Autoclave Technology Integration with Digital Platforms
SEP 12, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave-Digital Integration Background and Objectives
Autoclave technology has evolved significantly since its inception in the early 20th century, transitioning from basic pressure vessels to sophisticated systems critical in industries such as aerospace, composites manufacturing, and medical sterilization. The integration of digital platforms with autoclave technology represents a pivotal advancement in this evolution, marking the transition from purely mechanical systems to smart, connected manufacturing assets capable of real-time monitoring, predictive maintenance, and process optimization.
The historical trajectory of autoclave technology shows a clear progression toward greater precision, efficiency, and automation. Early autoclaves relied on manual controls and basic pressure/temperature gauges, while modern systems incorporate advanced sensors, computerized control systems, and data logging capabilities. However, these advancements have largely occurred in isolation, with digital capabilities added incrementally rather than through comprehensive integration strategies.
Current technological trends indicate a convergence of industrial equipment with Industry 4.0 principles, including the Internet of Things (IoT), artificial intelligence, cloud computing, and digital twin technologies. This convergence creates unprecedented opportunities for autoclave technology to become fully integrated into digital manufacturing ecosystems, enabling capabilities such as remote operation, predictive quality control, and adaptive processing parameters.
The primary objective of autoclave-digital integration is to transform traditional batch processing equipment into intelligent, connected systems that can communicate with broader manufacturing execution systems (MES) and enterprise resource planning (ERP) platforms. This integration aims to address persistent challenges in autoclave operations, including energy efficiency, cycle time optimization, quality consistency, and maintenance scheduling.
Technical goals for this integration include developing standardized communication protocols for autoclave systems, creating robust sensor networks capable of withstanding extreme pressure and temperature environments, implementing edge computing capabilities for real-time process control, and establishing secure data architectures that protect proprietary manufacturing information while enabling analytics-driven insights.
The broader vision encompasses creating a seamless digital thread throughout the autoclave manufacturing process, from material preparation through curing cycles to final quality inspection. This digital continuity would enable comprehensive traceability, process validation, and continuous improvement methodologies previously impossible with isolated autoclave systems.
As manufacturing industries face increasing pressure for sustainability, customization, and efficiency, the integration of autoclave technology with digital platforms represents not merely a technical enhancement but a strategic imperative for maintaining competitiveness and meeting evolving market demands.
The historical trajectory of autoclave technology shows a clear progression toward greater precision, efficiency, and automation. Early autoclaves relied on manual controls and basic pressure/temperature gauges, while modern systems incorporate advanced sensors, computerized control systems, and data logging capabilities. However, these advancements have largely occurred in isolation, with digital capabilities added incrementally rather than through comprehensive integration strategies.
Current technological trends indicate a convergence of industrial equipment with Industry 4.0 principles, including the Internet of Things (IoT), artificial intelligence, cloud computing, and digital twin technologies. This convergence creates unprecedented opportunities for autoclave technology to become fully integrated into digital manufacturing ecosystems, enabling capabilities such as remote operation, predictive quality control, and adaptive processing parameters.
The primary objective of autoclave-digital integration is to transform traditional batch processing equipment into intelligent, connected systems that can communicate with broader manufacturing execution systems (MES) and enterprise resource planning (ERP) platforms. This integration aims to address persistent challenges in autoclave operations, including energy efficiency, cycle time optimization, quality consistency, and maintenance scheduling.
Technical goals for this integration include developing standardized communication protocols for autoclave systems, creating robust sensor networks capable of withstanding extreme pressure and temperature environments, implementing edge computing capabilities for real-time process control, and establishing secure data architectures that protect proprietary manufacturing information while enabling analytics-driven insights.
The broader vision encompasses creating a seamless digital thread throughout the autoclave manufacturing process, from material preparation through curing cycles to final quality inspection. This digital continuity would enable comprehensive traceability, process validation, and continuous improvement methodologies previously impossible with isolated autoclave systems.
As manufacturing industries face increasing pressure for sustainability, customization, and efficiency, the integration of autoclave technology with digital platforms represents not merely a technical enhancement but a strategic imperative for maintaining competitiveness and meeting evolving market demands.
Market Demand Analysis for Smart Sterilization Solutions
The global market for smart sterilization solutions is experiencing significant growth, driven by increasing demands for enhanced infection control and operational efficiency across multiple sectors. Healthcare facilities, including hospitals, clinics, and dental practices, represent the primary market segment, accounting for approximately 60% of the current demand. These institutions are actively seeking autoclave systems that can integrate with their existing digital infrastructure to streamline sterilization processes, reduce human error, and ensure compliance with increasingly stringent regulatory requirements.
The pharmaceutical and biotechnology industries constitute another substantial market segment, where the need for validated sterilization processes with comprehensive digital documentation capabilities is paramount. Research indicates that facilities implementing digitally-integrated autoclave systems report efficiency improvements of 25-30% compared to traditional manual monitoring systems, creating a compelling value proposition for adoption.
Consumer awareness regarding infection prevention has dramatically increased following the COVID-19 pandemic, expanding market opportunities beyond traditional healthcare settings. Food processing, cosmetics manufacturing, and laboratory services are emerging as significant growth areas for smart sterilization technologies, collectively representing a market expansion opportunity of approximately 15% annually through 2027.
Regional analysis reveals that North America currently leads market demand with 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is projected to demonstrate the highest growth rate over the next five years due to rapid healthcare infrastructure development and increasing adoption of digital healthcare solutions in countries like China, India, and Singapore.
Key market drivers include the growing emphasis on healthcare-associated infection prevention, increasing regulatory requirements for sterilization validation and documentation, and the broader digital transformation initiatives across healthcare systems globally. The demand for remote monitoring capabilities has accelerated significantly, with 78% of healthcare facilities expressing interest in autoclave systems offering real-time monitoring and alert functionalities accessible via mobile applications or centralized dashboards.
Cost considerations remain a significant factor influencing adoption rates, particularly among smaller healthcare facilities and developing markets. Market research indicates that while initial investment concerns exist, facilities increasingly recognize the long-term operational cost benefits through reduced cycle times, lower energy consumption, and decreased sterilization failures. The return on investment period for smart autoclave systems typically ranges between 18-24 months, making them increasingly attractive despite higher upfront costs compared to conventional systems.
The pharmaceutical and biotechnology industries constitute another substantial market segment, where the need for validated sterilization processes with comprehensive digital documentation capabilities is paramount. Research indicates that facilities implementing digitally-integrated autoclave systems report efficiency improvements of 25-30% compared to traditional manual monitoring systems, creating a compelling value proposition for adoption.
Consumer awareness regarding infection prevention has dramatically increased following the COVID-19 pandemic, expanding market opportunities beyond traditional healthcare settings. Food processing, cosmetics manufacturing, and laboratory services are emerging as significant growth areas for smart sterilization technologies, collectively representing a market expansion opportunity of approximately 15% annually through 2027.
Regional analysis reveals that North America currently leads market demand with 35% share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is projected to demonstrate the highest growth rate over the next five years due to rapid healthcare infrastructure development and increasing adoption of digital healthcare solutions in countries like China, India, and Singapore.
Key market drivers include the growing emphasis on healthcare-associated infection prevention, increasing regulatory requirements for sterilization validation and documentation, and the broader digital transformation initiatives across healthcare systems globally. The demand for remote monitoring capabilities has accelerated significantly, with 78% of healthcare facilities expressing interest in autoclave systems offering real-time monitoring and alert functionalities accessible via mobile applications or centralized dashboards.
Cost considerations remain a significant factor influencing adoption rates, particularly among smaller healthcare facilities and developing markets. Market research indicates that while initial investment concerns exist, facilities increasingly recognize the long-term operational cost benefits through reduced cycle times, lower energy consumption, and decreased sterilization failures. The return on investment period for smart autoclave systems typically ranges between 18-24 months, making them increasingly attractive despite higher upfront costs compared to conventional systems.
Current Challenges in Autoclave-Digital Platform Integration
The integration of autoclave technology with digital platforms faces significant technical challenges that impede seamless implementation across industries. Legacy autoclave systems, many designed decades ago, lack standardized communication protocols necessary for modern digital integration. This incompatibility creates substantial barriers when attempting to connect these systems to contemporary IoT frameworks, cloud platforms, or enterprise management software.
Data acquisition represents another critical challenge, as traditional autoclaves typically feature limited sensing capabilities. The harsh operating environments—characterized by high temperatures, pressures, and sometimes corrosive conditions—severely restrict sensor deployment and reliability. This limitation creates significant blind spots in process monitoring and control, hampering the development of data-driven optimization strategies.
Real-time processing capabilities present further complications. The complex thermodynamic processes occurring within autoclaves generate substantial data volumes that require immediate analysis for effective control. Current edge computing solutions often struggle with the computational demands of processing this information while maintaining the millisecond response times necessary for critical process adjustments.
Security vulnerabilities emerge as particularly concerning when connecting previously isolated industrial equipment to networked systems. Autoclave operations frequently involve proprietary processes or handle sensitive materials, making them potential targets for industrial espionage or sabotage. The security protocols designed for standard IT environments often prove inadequate for these specialized industrial applications.
Integration with existing enterprise systems presents additional hurdles. Manufacturing execution systems (MES), enterprise resource planning (ERP) platforms, and quality management systems typically operate with data models and workflows not designed to accommodate the unique operational parameters of autoclave processes. This disconnect creates significant challenges in achieving end-to-end process visibility and optimization.
Regulatory compliance adds another layer of complexity, particularly in highly regulated industries such as aerospace, medical device manufacturing, and pharmaceuticals. Digital modifications to validated processes require extensive documentation and verification, creating substantial barriers to technological advancement. Many organizations find themselves caught between regulatory requirements for process consistency and the competitive pressure to implement digital transformation initiatives.
Workforce adaptation represents a frequently overlooked challenge. The specialized knowledge required to operate both autoclave systems and digital platforms creates a significant skills gap in many organizations. Technicians proficient in traditional autoclave operation often lack digital literacy, while IT specialists may not understand the nuances of autoclave processes, creating communication barriers that impede successful integration projects.
Data acquisition represents another critical challenge, as traditional autoclaves typically feature limited sensing capabilities. The harsh operating environments—characterized by high temperatures, pressures, and sometimes corrosive conditions—severely restrict sensor deployment and reliability. This limitation creates significant blind spots in process monitoring and control, hampering the development of data-driven optimization strategies.
Real-time processing capabilities present further complications. The complex thermodynamic processes occurring within autoclaves generate substantial data volumes that require immediate analysis for effective control. Current edge computing solutions often struggle with the computational demands of processing this information while maintaining the millisecond response times necessary for critical process adjustments.
Security vulnerabilities emerge as particularly concerning when connecting previously isolated industrial equipment to networked systems. Autoclave operations frequently involve proprietary processes or handle sensitive materials, making them potential targets for industrial espionage or sabotage. The security protocols designed for standard IT environments often prove inadequate for these specialized industrial applications.
Integration with existing enterprise systems presents additional hurdles. Manufacturing execution systems (MES), enterprise resource planning (ERP) platforms, and quality management systems typically operate with data models and workflows not designed to accommodate the unique operational parameters of autoclave processes. This disconnect creates significant challenges in achieving end-to-end process visibility and optimization.
Regulatory compliance adds another layer of complexity, particularly in highly regulated industries such as aerospace, medical device manufacturing, and pharmaceuticals. Digital modifications to validated processes require extensive documentation and verification, creating substantial barriers to technological advancement. Many organizations find themselves caught between regulatory requirements for process consistency and the competitive pressure to implement digital transformation initiatives.
Workforce adaptation represents a frequently overlooked challenge. The specialized knowledge required to operate both autoclave systems and digital platforms creates a significant skills gap in many organizations. Technicians proficient in traditional autoclave operation often lack digital literacy, while IT specialists may not understand the nuances of autoclave processes, creating communication barriers that impede successful integration projects.
Existing Digital Integration Solutions for Autoclaves
01 Autoclave monitoring and control systems
Advanced monitoring and control systems for autoclaves that integrate sensors, data analytics, and automation to optimize sterilization processes. These systems provide real-time monitoring of critical parameters such as temperature, pressure, and cycle time, ensuring consistent sterilization results while improving efficiency and safety. The integration of digital controls allows for precise adjustment of process parameters and automated documentation for regulatory compliance.- Autoclave Control Systems and Automation: Modern autoclave technology integrates advanced control systems that automate sterilization processes. These systems include programmable logic controllers, digital interfaces, and remote monitoring capabilities that ensure precise control of temperature, pressure, and cycle times. The automation allows for standardized protocols, reduced human error, and improved efficiency in sterilization processes across various industries including healthcare and manufacturing.
- Integration with Data Management Systems: Autoclave technology now incorporates sophisticated data management systems that enable tracking, recording, and analysis of sterilization cycles. These integrated systems provide real-time monitoring, documentation of sterilization parameters, and compliance reporting. The technology allows for seamless integration with hospital information systems, manufacturing execution systems, and quality management platforms, enhancing traceability and regulatory compliance.
- Energy Efficiency and Sustainability Features: Modern autoclaves incorporate energy-efficient technologies that reduce resource consumption while maintaining sterilization efficacy. These innovations include heat recovery systems, water recirculation mechanisms, and optimized cycle designs. The integration of sustainable features such as improved insulation, energy-saving modes, and eco-friendly materials contributes to reduced environmental impact and operational costs in sterilization processes.
- Mobile and IoT-Enabled Autoclave Solutions: The integration of Internet of Things (IoT) technology with autoclaves enables remote monitoring, control, and maintenance of sterilization equipment. These smart autoclave systems feature wireless connectivity, mobile applications for status updates, and predictive maintenance capabilities. The technology allows operators to receive alerts, adjust parameters remotely, and access sterilization data from anywhere, improving operational flexibility and equipment uptime.
- Specialized Autoclave Designs for Specific Applications: Autoclave technology has evolved to address specialized needs across different industries through customized designs and integration capabilities. These include laboratory autoclaves with research-specific features, industrial autoclaves for composite material processing, and medical autoclaves with enhanced infection control capabilities. The specialized designs incorporate industry-specific requirements, materials compatibility considerations, and process optimization features tailored to particular applications.
02 Integration of autoclaves with IoT and cloud technologies
Autoclave systems connected to Internet of Things (IoT) platforms and cloud services that enable remote monitoring, control, and data management. These integrated solutions allow operators to access autoclave status and performance metrics from anywhere, receive alerts for cycle completion or anomalies, and store sterilization records securely in the cloud. The technology facilitates predictive maintenance, resource optimization, and enhanced operational visibility across multiple facilities.Expand Specific Solutions03 Sustainable autoclave technologies
Environmentally friendly autoclave systems that integrate energy recovery, water recycling, and resource optimization technologies. These innovations reduce the environmental footprint of sterilization processes by minimizing water consumption, recovering heat energy, and optimizing cycle times. The integration of smart controls allows for dynamic adjustment of resource usage based on load characteristics, further enhancing sustainability while maintaining sterilization efficacy.Expand Specific Solutions04 Autoclave integration with workflow management systems
Solutions that integrate autoclaves with broader workflow management systems in healthcare, laboratory, and manufacturing environments. These integrated systems coordinate sterilization processes with inventory management, scheduling, and tracking systems to optimize operational efficiency. The technology enables automated documentation of sterilization cycles, instrument tracking via RFID or barcodes, and seamless integration with quality management systems to ensure regulatory compliance.Expand Specific Solutions05 Specialized autoclave designs for specific applications
Custom autoclave technologies designed for integration into specific industrial or scientific processes. These specialized systems feature adaptations for particular materials, products, or environments, such as pharmaceutical manufacturing, composite curing, or food processing. The integration focuses on optimizing parameters for specific applications while ensuring compatibility with existing production lines and quality control systems.Expand Specific Solutions
Leading Manufacturers and Technology Providers Analysis
The autoclave technology integration with digital platforms market is currently in a growth phase, characterized by increasing adoption across manufacturing, healthcare, and automotive sectors. The global market size is expanding rapidly, driven by Industry 4.0 initiatives and demand for enhanced process efficiency. Regarding technological maturity, established industrial leaders like Siemens AG and AVEVA Software are spearheading integration solutions with advanced IoT capabilities, while Toyota Motor Corp and Alibaba Group are developing innovative digital platform connections. Companies such as Steriflow SAS and Olympus Corp offer specialized autoclave solutions with varying degrees of digital integration. The competitive landscape shows a mix of traditional industrial equipment manufacturers evolving their offerings alongside technology firms entering the space, creating a dynamic ecosystem of partnerships and proprietary solutions.
Siemens AG
Technical Solution: Siemens has developed a comprehensive Digital Twin platform for autoclave processes that integrates physical autoclave operations with digital monitoring and control systems. Their solution combines IoT sensors, edge computing, and cloud-based analytics to create a virtual representation of autoclave operations in real-time. The Siemens Autoclave Suite includes predictive maintenance capabilities, process optimization algorithms, and remote monitoring interfaces that allow operators to visualize critical parameters such as temperature distribution, pressure gradients, and cure cycles. The platform leverages MindSphere, Siemens' cloud-based IoT operating system, to enable data-driven decision making and process improvements[1]. Their solution also incorporates digital workflow management that guides operators through standardized procedures while automatically documenting compliance with industry regulations and quality standards[3].
Strengths: Comprehensive end-to-end integration with existing industrial automation systems; robust security protocols for sensitive manufacturing data; extensive experience in industrial digitalization. Weaknesses: Higher implementation costs compared to specialized solutions; potential vendor lock-in with proprietary systems; requires significant customization for specialized autoclave applications.
Steriflow SAS
Technical Solution: Steriflow SAS has pioneered an integrated digital platform specifically for pharmaceutical and food processing autoclaves. Their solution combines advanced PLC controls with a comprehensive software suite that enables complete process traceability and validation. The Steriflow Digital Autoclave Management System (SDAMS) features real-time monitoring of critical parameters including temperature, pressure, and F0 values through a network of high-precision sensors distributed throughout the autoclave chamber. Data is continuously logged and analyzed to ensure product quality and regulatory compliance. The platform includes a sophisticated recipe management system that allows operators to create, store, and deploy standardized sterilization protocols across multiple units[5]. Steriflow's solution also incorporates automated batch reporting that generates FDA 21 CFR Part 11 compliant documentation, significantly reducing the administrative burden associated with validation processes. Their cloud-based architecture enables remote monitoring and troubleshooting capabilities, allowing technical support to diagnose issues without being physically present at the facility[6].
Strengths: Specialized expertise in pharmaceutical and food processing applications; superior validation documentation capabilities; highly accurate sensor technology for critical process parameters. Weaknesses: Less suitable for non-sterilization autoclave applications; limited integration with third-party enterprise systems; higher initial cost compared to generic control systems.
Key Technical Innovations in Smart Autoclave Systems
Autoclave system and method
PatentActiveUS20200147568A1
Innovation
- A heat exchanger is interposed between the autoclave vessel and the external support system, utilizing thermal exchange conduits and a medium to cool and manage both leaching and venting fluids, reducing their temperature and pressure before reintroduction, while also pre-heating the leaching fluid for efficient operation.
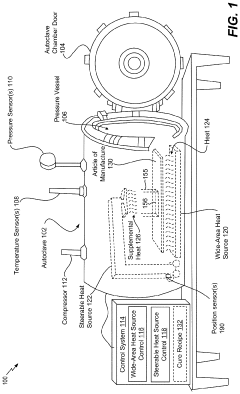
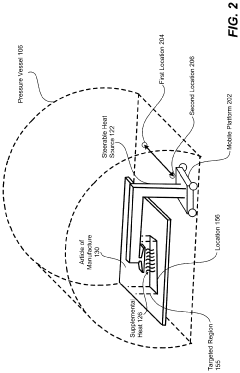
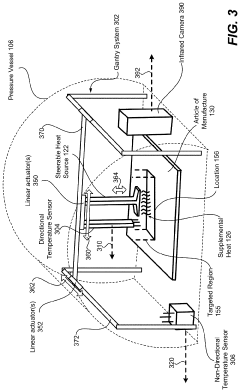
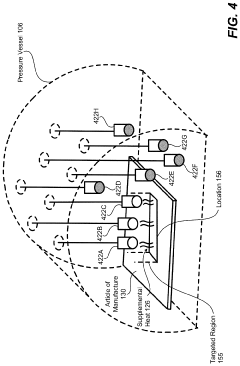
Steerable heat source
PatentActiveUS20200307035A1
Innovation
- A steerable heat source is integrated within the autoclave, coupled with a control system that directs supplemental heat to targeted regions using temperature sensors and a compressor to regulate pressure, ensuring precise temperature control and uniform heating.
Regulatory Compliance for Digital Medical Sterilization
The integration of autoclave technology with digital platforms necessitates strict adherence to regulatory frameworks governing medical sterilization processes. Healthcare facilities must comply with standards set by organizations such as the FDA, EU MDR, and ISO, which have established comprehensive guidelines for sterilization validation, monitoring, and documentation in digital environments.
FDA 21 CFR Part 11 specifically addresses electronic records and signatures, requiring digital autoclave systems to implement robust authentication protocols, audit trails, and data integrity measures. These regulations ensure that digital sterilization records maintain the same level of trustworthiness and legal standing as traditional paper documentation.
The EU Medical Device Regulation (MDR) imposes additional requirements for medical sterilization processes that utilize digital platforms, emphasizing risk management and post-market surveillance. Digital autoclave systems must incorporate features that enable continuous monitoring and reporting of sterilization parameters to meet these regulatory expectations.
ISO 17665 provides specific guidelines for moist heat sterilization validation and routine monitoring, which must be adapted for digital implementation. When autoclave technology interfaces with digital platforms, the validation processes must demonstrate that electronic monitoring systems accurately capture critical parameters such as temperature, pressure, and time with the same precision as traditional methods.
Cybersecurity regulations have become increasingly relevant as autoclave systems connect to hospital networks and cloud platforms. HIPAA compliance in the US and GDPR in Europe mandate specific data protection measures when patient-related information intersects with sterilization records. Digital autoclave platforms must implement encryption, access controls, and secure data transmission protocols to protect sensitive information.
Regulatory bodies are increasingly focusing on software validation requirements for medical technology. IEC 62304 establishes a framework for medical device software lifecycle processes that applies to digital components of autoclave systems. Manufacturers must demonstrate that software controlling or monitoring sterilization processes meets safety and performance requirements through documented verification and validation activities.
Compliance with these regulations requires implementing quality management systems that encompass both physical autoclave operations and their digital counterparts. ISO 13485 provides a comprehensive framework for quality management in medical device manufacturing and related services, including sterilization processes enhanced by digital technologies.
As regulatory landscapes evolve, digital autoclave platforms must incorporate adaptable compliance frameworks that can respond to changing requirements. This includes designing systems with configurable parameters that can be adjusted to meet regional variations in sterilization standards and documentation requirements across global markets.
FDA 21 CFR Part 11 specifically addresses electronic records and signatures, requiring digital autoclave systems to implement robust authentication protocols, audit trails, and data integrity measures. These regulations ensure that digital sterilization records maintain the same level of trustworthiness and legal standing as traditional paper documentation.
The EU Medical Device Regulation (MDR) imposes additional requirements for medical sterilization processes that utilize digital platforms, emphasizing risk management and post-market surveillance. Digital autoclave systems must incorporate features that enable continuous monitoring and reporting of sterilization parameters to meet these regulatory expectations.
ISO 17665 provides specific guidelines for moist heat sterilization validation and routine monitoring, which must be adapted for digital implementation. When autoclave technology interfaces with digital platforms, the validation processes must demonstrate that electronic monitoring systems accurately capture critical parameters such as temperature, pressure, and time with the same precision as traditional methods.
Cybersecurity regulations have become increasingly relevant as autoclave systems connect to hospital networks and cloud platforms. HIPAA compliance in the US and GDPR in Europe mandate specific data protection measures when patient-related information intersects with sterilization records. Digital autoclave platforms must implement encryption, access controls, and secure data transmission protocols to protect sensitive information.
Regulatory bodies are increasingly focusing on software validation requirements for medical technology. IEC 62304 establishes a framework for medical device software lifecycle processes that applies to digital components of autoclave systems. Manufacturers must demonstrate that software controlling or monitoring sterilization processes meets safety and performance requirements through documented verification and validation activities.
Compliance with these regulations requires implementing quality management systems that encompass both physical autoclave operations and their digital counterparts. ISO 13485 provides a comprehensive framework for quality management in medical device manufacturing and related services, including sterilization processes enhanced by digital technologies.
As regulatory landscapes evolve, digital autoclave platforms must incorporate adaptable compliance frameworks that can respond to changing requirements. This includes designing systems with configurable parameters that can be adjusted to meet regional variations in sterilization standards and documentation requirements across global markets.
Cybersecurity Considerations for Connected Autoclaves
As the integration of autoclaves with digital platforms accelerates, cybersecurity emerges as a critical concern that cannot be overlooked. Connected autoclaves present unique security challenges due to their operation in sterile environments and their critical role in healthcare, pharmaceutical, and industrial applications. These devices now incorporate sensors, remote monitoring capabilities, and cloud connectivity, expanding their attack surface significantly.
The primary security vulnerabilities in connected autoclaves include unauthorized access points, data transmission interception, firmware exploitation, and supply chain compromises. Recent security assessments have revealed that many autoclave systems lack basic security controls such as encrypted communications, secure authentication mechanisms, and regular security patches, making them susceptible to various cyber threats.
Threat actors targeting connected autoclaves may include nation-states seeking intellectual property, cybercriminals pursuing ransomware opportunities, or insiders with malicious intent. The consequences of a successful attack could be severe, ranging from operational disruption and data breaches to potential safety hazards if sterilization processes are compromised.
Implementing a defense-in-depth strategy is essential for protecting connected autoclaves. This approach should include network segmentation to isolate autoclave systems from general IT networks, strong authentication protocols requiring multi-factor verification, and end-to-end encryption for all data transmissions. Regular security assessments and penetration testing should be conducted to identify and remediate vulnerabilities before they can be exploited.
Regulatory frameworks are evolving to address these concerns. The FDA's guidance on medical device cybersecurity, the EU's Medical Device Regulation, and the IEC 80001 standard all provide frameworks for securing connected medical equipment, including autoclaves. Compliance with these standards is becoming increasingly important for manufacturers and operators alike.
Future security developments for connected autoclaves will likely include AI-powered anomaly detection systems capable of identifying unusual operational patterns that might indicate a security breach. Blockchain technology may also be employed to create immutable records of sterilization cycles, ensuring data integrity and preventing tampering with critical process parameters.
Organizations implementing connected autoclave solutions must develop comprehensive security policies that address device lifecycle management, incident response procedures, and regular security training for personnel. A collaborative approach involving IT security teams, biomedical engineering, and operational staff is essential for maintaining robust security postures in increasingly connected sterilization environments.
The primary security vulnerabilities in connected autoclaves include unauthorized access points, data transmission interception, firmware exploitation, and supply chain compromises. Recent security assessments have revealed that many autoclave systems lack basic security controls such as encrypted communications, secure authentication mechanisms, and regular security patches, making them susceptible to various cyber threats.
Threat actors targeting connected autoclaves may include nation-states seeking intellectual property, cybercriminals pursuing ransomware opportunities, or insiders with malicious intent. The consequences of a successful attack could be severe, ranging from operational disruption and data breaches to potential safety hazards if sterilization processes are compromised.
Implementing a defense-in-depth strategy is essential for protecting connected autoclaves. This approach should include network segmentation to isolate autoclave systems from general IT networks, strong authentication protocols requiring multi-factor verification, and end-to-end encryption for all data transmissions. Regular security assessments and penetration testing should be conducted to identify and remediate vulnerabilities before they can be exploited.
Regulatory frameworks are evolving to address these concerns. The FDA's guidance on medical device cybersecurity, the EU's Medical Device Regulation, and the IEC 80001 standard all provide frameworks for securing connected medical equipment, including autoclaves. Compliance with these standards is becoming increasingly important for manufacturers and operators alike.
Future security developments for connected autoclaves will likely include AI-powered anomaly detection systems capable of identifying unusual operational patterns that might indicate a security breach. Blockchain technology may also be employed to create immutable records of sterilization cycles, ensuring data integrity and preventing tampering with critical process parameters.
Organizations implementing connected autoclave solutions must develop comprehensive security policies that address device lifecycle management, incident response procedures, and regular security training for personnel. A collaborative approach involving IT security teams, biomedical engineering, and operational staff is essential for maintaining robust security postures in increasingly connected sterilization environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!