Autoclave vs. Cold Sterile Processing: Pros and Cons Explained
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sterilization Technology Evolution and Objectives
Sterilization technologies have evolved significantly over the past century, transitioning from basic heat applications to sophisticated automated systems. The earliest documented sterilization methods date back to the late 19th century when steam under pressure was first utilized for medical purposes. This marked the beginning of autoclave technology, which has remained a cornerstone of sterilization processes across healthcare, laboratory, and industrial settings.
The evolution of sterilization technology can be traced through several distinct phases. The initial phase (1880s-1940s) focused on establishing fundamental principles of thermal sterilization. The second phase (1950s-1970s) saw the introduction of chemical sterilization methods, including ethylene oxide and glutaraldehyde. The third phase (1980s-2000s) brought significant automation improvements and validation protocols, while the current phase (2000s-present) emphasizes energy efficiency, reduced processing times, and environmentally sustainable approaches.
Autoclave technology has undergone substantial refinements, evolving from simple pressure cookers to sophisticated computer-controlled systems with precise parameter monitoring. Simultaneously, cold sterilization methods have advanced from basic chemical immersion to complex low-temperature gas plasma systems and advanced chemical sterilants with improved material compatibility and reduced processing times.
The primary objectives driving sterilization technology development include achieving complete microbial elimination while preserving instrument integrity, reducing cycle times to improve operational efficiency, minimizing environmental impact through reduced water and energy consumption, and ensuring compatibility with increasingly complex and delicate medical devices and materials.
Current technological goals focus on developing systems that can effectively sterilize temperature-sensitive electronics and polymers without degradation, creating rapid sterilization methods for emergency situations and point-of-care applications, implementing real-time monitoring systems for verification of sterilization efficacy, and reducing operational costs while maintaining or improving sterilization standards.
The tension between autoclave and cold sterilization technologies represents a fundamental challenge in the field: balancing the proven reliability and simplicity of heat-based methods against the material preservation advantages of chemical approaches. This technological dichotomy continues to drive innovation as researchers and manufacturers seek optimal solutions that combine the best aspects of both approaches while minimizing their respective limitations.
The evolution of sterilization technology can be traced through several distinct phases. The initial phase (1880s-1940s) focused on establishing fundamental principles of thermal sterilization. The second phase (1950s-1970s) saw the introduction of chemical sterilization methods, including ethylene oxide and glutaraldehyde. The third phase (1980s-2000s) brought significant automation improvements and validation protocols, while the current phase (2000s-present) emphasizes energy efficiency, reduced processing times, and environmentally sustainable approaches.
Autoclave technology has undergone substantial refinements, evolving from simple pressure cookers to sophisticated computer-controlled systems with precise parameter monitoring. Simultaneously, cold sterilization methods have advanced from basic chemical immersion to complex low-temperature gas plasma systems and advanced chemical sterilants with improved material compatibility and reduced processing times.
The primary objectives driving sterilization technology development include achieving complete microbial elimination while preserving instrument integrity, reducing cycle times to improve operational efficiency, minimizing environmental impact through reduced water and energy consumption, and ensuring compatibility with increasingly complex and delicate medical devices and materials.
Current technological goals focus on developing systems that can effectively sterilize temperature-sensitive electronics and polymers without degradation, creating rapid sterilization methods for emergency situations and point-of-care applications, implementing real-time monitoring systems for verification of sterilization efficacy, and reducing operational costs while maintaining or improving sterilization standards.
The tension between autoclave and cold sterilization technologies represents a fundamental challenge in the field: balancing the proven reliability and simplicity of heat-based methods against the material preservation advantages of chemical approaches. This technological dichotomy continues to drive innovation as researchers and manufacturers seek optimal solutions that combine the best aspects of both approaches while minimizing their respective limitations.
Market Demand Analysis for Medical Sterilization Solutions
The global medical sterilization solutions market is experiencing robust growth, driven by increasing healthcare-associated infections, rising surgical procedures, and stringent regulatory requirements for infection control. Current market valuation stands at approximately $7.1 billion, with projections indicating a compound annual growth rate of 6.8% through 2028, potentially reaching $10.5 billion.
Hospital-acquired infections affect millions of patients annually, creating substantial demand for effective sterilization technologies. The COVID-19 pandemic has further accelerated this demand, with healthcare facilities worldwide implementing enhanced sterilization protocols. This has particularly benefited manufacturers of both autoclave and cold sterilization solutions.
Surgical instrument sterilization represents the largest application segment, accounting for nearly 40% of the market share. The growing complexity of modern surgical instruments, many containing heat-sensitive components, has created specific demand for cold sterilization methods that can effectively process these delicate tools without damage.
Regional analysis reveals North America as the dominant market, holding approximately 35% of global market share, followed by Europe at 30% and Asia-Pacific as the fastest-growing region with 9.2% annual growth. This geographic distribution reflects varying healthcare infrastructure development and regulatory frameworks across regions.
End-user segmentation shows hospitals and clinics as primary consumers, representing 65% of the market. However, ambulatory surgical centers are emerging as the fastest-growing segment due to the increasing shift toward outpatient procedures and cost-effective healthcare delivery models.
Market demand is increasingly influenced by sustainability concerns, with healthcare facilities seeking sterilization solutions that minimize environmental impact. This trend favors technologies with reduced water consumption, energy efficiency, and decreased chemical waste - factors that influence the autoclave versus cold sterilization decision matrix.
Cost considerations remain paramount in market dynamics, with healthcare facilities balancing initial equipment investment against operational expenses. While autoclaves typically require higher upfront costs but lower per-cycle expenses, cold sterilization often presents lower initial investment but higher recurring costs for consumables and specialized equipment maintenance.
The market is also witnessing growing demand for validation and monitoring systems that provide documentation of sterilization efficacy, driven by regulatory requirements and quality assurance protocols. This has created a complementary market segment for sterilization monitoring products and services.
Hospital-acquired infections affect millions of patients annually, creating substantial demand for effective sterilization technologies. The COVID-19 pandemic has further accelerated this demand, with healthcare facilities worldwide implementing enhanced sterilization protocols. This has particularly benefited manufacturers of both autoclave and cold sterilization solutions.
Surgical instrument sterilization represents the largest application segment, accounting for nearly 40% of the market share. The growing complexity of modern surgical instruments, many containing heat-sensitive components, has created specific demand for cold sterilization methods that can effectively process these delicate tools without damage.
Regional analysis reveals North America as the dominant market, holding approximately 35% of global market share, followed by Europe at 30% and Asia-Pacific as the fastest-growing region with 9.2% annual growth. This geographic distribution reflects varying healthcare infrastructure development and regulatory frameworks across regions.
End-user segmentation shows hospitals and clinics as primary consumers, representing 65% of the market. However, ambulatory surgical centers are emerging as the fastest-growing segment due to the increasing shift toward outpatient procedures and cost-effective healthcare delivery models.
Market demand is increasingly influenced by sustainability concerns, with healthcare facilities seeking sterilization solutions that minimize environmental impact. This trend favors technologies with reduced water consumption, energy efficiency, and decreased chemical waste - factors that influence the autoclave versus cold sterilization decision matrix.
Cost considerations remain paramount in market dynamics, with healthcare facilities balancing initial equipment investment against operational expenses. While autoclaves typically require higher upfront costs but lower per-cycle expenses, cold sterilization often presents lower initial investment but higher recurring costs for consumables and specialized equipment maintenance.
The market is also witnessing growing demand for validation and monitoring systems that provide documentation of sterilization efficacy, driven by regulatory requirements and quality assurance protocols. This has created a complementary market segment for sterilization monitoring products and services.
Current Sterilization Methods and Technical Limitations
Sterilization is a critical process in healthcare, laboratory, and industrial settings, ensuring the elimination of all microorganisms including bacteria, viruses, fungi, and spores. Currently, two predominant methods dominate the sterilization landscape: autoclave (steam sterilization) and cold sterile processing.
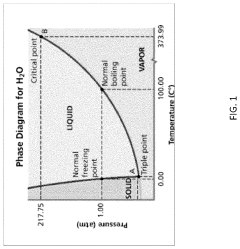
Autoclave sterilization utilizes pressurized steam at 121-134°C (250-273°F) for 15-30 minutes, achieving sterilization through protein denaturation and coagulation. This method remains the gold standard for heat-resistant items due to its reliability, cost-effectiveness, and environmental friendliness. However, it presents significant limitations including the inability to process heat-sensitive materials, potential for moisture damage, and considerable energy consumption.
Cold sterile processing encompasses several techniques that operate at lower temperatures. Ethylene oxide (EtO) sterilization functions at 37-63°C using a gaseous alkylating agent that disrupts cellular metabolism. While effective for heat-sensitive items, EtO requires extensive aeration time (8-12 hours) and poses environmental and occupational hazards due to its carcinogenic properties.
Hydrogen peroxide sterilization, another cold method, employs vaporized H₂O₂ (30-35%) at temperatures below 50°C. This technique offers rapid cycle times (28-55 minutes) and leaves no toxic residues, converting to water and oxygen. However, it demonstrates limited penetration capabilities for long, narrow lumens and requires specialized equipment with high operational costs.
Gamma irradiation, utilizing Cobalt-60 or Cesium-137 sources, provides excellent penetration for dense materials and packaged products. While highly effective, this method requires substantial infrastructure investment and raises safety concerns regarding radioactive material handling.
Technical limitations across current sterilization methods include material compatibility issues, with certain polymers degrading under high temperatures or oxidative conditions. Efficacy validation presents challenges, particularly for novel materials and complex geometries where sterilant penetration may be compromised.
Process monitoring limitations exist in real-time verification of sterilization parameters, especially for cold methods where biological indicators require incubation periods of 24-48 hours before confirming successful sterilization. Additionally, regulatory compliance demands increasingly stringent documentation and validation protocols, adding complexity to implementation.
Sustainability concerns have emerged as significant limitations, with EtO facing environmental restrictions and autoclave processes consuming substantial water and energy resources. The healthcare industry increasingly seeks sterilization methods with reduced environmental footprints while maintaining efficacy and safety standards.
Autoclave sterilization utilizes pressurized steam at 121-134°C (250-273°F) for 15-30 minutes, achieving sterilization through protein denaturation and coagulation. This method remains the gold standard for heat-resistant items due to its reliability, cost-effectiveness, and environmental friendliness. However, it presents significant limitations including the inability to process heat-sensitive materials, potential for moisture damage, and considerable energy consumption.
Cold sterile processing encompasses several techniques that operate at lower temperatures. Ethylene oxide (EtO) sterilization functions at 37-63°C using a gaseous alkylating agent that disrupts cellular metabolism. While effective for heat-sensitive items, EtO requires extensive aeration time (8-12 hours) and poses environmental and occupational hazards due to its carcinogenic properties.
Hydrogen peroxide sterilization, another cold method, employs vaporized H₂O₂ (30-35%) at temperatures below 50°C. This technique offers rapid cycle times (28-55 minutes) and leaves no toxic residues, converting to water and oxygen. However, it demonstrates limited penetration capabilities for long, narrow lumens and requires specialized equipment with high operational costs.
Gamma irradiation, utilizing Cobalt-60 or Cesium-137 sources, provides excellent penetration for dense materials and packaged products. While highly effective, this method requires substantial infrastructure investment and raises safety concerns regarding radioactive material handling.
Technical limitations across current sterilization methods include material compatibility issues, with certain polymers degrading under high temperatures or oxidative conditions. Efficacy validation presents challenges, particularly for novel materials and complex geometries where sterilant penetration may be compromised.
Process monitoring limitations exist in real-time verification of sterilization parameters, especially for cold methods where biological indicators require incubation periods of 24-48 hours before confirming successful sterilization. Additionally, regulatory compliance demands increasingly stringent documentation and validation protocols, adding complexity to implementation.
Sustainability concerns have emerged as significant limitations, with EtO facing environmental restrictions and autoclave processes consuming substantial water and energy resources. The healthcare industry increasingly seeks sterilization methods with reduced environmental footprints while maintaining efficacy and safety standards.
Comparative Analysis of Autoclave vs Cold Sterilization
01 Autoclave sterilization effectiveness
Autoclave sterilization uses high-pressure steam to eliminate microorganisms. The effectiveness of this method depends on temperature, pressure, and exposure time. Modern autoclaves typically operate at 121-134°C under pressure, ensuring the destruction of bacteria, viruses, fungi, and spores. This method is particularly effective for heat-resistant materials and provides reliable sterilization for medical instruments and laboratory equipment.- Autoclave sterilization methods and effectiveness: Autoclave sterilization uses high-pressure steam to eliminate microorganisms. This method is highly effective for heat-resistant materials and is considered the gold standard for sterilization in many medical and laboratory settings. The effectiveness depends on temperature, pressure, and exposure time. Typically operating at 121-134°C under pressure, autoclaves can achieve complete sterilization by denaturing proteins and destroying cell membranes of microorganisms. The process is validated through biological indicators and physical monitors to ensure sterilization efficacy.
- Cold sterilization techniques and applications: Cold sterilization methods provide alternatives for heat-sensitive materials that cannot withstand autoclave conditions. These techniques include chemical sterilants, gas plasma, ethylene oxide, and radiation. Cold sterilization is particularly valuable for electronic devices, plastic components, and certain pharmaceutical products. The effectiveness varies depending on the specific method, concentration of sterilizing agents, exposure time, and material compatibility. While generally less reliable than heat-based methods, cold sterilization can achieve acceptable sterility assurance levels when properly validated and controlled.
- Monitoring and validation of sterilization processes: Effective sterilization requires robust monitoring and validation protocols to ensure consistent results. This includes the use of biological indicators containing resistant bacterial spores, chemical indicators that change color upon exposure to sterilizing conditions, and physical monitors that track parameters like temperature, pressure, and time. Validation methods may involve periodic testing, parametric release systems, and documentation of sterilization cycles. These monitoring approaches help verify that sterilization processes consistently achieve the required sterility assurance level (SAL) and comply with regulatory standards.
- Innovations in sterilization equipment and systems: Recent innovations in sterilization technology focus on improving efficiency, reducing cycle times, and enhancing safety. These include advanced autoclave designs with better steam distribution, rapid cooling systems, and improved door safety mechanisms. For cold sterilization, developments include new low-temperature hydrogen peroxide plasma systems, improved ethylene oxide chambers with better aeration, and novel chemical sterilants with reduced toxicity. Automated loading systems, computerized cycle control, and remote monitoring capabilities further enhance sterilization processes. These innovations aim to increase throughput while maintaining or improving sterilization effectiveness.
- Comparative effectiveness of sterilization methods: Studies comparing autoclave and cold sterilization methods reveal significant differences in effectiveness across various applications. Autoclaves generally provide superior microbial kill rates but are limited to heat-resistant items. Cold sterilization methods offer versatility for heat-sensitive materials but may require longer exposure times or have material compatibility issues. Factors affecting comparative effectiveness include the target microorganisms, material properties, process parameters, and environmental conditions. The selection of optimal sterilization method depends on balancing these factors against practical considerations such as cost, processing time, and safety requirements.
02 Cold sterilization processes and chemical agents
Cold sterilization involves using chemical agents at lower temperatures to achieve sterilization. Common methods include ethylene oxide gas, hydrogen peroxide vapor, peracetic acid, and glutaraldehyde solutions. These methods are suitable for heat-sensitive materials that cannot withstand autoclave conditions. The effectiveness depends on concentration of the sterilant, contact time, temperature, and humidity levels during the process.Expand Specific Solutions03 Sterilization validation and monitoring systems
Effective sterilization requires proper validation and monitoring systems to ensure consistent results. These systems include biological indicators containing resistant spores, chemical indicators that change color when exposed to sterilization conditions, and parametric monitoring devices that track critical parameters like temperature, pressure, and time. Regular validation testing helps maintain sterilization effectiveness and ensures compliance with regulatory standards.Expand Specific Solutions04 Sterilization equipment design and innovation
Advancements in sterilization equipment design have improved effectiveness and efficiency. Modern systems feature precise control mechanisms, uniform distribution of sterilizing agents, optimized chamber designs, and automated cycle management. Innovations include rapid cycle technologies, improved sealing mechanisms, and integrated monitoring systems that enhance reliability while reducing processing time and resource consumption.Expand Specific Solutions05 Comparative effectiveness of sterilization methods
Different sterilization methods show varying effectiveness depending on the target materials and microorganisms. Autoclaves provide reliable sterilization for heat-resistant items but may damage sensitive materials. Cold sterilization offers alternatives for heat-sensitive items but may require longer processing times or have material compatibility issues. The selection of appropriate sterilization method should consider factors such as material compatibility, required sterility assurance level, processing time, and cost-effectiveness.Expand Specific Solutions
Leading Manufacturers and Market Competition
The sterilization technology market is in a mature growth phase, with a global market size exceeding $7 billion and steady annual growth of 5-8%. Autoclave technology dominates with approximately 65% market share, while cold sterilization methods are gaining traction due to their applicability to heat-sensitive materials. Leading autoclave manufacturers include Fedegari Autoclavi SpA and Eschmann Holdings Ltd., who have established robust market positions through decades of innovation. Cold sterilization is seeing technological advancement from companies like Olympus Corp. and SANYO Electric, particularly in healthcare settings. The competitive landscape is characterized by established players with specialized expertise, while research institutions like China Agricultural University and Shinshu University are driving next-generation sterilization technologies that balance efficacy, material compatibility, and operational efficiency.
Fedegari Autoclavi SpA
Technical Solution: Fedegari specializes in advanced autoclave technology for pharmaceutical and biotech industries. Their GMP-compliant steam sterilizers utilize saturated steam under pressure (typically 121-134°C) to achieve sterility assurance levels (SAL) of 10^-6. Fedegari's systems incorporate sophisticated process control with THEMA4 software that enables precise parameter management and comprehensive data recording for regulatory compliance. Their autoclaves feature innovative air-steam mixture cycles that improve temperature distribution and penetration in porous loads, addressing traditional autoclave limitations. Fedegari has also developed hybrid systems that combine autoclave technology with other sterilization methods to optimize processing for different materials, reducing cycle times by up to 30% compared to conventional autoclaves.
Strengths: Superior validation capabilities with comprehensive documentation systems; advanced process control technology; energy-efficient designs that reduce water and steam consumption by up to 40%. Weaknesses: Higher initial capital investment compared to cold sterilization systems; not suitable for heat-sensitive materials; requires significant utility infrastructure including steam generation and water treatment systems.
Olympus Corp.
Technical Solution: Olympus has developed advanced endoscope reprocessing systems that utilize both thermal and chemical sterilization methods. Their OER-Pro series combines automated high-level disinfection with optional terminal sterilization capabilities. For heat-resistant devices, Olympus offers autoclave-compatible endoscopes that withstand temperatures up to 134°C. For heat-sensitive equipment, they've pioneered low-temperature hydrogen peroxide plasma sterilization systems that operate at 50-55°C, achieving sterilization in approximately 28-75 minutes depending on the cycle. Olympus has also developed proprietary chemical formulations optimized for their automated endoscope reprocessors, which achieve high-level disinfection while minimizing damage to sensitive optical components and electronic sensors. Their systems incorporate comprehensive tracking software that documents reprocessing parameters for each instrument.
Strengths: Versatile solutions for both heat-resistant and heat-sensitive instruments; automated systems reduce human error; comprehensive tracking capabilities for regulatory compliance. Weaknesses: Chemical sterilization methods require careful handling of potentially hazardous substances; some low-temperature processes have longer cycle times than autoclaving; recurring costs for consumable disinfectants and sterilants.
Key Patents and Innovations in Sterilization Technology
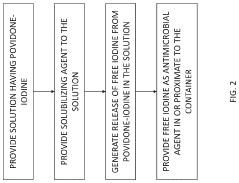
System, method and process for self-sterilization of iodine-containing devices
PatentPendingUS20230390397A1
Innovation
- A self-sterilization process using a closed container system heated to low temperatures (60-100°C) under high pressure to generate free elemental iodine, which diffuses and reacts to kill bacteria and viruses, maintaining solution purity and preventing contamination without creating new impurities.
Sterilisation device and corresponding method
PatentWO2010128217A1
Innovation
- A sterilization device using wave emission, specifically S-band microwaves, that employs a temperature variation protocol involving a first rise, a decrease, and a second rise to effectively sterilize products without the need for high pressure and temperature, allowing for automatic control and safe operation.
Regulatory Compliance and Safety Standards
Regulatory compliance for sterilization processes is governed by stringent international and national standards that ensure patient safety and infection control. The FDA in the United States requires medical facilities to adhere to specific guidelines outlined in 21 CFR Part 820 for autoclave sterilization, while cold sterilization must comply with EPA regulations for high-level disinfectants. Similarly, the European Union enforces EN 285 and EN 13060 standards for steam sterilizers, with additional requirements under the Medical Device Regulation (MDR).
For autoclave sterilization, compliance involves regular validation through biological indicators, typically Geobacillus stearothermophilus spores, which must be tested at least weekly according to CDC recommendations. Documentation requirements include cycle parameters (temperature, pressure, time), maintenance records, and staff training certifications. The Joint Commission mandates that healthcare facilities maintain comprehensive sterilization logs for at least three years.
Cold sterilization processes face more complex regulatory challenges due to the chemical nature of the disinfectants used. OSHA regulations require proper ventilation systems, personal protective equipment, and chemical exposure monitoring. The EPA's FIFRA (Federal Insecticide, Fungicide, and Rodenticide Act) governs registration and labeling of chemical sterilants, with specific contact time requirements that must be strictly followed to achieve claimed efficacy levels.
Safety standards for autoclave operation include ANSI/AAMI ST79 guidelines, which detail requirements for steam quality, chamber design, and safety features such as pressure relief valves and door interlocks. Facilities must implement regular preventive maintenance schedules and operator certification programs to minimize risks of burns, explosions, or sterilization failures.
For cold sterilization, safety standards focus on chemical exposure limits established by NIOSH and ACGIH. These include Threshold Limit Values (TLVs) for glutaraldehyde, hydrogen peroxide, and peracetic acid. Material Safety Data Sheets must be readily accessible, and emergency protocols for chemical spills must be established and regularly practiced.
International harmonization efforts through ISO 17665 (for moist heat sterilization) and ISO 11135 (for ethylene oxide sterilization) provide global benchmarks for validation methodologies. Healthcare facilities increasingly face pressure to demonstrate compliance with these standards through third-party accreditation programs such as those offered by DNV GL or AAAHC.
Regulatory non-compliance carries significant consequences, including potential facility closure, financial penalties, and legal liability. More importantly, sterilization failures linked to regulatory violations can lead to healthcare-associated infections, which affect approximately 1.7 million patients annually in the United States alone, with mortality rates between 5-10% according to CDC statistics.
For autoclave sterilization, compliance involves regular validation through biological indicators, typically Geobacillus stearothermophilus spores, which must be tested at least weekly according to CDC recommendations. Documentation requirements include cycle parameters (temperature, pressure, time), maintenance records, and staff training certifications. The Joint Commission mandates that healthcare facilities maintain comprehensive sterilization logs for at least three years.
Cold sterilization processes face more complex regulatory challenges due to the chemical nature of the disinfectants used. OSHA regulations require proper ventilation systems, personal protective equipment, and chemical exposure monitoring. The EPA's FIFRA (Federal Insecticide, Fungicide, and Rodenticide Act) governs registration and labeling of chemical sterilants, with specific contact time requirements that must be strictly followed to achieve claimed efficacy levels.
Safety standards for autoclave operation include ANSI/AAMI ST79 guidelines, which detail requirements for steam quality, chamber design, and safety features such as pressure relief valves and door interlocks. Facilities must implement regular preventive maintenance schedules and operator certification programs to minimize risks of burns, explosions, or sterilization failures.
For cold sterilization, safety standards focus on chemical exposure limits established by NIOSH and ACGIH. These include Threshold Limit Values (TLVs) for glutaraldehyde, hydrogen peroxide, and peracetic acid. Material Safety Data Sheets must be readily accessible, and emergency protocols for chemical spills must be established and regularly practiced.
International harmonization efforts through ISO 17665 (for moist heat sterilization) and ISO 11135 (for ethylene oxide sterilization) provide global benchmarks for validation methodologies. Healthcare facilities increasingly face pressure to demonstrate compliance with these standards through third-party accreditation programs such as those offered by DNV GL or AAAHC.
Regulatory non-compliance carries significant consequences, including potential facility closure, financial penalties, and legal liability. More importantly, sterilization failures linked to regulatory violations can lead to healthcare-associated infections, which affect approximately 1.7 million patients annually in the United States alone, with mortality rates between 5-10% according to CDC statistics.
Environmental Impact and Sustainability Considerations
The environmental impact of sterilization methods represents a critical consideration in healthcare and industrial settings, with both autoclave and cold sterilization processes presenting distinct ecological footprints. Autoclave sterilization, while highly effective, consumes significant energy resources due to its high-temperature steam requirements. A standard medical autoclave cycle typically consumes between 1.5-3 kWh of electricity and 20-30 gallons of water per cycle, contributing substantially to healthcare facilities' carbon footprint and operational costs.
Water consumption presents another environmental concern for autoclave operations. Beyond the direct water used in steam generation, additional water resources are required for cooling systems. Some modern autoclave systems have implemented water recycling technologies, reducing consumption by up to 60%, though these advanced systems remain less common in resource-constrained settings.
Cold sterilization methods generally demonstrate lower direct energy consumption profiles, operating at ambient temperatures without requiring heat generation. However, the environmental impact shifts to chemical production and disposal considerations. Many cold sterilants, particularly ethylene oxide and hydrogen peroxide, require energy-intensive manufacturing processes and create potential environmental hazards during disposal phases.
Chemical waste management represents a significant environmental challenge for cold sterilization approaches. Glutaraldehyde and peracetic acid solutions require specialized disposal protocols to prevent waterway contamination. Studies indicate that improper disposal of these chemicals can disrupt aquatic ecosystems and potentially enter groundwater systems, creating long-term environmental liabilities.
Life cycle assessment (LCA) studies comparing both sterilization approaches reveal complex sustainability profiles. While autoclaves demonstrate higher operational energy consumption, their equipment typically maintains longer service lives (15-20 years) compared to cold sterilization systems (7-10 years). This extended operational lifespan partially offsets manufacturing-related environmental impacts through reduced replacement frequency.
Recent technological innovations are addressing sustainability concerns across both sterilization paradigms. Energy-efficient autoclaves incorporating heat recovery systems can reduce energy consumption by 25-40%, while advanced cold sterilization systems have developed closed-loop chemical recycling capabilities, minimizing waste generation by up to 80% compared to conventional systems.
Regulatory frameworks increasingly emphasize environmental performance alongside sterilization efficacy. The European Union's Medical Device Regulation and the United States EPA guidelines now incorporate sustainability metrics into compliance requirements, accelerating industry adoption of eco-friendly sterilization technologies and operational practices across global healthcare systems.
Water consumption presents another environmental concern for autoclave operations. Beyond the direct water used in steam generation, additional water resources are required for cooling systems. Some modern autoclave systems have implemented water recycling technologies, reducing consumption by up to 60%, though these advanced systems remain less common in resource-constrained settings.
Cold sterilization methods generally demonstrate lower direct energy consumption profiles, operating at ambient temperatures without requiring heat generation. However, the environmental impact shifts to chemical production and disposal considerations. Many cold sterilants, particularly ethylene oxide and hydrogen peroxide, require energy-intensive manufacturing processes and create potential environmental hazards during disposal phases.
Chemical waste management represents a significant environmental challenge for cold sterilization approaches. Glutaraldehyde and peracetic acid solutions require specialized disposal protocols to prevent waterway contamination. Studies indicate that improper disposal of these chemicals can disrupt aquatic ecosystems and potentially enter groundwater systems, creating long-term environmental liabilities.
Life cycle assessment (LCA) studies comparing both sterilization approaches reveal complex sustainability profiles. While autoclaves demonstrate higher operational energy consumption, their equipment typically maintains longer service lives (15-20 years) compared to cold sterilization systems (7-10 years). This extended operational lifespan partially offsets manufacturing-related environmental impacts through reduced replacement frequency.
Recent technological innovations are addressing sustainability concerns across both sterilization paradigms. Energy-efficient autoclaves incorporating heat recovery systems can reduce energy consumption by 25-40%, while advanced cold sterilization systems have developed closed-loop chemical recycling capabilities, minimizing waste generation by up to 80% compared to conventional systems.
Regulatory frameworks increasingly emphasize environmental performance alongside sterilization efficacy. The European Union's Medical Device Regulation and the United States EPA guidelines now incorporate sustainability metrics into compliance requirements, accelerating industry adoption of eco-friendly sterilization technologies and operational practices across global healthcare systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!