycle times, ensuring that sterilization processes meet required standards. The automated systems can alert operators to deviations from safety protocols and maintain digital records for compliance verification and audit purposes.
- Safety alert and notification mechanisms: Systems designed to provide immediate alerts and notifications when autoclave parameters deviate from safety standards. These mechanisms include visual and audible alarms, mobile notifications, and automated emergency protocols that activate when dangerous conditions are detected. The notification systems ensure timely intervention to prevent accidents, equipment damage, or sterilization failures, enhancing overall safety compliance.
- Training and certification verification systems: Digital platforms that manage operator training, certification, and authorization for autoclave use. These systems track personnel qualifications, provide training modules on safety protocols, and verify that only properly trained individuals operate autoclaves. They maintain records of training completion, certification status, and competency assessments, ensuring compliance with regulatory requirements for operator qualification.
- Predictive maintenance and risk assessment: Systems that analyze operational data to predict potential equipment failures or safety risks before they occur. These solutions use algorithms to identify patterns indicating maintenance needs or developing issues in autoclave systems. By enabling proactive maintenance and risk mitigation, these technologies help prevent safety incidents, ensure continuous compliance with protocols, and extend equipment lifespan.
- Documentation and audit trail systems: Digital solutions that automatically generate and maintain comprehensive documentation of autoclave operations for regulatory compliance. These systems create secure, tamper-proof records of sterilization cycles, maintenance activities, safety checks, and protocol adherence. The documentation includes time-stamped events, operator identification, and parameter logs that facilitate regulatory inspections and demonstrate ongoing compliance with safety standards.
02 Real-time alert and notification systems
Systems designed to provide immediate alerts when autoclave parameters fall outside of safe operating ranges. These notification systems can send alerts to multiple stakeholders through various channels including mobile devices, control panels, and centralized monitoring stations. This ensures rapid response to potential safety issues before they escalate into hazardous situations.Expand Specific Solutions03 Training and certification management for autoclave operators
Comprehensive systems for managing the training, certification, and competency assessment of personnel who operate autoclaves. These solutions track completion of required safety training, maintain certification records, and ensure that only qualified individuals operate sterilization equipment. Regular recertification requirements help maintain high safety standards and protocol compliance.Expand Specific Solutions04 Digital record-keeping and compliance documentation
Electronic systems for maintaining detailed records of autoclave operations, maintenance activities, and safety inspections. These solutions create tamper-proof audit trails that demonstrate regulatory compliance and facilitate reporting. The digital documentation includes cycle parameters, validation results, maintenance history, and safety incident reports, all essential for regulatory inspections.Expand Specific Solutions05 Predictive maintenance and risk assessment for autoclave safety
Advanced analytical systems that use operational data to predict potential equipment failures before they occur. These solutions analyze patterns in performance metrics to identify early warning signs of safety issues, schedule preventive maintenance, and assess compliance risks. By anticipating problems rather than reacting to failures, these systems significantly enhance autoclave safety and reliability.Expand Specific Solutions
Leading Autoclave Manufacturers and Compliance Providers
The autoclave safety protocols market in biotech is currently in a growth phase, driven by increasing regulatory scrutiny and expanding biotech applications. The market size is estimated to be substantial, with projections showing continued expansion due to heightened focus on compliance and safety standards. Technologically, the field demonstrates moderate maturity with established protocols, but continuous innovation is evident. Key players shaping the competitive landscape include Baxter International and Baxter Healthcare SA with their comprehensive healthcare systems, Cytiva (Global Life Sciences Solutions) offering specialized bioprocessing solutions, SCHOTT AG providing high-quality glass products for autoclaves, and Olympus Corp. contributing precision equipment. Smaller specialized firms like Thermal Compliance Ltd. and S.M.I. Medical S.R.L. are carving niches in compliance monitoring and sterilization equipment, respectively.
Olympus Corp.
Technical Solution: Olympus has pioneered an advanced Autoclave Safety Protocol system specifically for biotech applications, focusing on their line of laboratory autoclaves with enhanced safety features. Their technology incorporates AI-driven predictive maintenance algorithms that analyze operational patterns to identify potential safety issues before they occur. The system features multi-zone temperature and pressure monitoring with independent sensors that provide redundant verification of critical parameters. Olympus has implemented a proprietary "SafetyLock" mechanism that prevents door opening until pressure equalization and temperature reduction meet predetermined safety thresholds. Their protocol management software includes automated compliance reporting tools that generate documentation aligned with FDA 21 CFR Part 11, EU GMP Annex 11, and ISO 13485 requirements, streamlining regulatory inspections and audits.
Strengths: Superior predictive maintenance capabilities reducing downtime and safety incidents; intuitive user interface requiring minimal training; comprehensive documentation system that simplifies compliance verification. Weaknesses: Premium pricing positions their solutions out of reach for smaller biotech operations; proprietary systems can create integration challenges with existing laboratory information management systems.
Baxter International, Inc.
Technical Solution: Baxter International has developed comprehensive Autoclave Safety Protocol Management Systems specifically designed for biotech applications. Their approach integrates real-time monitoring technology with automated documentation systems to ensure compliance with FDA and international regulations. The system features multi-parameter monitoring (temperature, pressure, time, and steam quality) with wireless sensors that provide continuous data streams to a centralized validation platform. Baxter's protocol includes pre-programmed cycle parameters for different biological materials and containment levels, with automatic alerts for deviations from validated parameters. Their solution incorporates blockchain-based documentation that creates tamper-proof records of each sterilization cycle, addressing the increasing regulatory focus on data integrity in biotech operations.
Strengths: Comprehensive integration with existing biotech quality management systems; robust data integrity features; adaptable to various regulatory frameworks globally. Weaknesses: Higher implementation costs compared to standard systems; requires significant staff training for optimal utilization; complex validation process for specialized biotech applications.
Critical Patents and Innovations in Autoclave Safety
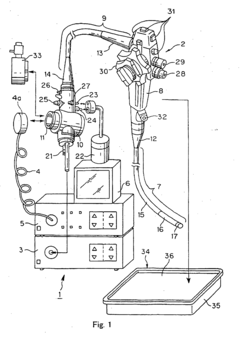
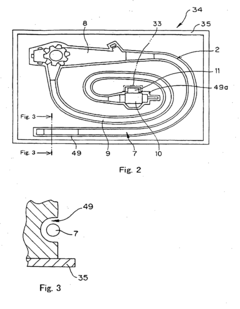
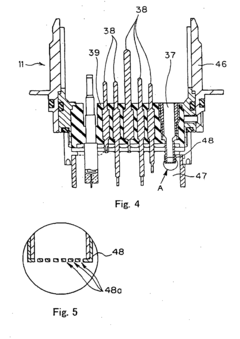
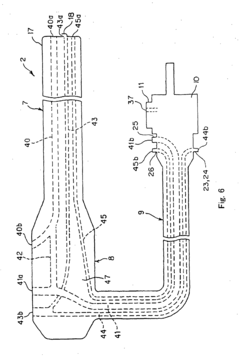
Process challenge device for a high-pressure steam sterilizer and sheet for a challenge device
PatentInactiveUS8333933B2
Innovation
- A process challenge device comprising steam permeable bodies and a holder, where the steam permeable bodies form a cavity with openings that communicate with each other, allowing steam to reach an indicator, which changes appearance upon exposure to a predetermined temperature, and can be reused without damaging the components, with the indicator being easily removable and user-selectable.
Autoclave sterilization treatment method and autoclave sterilization apparatus using this treatment method
PatentInactiveEP1574223B1
Innovation
- An autoclave sterilization treatment method that includes a check step with a display member, such as a touch panel, to confirm necessary precautions before proceeding with high-temperature and high-pressure steam sterilization, ensuring that operators complete specific preparatory processes like opening steam vents or using waterproofing caps with check valves, and preventing the sterilization process from starting if these checks are not completed.
Regulatory Requirements for Autoclave Operation
Regulatory frameworks governing autoclave operation in biotechnology settings are complex and multifaceted, requiring strict adherence to ensure both safety and compliance. At the federal level, the Occupational Safety and Health Administration (OSHA) establishes baseline requirements through 29 CFR 1910.132-138, mandating appropriate personal protective equipment and training for autoclave operators. These regulations are complemented by the Centers for Disease Control and Prevention (CDC) guidelines, particularly those outlined in Biosafety in Microbiological and Biomedical Laboratories (BMBL), which provides comprehensive protocols for sterilization validation and documentation.
The Food and Drug Administration (FDA) imposes additional requirements for facilities involved in pharmaceutical or medical device production through 21 CFR Part 820, emphasizing equipment validation, calibration, and maintenance records. For research institutions receiving federal funding, compliance with NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules is mandatory, with specific provisions for decontamination of potentially biohazardous materials.
State and local regulations introduce another layer of compliance requirements, with significant variations across jurisdictions. California, Massachusetts, and New York have implemented particularly stringent standards through their respective Departments of Public Health, often exceeding federal baselines. Local fire codes and waste management ordinances frequently contain specific provisions for autoclave installation, operation, and waste disposal.
International standards provide globally recognized frameworks for autoclave operation, with ISO 17665 establishing parameters for moist heat sterilization in healthcare facilities. The European Standard EN 285 specifies requirements for large steam sterilizers, while EN 13060 addresses small steam sterilizers. These standards are increasingly adopted as benchmarks even in non-European facilities seeking to demonstrate best practices.
Industry-specific guidelines further refine regulatory expectations, with the Parenteral Drug Association (PDA) Technical Report No. 1 offering detailed guidance on validation of moist heat sterilization processes. The Association for the Advancement of Medical Instrumentation (AAMI) provides comprehensive standards through ST79, detailing comprehensive steam sterilization protocols particularly relevant to medical device manufacturers.
Compliance documentation requirements are extensive, necessitating maintenance of standard operating procedures (SOPs), validation protocols, calibration records, maintenance logs, and operator training documentation. Regulatory bodies increasingly expect electronic record-keeping systems with appropriate data integrity controls, particularly for facilities subject to FDA oversight.
Regulatory trends indicate movement toward risk-based approaches to compliance, with greater emphasis on process understanding rather than prescriptive requirements. Harmonization efforts between international regulatory frameworks continue to evolve, potentially simplifying compliance for multinational organizations while raising standards in emerging markets.
The Food and Drug Administration (FDA) imposes additional requirements for facilities involved in pharmaceutical or medical device production through 21 CFR Part 820, emphasizing equipment validation, calibration, and maintenance records. For research institutions receiving federal funding, compliance with NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules is mandatory, with specific provisions for decontamination of potentially biohazardous materials.
State and local regulations introduce another layer of compliance requirements, with significant variations across jurisdictions. California, Massachusetts, and New York have implemented particularly stringent standards through their respective Departments of Public Health, often exceeding federal baselines. Local fire codes and waste management ordinances frequently contain specific provisions for autoclave installation, operation, and waste disposal.
International standards provide globally recognized frameworks for autoclave operation, with ISO 17665 establishing parameters for moist heat sterilization in healthcare facilities. The European Standard EN 285 specifies requirements for large steam sterilizers, while EN 13060 addresses small steam sterilizers. These standards are increasingly adopted as benchmarks even in non-European facilities seeking to demonstrate best practices.
Industry-specific guidelines further refine regulatory expectations, with the Parenteral Drug Association (PDA) Technical Report No. 1 offering detailed guidance on validation of moist heat sterilization processes. The Association for the Advancement of Medical Instrumentation (AAMI) provides comprehensive standards through ST79, detailing comprehensive steam sterilization protocols particularly relevant to medical device manufacturers.
Compliance documentation requirements are extensive, necessitating maintenance of standard operating procedures (SOPs), validation protocols, calibration records, maintenance logs, and operator training documentation. Regulatory bodies increasingly expect electronic record-keeping systems with appropriate data integrity controls, particularly for facilities subject to FDA oversight.
Regulatory trends indicate movement toward risk-based approaches to compliance, with greater emphasis on process understanding rather than prescriptive requirements. Harmonization efforts between international regulatory frameworks continue to evolve, potentially simplifying compliance for multinational organizations while raising standards in emerging markets.
Risk Management Strategies for Autoclave Compliance
Effective risk management is essential for maintaining autoclave compliance in biotech environments. Organizations must implement a comprehensive risk assessment framework that identifies potential hazards associated with autoclave operations, including mechanical failures, operator errors, and process deviations. This assessment should categorize risks based on severity and likelihood, enabling prioritization of mitigation efforts toward high-impact areas.
A multi-layered approach to risk mitigation proves most effective, combining engineering controls, administrative measures, and personal protective equipment. Engineering controls include installing pressure relief valves, temperature monitoring systems, and automatic shut-off mechanisms. These technical safeguards provide the first line of defense against catastrophic failures and process deviations.
Administrative controls complement the technical measures through documented procedures, regular training programs, and clear communication protocols. Standard Operating Procedures (SOPs) should detail step-by-step instructions for normal operations, maintenance activities, and emergency responses. These procedures must be regularly reviewed and updated to incorporate lessons learned and evolving best practices.
Validation and verification processes form another critical component of risk management. Regular testing of safety systems, calibration of monitoring equipment, and performance qualification of autoclaves ensure that control measures remain effective over time. Documentation of these activities provides evidence of compliance and facilitates regulatory inspections.
Continuous monitoring systems represent an advanced approach to risk management, allowing real-time detection of deviations before they escalate into serious incidents. Modern autoclaves equipped with digital monitoring capabilities can track critical parameters throughout sterilization cycles and alert operators to potential issues. Integration with laboratory information management systems enables trend analysis and predictive maintenance.
Incident response planning completes the risk management framework. Despite preventive measures, organizations must prepare for potential failures by developing clear emergency procedures, conducting regular drills, and maintaining appropriate response equipment. Post-incident analysis should identify root causes and inform improvements to the risk management system.
Cross-functional collaboration enhances risk management effectiveness. Safety committees comprising representatives from operations, quality assurance, engineering, and management should regularly review risk assessments and compliance metrics. This collaborative approach ensures comprehensive risk identification and promotes a culture of safety throughout the organization.
A multi-layered approach to risk mitigation proves most effective, combining engineering controls, administrative measures, and personal protective equipment. Engineering controls include installing pressure relief valves, temperature monitoring systems, and automatic shut-off mechanisms. These technical safeguards provide the first line of defense against catastrophic failures and process deviations.
Administrative controls complement the technical measures through documented procedures, regular training programs, and clear communication protocols. Standard Operating Procedures (SOPs) should detail step-by-step instructions for normal operations, maintenance activities, and emergency responses. These procedures must be regularly reviewed and updated to incorporate lessons learned and evolving best practices.
Validation and verification processes form another critical component of risk management. Regular testing of safety systems, calibration of monitoring equipment, and performance qualification of autoclaves ensure that control measures remain effective over time. Documentation of these activities provides evidence of compliance and facilitates regulatory inspections.
Continuous monitoring systems represent an advanced approach to risk management, allowing real-time detection of deviations before they escalate into serious incidents. Modern autoclaves equipped with digital monitoring capabilities can track critical parameters throughout sterilization cycles and alert operators to potential issues. Integration with laboratory information management systems enables trend analysis and predictive maintenance.
Incident response planning completes the risk management framework. Despite preventive measures, organizations must prepare for potential failures by developing clear emergency procedures, conducting regular drills, and maintaining appropriate response equipment. Post-incident analysis should identify root causes and inform improvements to the risk management system.
Cross-functional collaboration enhances risk management effectiveness. Safety committees comprising representatives from operations, quality assurance, engineering, and management should regularly review risk assessments and compliance metrics. This collaborative approach ensures comprehensive risk identification and promotes a culture of safety throughout the organization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!