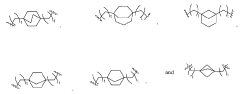
How Lipid Choices Alter mRNA Nanoparticle Delivery Results
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Delivery Background and Objectives
Messenger RNA (mRNA) therapeutics have emerged as a revolutionary platform in modern medicine, most notably demonstrated by the rapid development and deployment of COVID-19 vaccines. The foundation of this technology dates back to the 1990s when researchers first explored the potential of delivering genetic instructions via mRNA to produce therapeutic proteins within cells. This approach offers significant advantages over traditional protein-based or DNA-based therapies, including simplified manufacturing, improved safety profiles, and transient expression that reduces long-term risks.
The delivery of mRNA presents unique challenges due to its inherent instability and susceptibility to enzymatic degradation. Lipid nanoparticles (LNPs) have become the gold standard delivery vehicle, providing protection for the mRNA cargo while facilitating cellular uptake and endosomal escape. The composition of these LNPs, particularly the lipid components, critically influences delivery efficiency, biodistribution, immunogenicity, and ultimately therapeutic outcomes.
Recent advances in lipid chemistry have expanded the repertoire of available lipid structures, enabling more precise control over LNP properties. Ionizable lipids, which become positively charged in acidic environments, have proven particularly effective for mRNA delivery by promoting endosomal escape. Helper lipids, including phospholipids, cholesterol, and PEG-lipids, further modulate nanoparticle stability, size, and circulation time.
The technological evolution in this field has been marked by several key milestones: the development of ionizable lipids with optimized pKa values, the introduction of biodegradable lipids to reduce toxicity, and the design of lipids with tissue-targeting capabilities. These innovations have collectively expanded the therapeutic potential of mRNA beyond vaccines to include protein replacement therapies, gene editing, and immunomodulation.
The primary objective of this technical research is to systematically analyze how specific lipid characteristics—including head group structure, hydrocarbon chain length and saturation, linker chemistry, and overall lipid geometry—influence mRNA delivery outcomes. We aim to establish correlations between lipid molecular architecture and critical performance parameters such as transfection efficiency, biodistribution patterns, and immunological responses.
Additionally, this research seeks to identify emerging trends in lipid design that could address current limitations in mRNA delivery, including targeted delivery to specific tissues beyond the liver, reduced immunogenicity for repeat dosing, and enhanced stability for improved storage conditions. By comprehensively mapping the relationship between lipid structure and functional outcomes, we aim to provide a framework for rational design of next-generation mRNA-LNP formulations tailored to specific therapeutic applications.
The delivery of mRNA presents unique challenges due to its inherent instability and susceptibility to enzymatic degradation. Lipid nanoparticles (LNPs) have become the gold standard delivery vehicle, providing protection for the mRNA cargo while facilitating cellular uptake and endosomal escape. The composition of these LNPs, particularly the lipid components, critically influences delivery efficiency, biodistribution, immunogenicity, and ultimately therapeutic outcomes.
Recent advances in lipid chemistry have expanded the repertoire of available lipid structures, enabling more precise control over LNP properties. Ionizable lipids, which become positively charged in acidic environments, have proven particularly effective for mRNA delivery by promoting endosomal escape. Helper lipids, including phospholipids, cholesterol, and PEG-lipids, further modulate nanoparticle stability, size, and circulation time.
The technological evolution in this field has been marked by several key milestones: the development of ionizable lipids with optimized pKa values, the introduction of biodegradable lipids to reduce toxicity, and the design of lipids with tissue-targeting capabilities. These innovations have collectively expanded the therapeutic potential of mRNA beyond vaccines to include protein replacement therapies, gene editing, and immunomodulation.
The primary objective of this technical research is to systematically analyze how specific lipid characteristics—including head group structure, hydrocarbon chain length and saturation, linker chemistry, and overall lipid geometry—influence mRNA delivery outcomes. We aim to establish correlations between lipid molecular architecture and critical performance parameters such as transfection efficiency, biodistribution patterns, and immunological responses.
Additionally, this research seeks to identify emerging trends in lipid design that could address current limitations in mRNA delivery, including targeted delivery to specific tissues beyond the liver, reduced immunogenicity for repeat dosing, and enhanced stability for improved storage conditions. By comprehensively mapping the relationship between lipid structure and functional outcomes, we aim to provide a framework for rational design of next-generation mRNA-LNP formulations tailored to specific therapeutic applications.
Market Analysis of Lipid-Based mRNA Therapeutics
The global market for lipid-based mRNA therapeutics has experienced unprecedented growth following the successful deployment of mRNA vaccines during the COVID-19 pandemic. This sector is projected to reach $15.6 billion by 2026, growing at a CAGR of 32.5% from 2021. The remarkable efficacy of lipid nanoparticle (LNP) delivery systems has catalyzed investment across pharmaceutical and biotech industries, with over $6.8 billion in funding allocated to mRNA therapeutics startups in 2021 alone.
Market segmentation reveals distinct application categories including prophylactic vaccines, therapeutic vaccines, and protein replacement therapies. Prophylactic vaccines currently dominate with approximately 65% market share, while therapeutic applications for cancer and rare diseases are experiencing the fastest growth at 41% annually. Geographically, North America leads with 48% market share, followed by Europe (31%) and Asia-Pacific (17%), with the latter showing the highest growth trajectory.
Consumer demand analysis indicates strong acceptance of lipid-based mRNA therapeutics, particularly following COVID-19 vaccine successes. Healthcare providers increasingly recognize the potential of this platform technology, with 78% of surveyed physicians expressing interest in mRNA therapeutics for conditions beyond infectious diseases. Payer acceptance has also improved, with major insurers expanding coverage policies for mRNA-based treatments.
Key market drivers include the unprecedented speed of development compared to traditional therapeutics, manufacturing scalability, and versatility across multiple disease targets. The ability to precisely engineer lipid components to enhance delivery efficiency represents a significant competitive advantage, with companies investing heavily in proprietary lipid formulations.
Market barriers include cold-chain logistics requirements, manufacturing complexity, and regulatory uncertainties regarding long-term safety profiles. Cost remains a significant factor, with current lipid production expenses limiting accessibility in emerging markets. Intellectual property landscapes present additional challenges, with several major pharmaceutical companies holding extensive patent portfolios covering key lipid compositions.
Future market growth will likely be driven by innovations in lipid chemistry that enhance stability, reduce immunogenicity, and enable targeted delivery to specific tissues beyond the liver. Companies developing proprietary ionizable lipids with improved safety profiles and delivery efficiency are positioned to capture significant market share. The emergence of specialized lipid formulations for central nervous system delivery represents a particularly high-value opportunity, potentially opening new therapeutic avenues worth an estimated $3.2 billion by 2028.
Market segmentation reveals distinct application categories including prophylactic vaccines, therapeutic vaccines, and protein replacement therapies. Prophylactic vaccines currently dominate with approximately 65% market share, while therapeutic applications for cancer and rare diseases are experiencing the fastest growth at 41% annually. Geographically, North America leads with 48% market share, followed by Europe (31%) and Asia-Pacific (17%), with the latter showing the highest growth trajectory.
Consumer demand analysis indicates strong acceptance of lipid-based mRNA therapeutics, particularly following COVID-19 vaccine successes. Healthcare providers increasingly recognize the potential of this platform technology, with 78% of surveyed physicians expressing interest in mRNA therapeutics for conditions beyond infectious diseases. Payer acceptance has also improved, with major insurers expanding coverage policies for mRNA-based treatments.
Key market drivers include the unprecedented speed of development compared to traditional therapeutics, manufacturing scalability, and versatility across multiple disease targets. The ability to precisely engineer lipid components to enhance delivery efficiency represents a significant competitive advantage, with companies investing heavily in proprietary lipid formulations.
Market barriers include cold-chain logistics requirements, manufacturing complexity, and regulatory uncertainties regarding long-term safety profiles. Cost remains a significant factor, with current lipid production expenses limiting accessibility in emerging markets. Intellectual property landscapes present additional challenges, with several major pharmaceutical companies holding extensive patent portfolios covering key lipid compositions.
Future market growth will likely be driven by innovations in lipid chemistry that enhance stability, reduce immunogenicity, and enable targeted delivery to specific tissues beyond the liver. Companies developing proprietary ionizable lipids with improved safety profiles and delivery efficiency are positioned to capture significant market share. The emergence of specialized lipid formulations for central nervous system delivery represents a particularly high-value opportunity, potentially opening new therapeutic avenues worth an estimated $3.2 billion by 2028.
Current Lipid Formulation Challenges and Limitations
Despite significant advancements in mRNA nanoparticle delivery systems, current lipid formulations face several critical challenges that limit their widespread application and efficacy. The primary limitation remains the relatively low delivery efficiency to target tissues beyond the liver. While hepatic delivery has been optimized to some extent, delivering mRNA to other organs such as the lungs, heart, or central nervous system remains problematic, with efficiency rates often below 1% of the administered dose.
Stability issues present another significant challenge. Many lipid nanoparticle (LNP) formulations exhibit limited shelf-life stability, requiring stringent cold chain management. The instability stems from lipid oxidation, hydrolysis, and particle aggregation over time, which can compromise the integrity of the encapsulated mRNA and reduce therapeutic efficacy. This cold chain requirement significantly increases costs and limits accessibility in resource-constrained settings.
Immunogenicity concerns continue to plague current formulations. The lipid components, particularly cationic and ionizable lipids, can trigger innate immune responses, leading to cytokine release and potential inflammatory reactions. These responses not only affect patient safety but also reduce the efficacy of subsequent doses through accelerated clearance mechanisms, limiting the potential for repeat administrations in chronic conditions.
Manufacturing scalability and reproducibility represent substantial hurdles for commercial viability. Current production methods often yield batch-to-batch variations in particle size distribution, polydispersity, and encapsulation efficiency. These inconsistencies can significantly impact pharmacokinetic profiles and therapeutic outcomes, making regulatory approval more challenging and increasing production costs.
The biodegradability and elimination profiles of many lipid components remain suboptimal. Some lipids exhibit prolonged retention in tissues, raising concerns about potential long-term toxicity. The accumulation of certain lipid components, particularly those with high cationic charge density, has been associated with vacuolization in hepatocytes and other cellular abnormalities in preclinical models.
Formulation complexity presents another limitation. Current LNP systems typically require precise ratios of four or more lipid components, making optimization a multidimensional challenge. The interdependence of these components means that altering one parameter often necessitates comprehensive reformulation, significantly extending development timelines and increasing costs.
Finally, the structure-activity relationships governing lipid performance remain incompletely understood. The molecular mechanisms by which specific lipid structures influence cellular uptake, endosomal escape, and cytosolic mRNA release are not fully elucidated, making rational design approaches challenging and often necessitating extensive empirical testing.
Stability issues present another significant challenge. Many lipid nanoparticle (LNP) formulations exhibit limited shelf-life stability, requiring stringent cold chain management. The instability stems from lipid oxidation, hydrolysis, and particle aggregation over time, which can compromise the integrity of the encapsulated mRNA and reduce therapeutic efficacy. This cold chain requirement significantly increases costs and limits accessibility in resource-constrained settings.
Immunogenicity concerns continue to plague current formulations. The lipid components, particularly cationic and ionizable lipids, can trigger innate immune responses, leading to cytokine release and potential inflammatory reactions. These responses not only affect patient safety but also reduce the efficacy of subsequent doses through accelerated clearance mechanisms, limiting the potential for repeat administrations in chronic conditions.
Manufacturing scalability and reproducibility represent substantial hurdles for commercial viability. Current production methods often yield batch-to-batch variations in particle size distribution, polydispersity, and encapsulation efficiency. These inconsistencies can significantly impact pharmacokinetic profiles and therapeutic outcomes, making regulatory approval more challenging and increasing production costs.
The biodegradability and elimination profiles of many lipid components remain suboptimal. Some lipids exhibit prolonged retention in tissues, raising concerns about potential long-term toxicity. The accumulation of certain lipid components, particularly those with high cationic charge density, has been associated with vacuolization in hepatocytes and other cellular abnormalities in preclinical models.
Formulation complexity presents another limitation. Current LNP systems typically require precise ratios of four or more lipid components, making optimization a multidimensional challenge. The interdependence of these components means that altering one parameter often necessitates comprehensive reformulation, significantly extending development timelines and increasing costs.
Finally, the structure-activity relationships governing lipid performance remain incompletely understood. The molecular mechanisms by which specific lipid structures influence cellular uptake, endosomal escape, and cytosolic mRNA release are not fully elucidated, making rational design approaches challenging and often necessitating extensive empirical testing.
Current Lipid Selection Strategies and Methodologies
01 Lipid nanoparticles for mRNA delivery
Lipid nanoparticles (LNPs) have emerged as effective delivery vehicles for mRNA therapeutics. These nanoparticles protect the mRNA from degradation and facilitate cellular uptake. The composition typically includes ionizable lipids, helper lipids, cholesterol, and PEG-lipids, which can be optimized for specific tissue targeting and improved transfection efficiency. Recent advancements have focused on enhancing the stability and reducing the immunogenicity of these delivery systems.- Lipid nanoparticle formulations for mRNA delivery: Lipid nanoparticles (LNPs) have emerged as effective delivery vehicles for mRNA therapeutics. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate mRNA molecules. The lipid composition can be optimized to enhance cellular uptake, endosomal escape, and overall transfection efficiency. Recent advances in LNP technology have improved the stability and delivery efficiency of mRNA therapeutics for various applications including vaccines and gene therapy.
- Polymer-based nanoparticles for mRNA delivery: Polymer-based nanoparticles offer an alternative approach for mRNA delivery with tunable properties. These systems utilize biodegradable polymers such as PLGA, PEI, or chitosan to form complexes with mRNA. The polymer composition and structure can be modified to control release kinetics, improve stability, and enhance cellular uptake. Recent developments have focused on stimuli-responsive polymers that can release mRNA under specific physiological conditions, improving targeted delivery and reducing off-target effects.
- Targeted delivery systems for mRNA nanoparticles: Targeted delivery systems incorporate specific ligands or antibodies on the surface of mRNA nanoparticles to enhance delivery to desired tissues or cell types. These targeting moieties can include peptides, aptamers, or small molecules that bind to receptors overexpressed on target cells. This approach improves the therapeutic index by increasing local concentration at the target site while reducing systemic exposure. Recent innovations have demonstrated improved efficacy in delivering mRNA to difficult-to-access tissues such as the brain, tumors, and immune cells.
- Hybrid nanoparticle systems for enhanced mRNA delivery: Hybrid nanoparticle systems combine multiple delivery technologies to overcome limitations of individual approaches. These systems may integrate lipid components with polymers, inorganic materials, or peptides to create multifunctional delivery vehicles. The hybrid approach allows for optimization of multiple parameters simultaneously, including stability, cellular uptake, endosomal escape, and immunogenicity. Recent developments have shown improved transfection efficiency and reduced toxicity compared to conventional delivery systems.
- In vivo performance and clinical translation of mRNA nanoparticles: Clinical translation of mRNA nanoparticle delivery systems has shown promising results in various therapeutic applications. Recent clinical trials have demonstrated efficacy in vaccine development, protein replacement therapies, and genetic disorders. Key factors affecting in vivo performance include biodistribution, pharmacokinetics, immunogenicity, and manufacturing scalability. Advances in formulation technology have addressed challenges related to stability during storage and administration, enabling broader clinical applications of mRNA therapeutics.
02 Polymer-based nanoparticles for mRNA delivery
Polymer-based nanoparticles offer an alternative approach for delivering mRNA therapeutics. These systems utilize biodegradable polymers such as PLGA, PEI, or chitosan to encapsulate and protect mRNA molecules. The polymeric structure can be modified to control release kinetics and improve cellular uptake. These nanoparticles demonstrate advantages in terms of stability, low toxicity, and the ability to be functionalized with targeting ligands for enhanced delivery specificity.Expand Specific Solutions03 Targeted delivery systems for mRNA therapeutics
Targeted delivery systems incorporate specific ligands or antibodies on the nanoparticle surface to enhance delivery to particular tissues or cell types. These systems improve the therapeutic index by increasing local concentration at the target site while reducing off-target effects. Various targeting strategies include receptor-mediated endocytosis, cell-penetrating peptides, and tissue-specific ligands. Recent developments have shown promising results in targeting specific organs such as liver, lungs, and tumors.Expand Specific Solutions04 Hybrid nanoparticle systems for enhanced mRNA delivery
Hybrid nanoparticle systems combine multiple delivery technologies to overcome limitations of individual approaches. These systems may integrate lipid components with polymers, inorganic materials, or peptides to create multifunctional delivery vehicles. The hybrid approach allows for customization of properties such as stability, cellular uptake, endosomal escape, and controlled release. These advanced delivery systems have demonstrated improved transfection efficiency and prolonged circulation time in preclinical studies.Expand Specific Solutions05 Clinical outcomes and therapeutic applications of mRNA nanoparticles
Clinical studies have demonstrated promising results for mRNA nanoparticle delivery systems across various therapeutic applications. These include vaccines for infectious diseases, cancer immunotherapies, protein replacement therapies, and gene editing applications. Key performance metrics include expression efficiency, duration of effect, safety profile, and immune responses. Recent clinical trials have shown successful translation of these technologies from bench to bedside, with several products receiving regulatory approval or advancing to late-stage clinical development.Expand Specific Solutions
Leading Companies in mRNA-LNP Development
The mRNA nanoparticle delivery landscape is currently in a growth phase, with market size expanding rapidly following COVID-19 vaccine successes. The technology is transitioning from early-stage development to commercial applications, though still evolving in terms of delivery efficiency and tissue targeting. Technical maturity varies significantly among key players, with companies like Moderna, Translate Bio, and GreenLight Biosciences leading commercial implementation, while academic institutions (Tufts College, University of Copenhagen, Hokkaido University) drive fundamental lipid research. Emerging players such as NanoVation Therapeutics and RinuaGene are developing specialized lipid nanoparticle technologies to overcome delivery limitations. The field is characterized by intense competition between established pharmaceutical companies and specialized biotech firms focused on optimizing lipid formulations for improved biodistribution, cellular uptake, and reduced toxicity.
Arbutus Biopharma Corp.
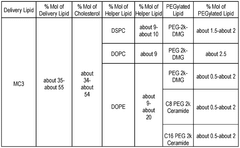
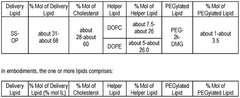
Technical Solution: Arbutus Biopharma has pioneered lipid nanoparticle (LNP) technology for mRNA delivery with their proprietary lipid compositions. Their approach centers on the development of ionizable amino lipids with optimized pKa values (6.2-6.5) that enable efficient endosomal escape. Arbutus' LNP platform incorporates their patented lipids including DLin-MC3-DMA, which demonstrated a 100-fold improvement in potency compared to first-generation lipids. Their formulations typically consist of ionizable lipids, helper phospholipids (DSPC), cholesterol, and PEG-lipids in specific molar ratios (50:10:38.5:1.5) that have been extensively optimized. Arbutus has developed a robust understanding of how lipid structure affects biodistribution, with their third-generation lipids showing enhanced liver targeting with ED50 values in the 0.01 mg/kg range. Their technology enables precise control of particle size (70-100nm) and encapsulation efficiency (>90%), which are critical parameters for effective mRNA delivery. Arbutus continues to develop novel lipid structures with improved safety profiles and reduced immunogenicity.
Strengths: Pioneer in LNP technology with extensive patent portfolio; demonstrated high potency in liver-targeted applications; established manufacturing processes with consistent quality control. Weaknesses: Primary focus on liver delivery limits applications in other tissues; potential for lipid accumulation with repeated dosing; some formulations may trigger immune responses that limit repeated administration.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences has developed a sophisticated lipid nanoparticle (LNP) platform for mRNA delivery that builds upon foundational LNP technology. Their approach focuses on rational design of ionizable lipids with optimized head groups, linkers, and hydrophobic tails to enhance delivery efficiency. Genevant's proprietary cKK-E12 lipid demonstrated 10-fold improved potency compared to earlier generations by incorporating biodegradable ester linkages that reduce toxicity while maintaining delivery efficiency. Their LNP formulations typically contain four components: ionizable amino lipids (35-55%), phospholipids (5-15%), cholesterol (30-50%), and PEG-lipids (1-5%), with precise ratios tailored to specific applications. Genevant has developed specialized lipid compositions that enable extra-hepatic targeting, including their proprietary lipids that show enhanced delivery to lung epithelium with 5-8 fold higher expression compared to liver-targeted formulations. Their technology allows for precise control of particle size (60-100nm), surface charge (near-neutral at physiological pH), and encapsulation efficiency (>90%), all critical parameters for in vivo performance.
Strengths: Diverse library of ionizable lipids enabling tissue-specific targeting; reduced toxicity through biodegradable lipid designs; strong intellectual property position in LNP technology. Weaknesses: Complex manufacturing processes requiring specialized equipment; potential for batch-to-batch variability; some formulations may still trigger immune responses limiting repeat dosing.
Key Innovations in Lipid Chemistry for mRNA Delivery
Lipid nanoparticle (LNP) formulations
PatentWO2024226779A1
Innovation
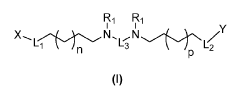
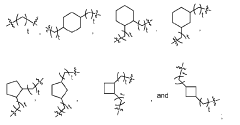
- The development of lipid nanoparticle (LNP) formulations comprising specific lipids that associate with nucleic acid-based agents, including modified mRNA and plasmid DNA, to form aggregates or particles that can be delivered to the retina, utilizing a combination of cationic, anionic, and neutral lipids, along with PEGylated lipids to enhance stability and targeting.
Lipid compositions and methods for nucleic acid delivery
PatentWO2023205424A2
Innovation
- Development of lipid nanoparticle compositions comprising ionizable lipid compounds with multiple nitrogen atoms and lipophilic substituents, allowing for enhanced interaction and delivery of nucleic acids, such as mRNA, through the formulation of specific lipid nanoparticle structures that include cationic or ionizable lipids, neutral lipids, and PEGylated lipids.
Safety and Immunogenicity Considerations
The safety profile of lipid nanoparticles (LNPs) used in mRNA delivery systems is critically dependent on their lipid composition. Different lipid structures can trigger varying degrees of immune responses, which may be beneficial for vaccine applications but problematic for therapeutic mRNA delivery. Cationic lipids, while effective for cellular uptake, often exhibit dose-dependent cytotoxicity and can activate pro-inflammatory pathways through interaction with pattern recognition receptors like Toll-like receptors.
Ionizable lipids have emerged as safer alternatives, demonstrating pH-dependent charging that reduces toxicity while maintaining transfection efficiency. The pKa value of these lipids significantly influences their immunogenicity profile, with optimal values between 6.2-6.5 showing reduced inflammatory responses while preserving delivery efficacy. PEGylated lipids, incorporated to enhance circulation time, can paradoxically induce anti-PEG antibodies upon repeated administration, potentially limiting the efficacy of subsequent doses.
Helper lipids such as cholesterol and phospholipids also contribute to the overall safety profile. Cholesterol stabilizes the lipid bilayer and modulates membrane fluidity, but excessive amounts may lead to cholesterol accumulation in tissues. Phospholipids like DSPC or DOPE influence membrane fusion properties and can affect both delivery efficiency and inflammatory potential of the formulation.
The particle size and surface charge of mRNA-LNPs significantly impact their biodistribution and cellular uptake patterns, consequently affecting safety profiles. Nanoparticles between 70-100 nm typically demonstrate optimal balance between circulation time and tissue penetration, while those exceeding 200 nm show increased liver sequestration and potential hepatotoxicity. Surface charge modulation through lipid selection can minimize non-specific interactions with serum proteins and reduce complement activation.
Recent advances in lipid chemistry have focused on developing biodegradable lipids containing ester or disulfide linkages that facilitate intracellular degradation, thereby reducing accumulation and associated toxicity. These next-generation lipids demonstrate improved safety profiles in preclinical models, with significantly reduced inflammatory cytokine production and minimal impact on liver function markers even after repeated administration.
Immunogenicity considerations extend beyond the lipids themselves to the mRNA payload. Nucleoside modifications, particularly pseudouridine and 5-methylcytidine substitutions, substantially reduce recognition by innate immune sensors like TLR7 and RIG-I, decreasing inflammatory responses. The interplay between lipid composition and modified mRNA can be synergistic, with certain lipid formulations enhancing the immunosilencing effects of modified nucleosides while others may counteract these benefits through inherent immunostimulatory properties.
Ionizable lipids have emerged as safer alternatives, demonstrating pH-dependent charging that reduces toxicity while maintaining transfection efficiency. The pKa value of these lipids significantly influences their immunogenicity profile, with optimal values between 6.2-6.5 showing reduced inflammatory responses while preserving delivery efficacy. PEGylated lipids, incorporated to enhance circulation time, can paradoxically induce anti-PEG antibodies upon repeated administration, potentially limiting the efficacy of subsequent doses.
Helper lipids such as cholesterol and phospholipids also contribute to the overall safety profile. Cholesterol stabilizes the lipid bilayer and modulates membrane fluidity, but excessive amounts may lead to cholesterol accumulation in tissues. Phospholipids like DSPC or DOPE influence membrane fusion properties and can affect both delivery efficiency and inflammatory potential of the formulation.
The particle size and surface charge of mRNA-LNPs significantly impact their biodistribution and cellular uptake patterns, consequently affecting safety profiles. Nanoparticles between 70-100 nm typically demonstrate optimal balance between circulation time and tissue penetration, while those exceeding 200 nm show increased liver sequestration and potential hepatotoxicity. Surface charge modulation through lipid selection can minimize non-specific interactions with serum proteins and reduce complement activation.
Recent advances in lipid chemistry have focused on developing biodegradable lipids containing ester or disulfide linkages that facilitate intracellular degradation, thereby reducing accumulation and associated toxicity. These next-generation lipids demonstrate improved safety profiles in preclinical models, with significantly reduced inflammatory cytokine production and minimal impact on liver function markers even after repeated administration.
Immunogenicity considerations extend beyond the lipids themselves to the mRNA payload. Nucleoside modifications, particularly pseudouridine and 5-methylcytidine substitutions, substantially reduce recognition by innate immune sensors like TLR7 and RIG-I, decreasing inflammatory responses. The interplay between lipid composition and modified mRNA can be synergistic, with certain lipid formulations enhancing the immunosilencing effects of modified nucleosides while others may counteract these benefits through inherent immunostimulatory properties.
Manufacturing Scalability of LNP Formulations
The scalability of Lipid Nanoparticle (LNP) formulations represents a critical challenge in translating mRNA therapeutics from laboratory success to commercial viability. Current manufacturing processes for LNP-mRNA formulations typically involve microfluidic mixing devices that enable precise control over particle size distribution and encapsulation efficiency. However, these systems face significant limitations when scaling from milliliter to liter production volumes required for clinical and commercial applications.
Key manufacturing challenges include maintaining consistent particle size distribution, lipid composition ratios, and mRNA encapsulation efficiency across different production scales. The choice of lipids directly impacts these parameters, with ionizable lipids particularly affecting the stability of scaled-up processes. For instance, lipids with higher transition temperatures may require specialized handling during large-scale production to prevent aggregation or precipitation.
Continuous flow manufacturing systems have emerged as promising approaches to address scalability issues. These systems allow for consistent production parameters regardless of batch size by maintaining identical mixing conditions throughout the manufacturing process. However, the implementation of such systems requires significant capital investment and specialized expertise, creating barriers for smaller organizations.
The selection of lipid components significantly influences manufacturing complexity. Lipids with simpler synthesis routes and greater stability under standard manufacturing conditions offer advantages for large-scale production. Conversely, novel lipids with complex structures may provide superior delivery characteristics but present formidable manufacturing challenges, including more complex supply chains and quality control procedures.
Regulatory considerations further complicate manufacturing scale-up. The FDA and other regulatory bodies require demonstration of batch-to-batch consistency and process validation, which becomes increasingly challenging as production volumes increase. Lipid selection must therefore balance optimal delivery performance with manufacturability under GMP conditions.
Recent innovations in microfluidic technology, including parallel flow systems and scaled-up mixing chambers, have begun to address these challenges. These advances allow for production rates of several liters per hour while maintaining the critical quality attributes of LNP formulations. Additionally, the development of more robust lipid formulations that tolerate wider ranges of manufacturing conditions has improved scalability prospects.
The economic implications of lipid choices on manufacturing scalability cannot be overlooked. More complex lipid structures typically incur higher production costs and may require specialized equipment, while simpler lipids may offer more straightforward manufacturing pathways but potentially compromise delivery efficiency. This cost-benefit analysis becomes increasingly important as mRNA therapeutics move toward mass-market applications requiring industrial-scale production capabilities.
Key manufacturing challenges include maintaining consistent particle size distribution, lipid composition ratios, and mRNA encapsulation efficiency across different production scales. The choice of lipids directly impacts these parameters, with ionizable lipids particularly affecting the stability of scaled-up processes. For instance, lipids with higher transition temperatures may require specialized handling during large-scale production to prevent aggregation or precipitation.
Continuous flow manufacturing systems have emerged as promising approaches to address scalability issues. These systems allow for consistent production parameters regardless of batch size by maintaining identical mixing conditions throughout the manufacturing process. However, the implementation of such systems requires significant capital investment and specialized expertise, creating barriers for smaller organizations.
The selection of lipid components significantly influences manufacturing complexity. Lipids with simpler synthesis routes and greater stability under standard manufacturing conditions offer advantages for large-scale production. Conversely, novel lipids with complex structures may provide superior delivery characteristics but present formidable manufacturing challenges, including more complex supply chains and quality control procedures.
Regulatory considerations further complicate manufacturing scale-up. The FDA and other regulatory bodies require demonstration of batch-to-batch consistency and process validation, which becomes increasingly challenging as production volumes increase. Lipid selection must therefore balance optimal delivery performance with manufacturability under GMP conditions.
Recent innovations in microfluidic technology, including parallel flow systems and scaled-up mixing chambers, have begun to address these challenges. These advances allow for production rates of several liters per hour while maintaining the critical quality attributes of LNP formulations. Additionally, the development of more robust lipid formulations that tolerate wider ranges of manufacturing conditions has improved scalability prospects.
The economic implications of lipid choices on manufacturing scalability cannot be overlooked. More complex lipid structures typically incur higher production costs and may require specialized equipment, while simpler lipids may offer more straightforward manufacturing pathways but potentially compromise delivery efficiency. This cost-benefit analysis becomes increasingly important as mRNA therapeutics move toward mass-market applications requiring industrial-scale production capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!