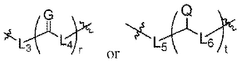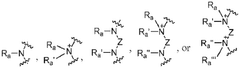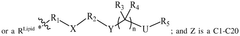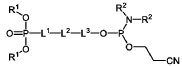mRNA Lipid Nanoparticle Delivery Mechanisms in Healthcare
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Evolution and Objectives
Messenger RNA (mRNA) technology has undergone a remarkable evolution over the past four decades, transforming from a theoretical concept to a revolutionary therapeutic platform. The journey began in the 1970s with the discovery of mRNA's role in protein synthesis, followed by pioneering work in the 1990s that demonstrated the potential for using synthetic mRNA to produce proteins in vivo. However, early applications faced significant challenges related to mRNA instability, immunogenicity, and inefficient delivery mechanisms.
The breakthrough came in the early 2000s with the development of modified nucleosides that reduced immunogenicity and increased translation efficiency. This innovation, coupled with advances in lipid nanoparticle (LNP) formulation techniques, created the foundation for viable mRNA therapeutics. LNPs emerged as the preferred delivery vehicle due to their ability to protect mRNA from degradation, facilitate cellular uptake, and enable endosomal escape for effective protein expression.
The COVID-19 pandemic served as a catalyst for mRNA technology, accelerating development timelines and demonstrating the platform's unprecedented speed and adaptability. The successful deployment of mRNA vaccines against SARS-CoV-2 validated decades of research and established mRNA-LNP technology as a transformative approach in modern medicine. This watershed moment has expanded the vision for mRNA applications beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disease treatments.
Current technological objectives focus on enhancing several critical aspects of mRNA-LNP delivery systems. Researchers aim to improve tissue targeting specificity to minimize off-target effects and reduce required dosages. Efforts are underway to develop LNP formulations that can effectively target tissues beyond the liver, which has traditionally been the primary destination for systemically administered LNPs. Engineering mRNA for extended half-life and controlled release profiles represents another important goal to achieve sustained therapeutic effects.
The field is also pursuing innovations in manufacturing scalability and stability, with objectives to develop room-temperature stable formulations that could revolutionize global distribution. Simultaneously, researchers are working to reduce production costs to improve accessibility, particularly in resource-limited settings. The ultimate technological vision encompasses personalized mRNA therapeutics tailored to individual genetic profiles and disease states.
As the technology continues to mature, interdisciplinary collaboration between molecular biologists, lipid chemists, pharmaceutical scientists, and clinicians will be essential to overcome remaining challenges and fully realize the transformative potential of mRNA-LNP delivery systems in healthcare.
The breakthrough came in the early 2000s with the development of modified nucleosides that reduced immunogenicity and increased translation efficiency. This innovation, coupled with advances in lipid nanoparticle (LNP) formulation techniques, created the foundation for viable mRNA therapeutics. LNPs emerged as the preferred delivery vehicle due to their ability to protect mRNA from degradation, facilitate cellular uptake, and enable endosomal escape for effective protein expression.
The COVID-19 pandemic served as a catalyst for mRNA technology, accelerating development timelines and demonstrating the platform's unprecedented speed and adaptability. The successful deployment of mRNA vaccines against SARS-CoV-2 validated decades of research and established mRNA-LNP technology as a transformative approach in modern medicine. This watershed moment has expanded the vision for mRNA applications beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disease treatments.
Current technological objectives focus on enhancing several critical aspects of mRNA-LNP delivery systems. Researchers aim to improve tissue targeting specificity to minimize off-target effects and reduce required dosages. Efforts are underway to develop LNP formulations that can effectively target tissues beyond the liver, which has traditionally been the primary destination for systemically administered LNPs. Engineering mRNA for extended half-life and controlled release profiles represents another important goal to achieve sustained therapeutic effects.
The field is also pursuing innovations in manufacturing scalability and stability, with objectives to develop room-temperature stable formulations that could revolutionize global distribution. Simultaneously, researchers are working to reduce production costs to improve accessibility, particularly in resource-limited settings. The ultimate technological vision encompasses personalized mRNA therapeutics tailored to individual genetic profiles and disease states.
As the technology continues to mature, interdisciplinary collaboration between molecular biologists, lipid chemists, pharmaceutical scientists, and clinicians will be essential to overcome remaining challenges and fully realize the transformative potential of mRNA-LNP delivery systems in healthcare.
Healthcare Market Demand for mRNA Delivery Systems
The global mRNA therapeutics market has experienced unprecedented growth following the successful deployment of mRNA-based COVID-19 vaccines, catalyzing significant interest in mRNA delivery technologies. Market analysis indicates that the global mRNA therapeutics market was valued at approximately $9.4 billion in 2022 and is projected to reach $37.8 billion by 2030, representing a compound annual growth rate of 19.2% during the forecast period.
Healthcare providers and pharmaceutical companies are increasingly recognizing the potential of mRNA-based therapies beyond vaccines, particularly in oncology, rare genetic disorders, and infectious diseases. The oncology segment currently dominates market demand, accounting for nearly 35% of the total market share, driven by the potential of mRNA therapeutics to enable personalized cancer treatments with reduced side effects compared to conventional chemotherapy.
Patient demand for less invasive and more effective treatment options has created a substantial market pull for advanced delivery systems. Traditional drug delivery methods often fail to protect mRNA from degradation or achieve targeted delivery to specific tissues, creating a critical need for innovative lipid nanoparticle (LNP) delivery technologies that can overcome these biological barriers.
Regulatory agencies worldwide have demonstrated increased receptiveness to mRNA-based therapeutics following the COVID-19 vaccine success, establishing accelerated pathways for review and approval. This regulatory environment has significantly reduced market entry barriers, further stimulating demand for effective delivery systems.
Healthcare systems are increasingly focused on cost-effective treatments that reduce hospitalization duration and improve patient outcomes. Economic analyses suggest that despite higher upfront costs, mRNA therapeutics delivered via optimized LNP systems could potentially reduce overall healthcare expenditures by preventing disease progression and minimizing complications, particularly for chronic conditions.
Geographic market analysis reveals that North America currently leads the demand for mRNA delivery systems, accounting for approximately 42% of the global market, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 23.5% during the forecast period, driven by increasing healthcare expenditure, growing awareness, and expanding research infrastructure.
Pharmaceutical companies are increasingly seeking partnerships with specialized LNP technology developers, creating a robust ecosystem of collaboration. Market research indicates that over 60% of major pharmaceutical companies have established strategic partnerships or made acquisitions related to mRNA delivery technologies in the past three years, highlighting the industry's recognition of delivery mechanisms as a critical competitive advantage.
Healthcare providers and pharmaceutical companies are increasingly recognizing the potential of mRNA-based therapies beyond vaccines, particularly in oncology, rare genetic disorders, and infectious diseases. The oncology segment currently dominates market demand, accounting for nearly 35% of the total market share, driven by the potential of mRNA therapeutics to enable personalized cancer treatments with reduced side effects compared to conventional chemotherapy.
Patient demand for less invasive and more effective treatment options has created a substantial market pull for advanced delivery systems. Traditional drug delivery methods often fail to protect mRNA from degradation or achieve targeted delivery to specific tissues, creating a critical need for innovative lipid nanoparticle (LNP) delivery technologies that can overcome these biological barriers.
Regulatory agencies worldwide have demonstrated increased receptiveness to mRNA-based therapeutics following the COVID-19 vaccine success, establishing accelerated pathways for review and approval. This regulatory environment has significantly reduced market entry barriers, further stimulating demand for effective delivery systems.
Healthcare systems are increasingly focused on cost-effective treatments that reduce hospitalization duration and improve patient outcomes. Economic analyses suggest that despite higher upfront costs, mRNA therapeutics delivered via optimized LNP systems could potentially reduce overall healthcare expenditures by preventing disease progression and minimizing complications, particularly for chronic conditions.
Geographic market analysis reveals that North America currently leads the demand for mRNA delivery systems, accounting for approximately 42% of the global market, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 23.5% during the forecast period, driven by increasing healthcare expenditure, growing awareness, and expanding research infrastructure.
Pharmaceutical companies are increasingly seeking partnerships with specialized LNP technology developers, creating a robust ecosystem of collaboration. Market research indicates that over 60% of major pharmaceutical companies have established strategic partnerships or made acquisitions related to mRNA delivery technologies in the past three years, highlighting the industry's recognition of delivery mechanisms as a critical competitive advantage.
Current Challenges in mRNA LNP Delivery Mechanisms
Despite the remarkable progress in mRNA lipid nanoparticle (LNP) technology, several significant challenges persist in the delivery mechanisms that limit their broader application in healthcare. One of the primary obstacles is the stability of mRNA molecules during storage and delivery. mRNA is inherently unstable and susceptible to degradation by ubiquitous ribonucleases, requiring sophisticated formulation strategies to maintain integrity until reaching target cells.
The immune response triggered by LNPs represents another substantial hurdle. Current LNP formulations can activate innate immune pathways, leading to inflammatory reactions and potential toxicity. This immunogenicity not only affects patient safety but also reduces therapeutic efficacy, particularly for treatments requiring repeated administration where immune memory can develop against LNP components.
Targeting specificity remains inadequate with existing delivery systems. While LNPs show natural tropism for the liver due to interactions with apolipoprotein E, achieving efficient delivery to other tissues and organs presents considerable difficulties. The inability to precisely direct mRNA therapeutics to specific cell types limits their application in treating conditions affecting tissues beyond the liver.
Endosomal escape efficiency constitutes a critical bottleneck in the delivery process. After cellular uptake via endocytosis, a significant portion of LNPs become trapped in endosomes and are ultimately degraded in lysosomes. Current formulations achieve only modest endosomal escape rates, estimated at less than 2% of internalized mRNA reaching the cytoplasm where translation occurs.
Manufacturing scalability and reproducibility pose substantial challenges for commercial viability. The complex multi-component nature of LNPs makes consistent large-scale production difficult, with batch-to-batch variations affecting critical quality attributes such as size distribution, encapsulation efficiency, and in vivo performance.
Regulatory hurdles further complicate advancement in this field. The novelty of mRNA-LNP platforms means regulatory frameworks are still evolving, creating uncertainty in development pathways. Safety concerns regarding potential off-target effects, biodistribution profiles, and long-term consequences of repeated administration require extensive characterization studies.
Cost considerations remain significant barriers to widespread adoption. Current manufacturing processes for GMP-grade mRNA and pharmaceutical-grade lipids are expensive, making treatments potentially unaffordable for many healthcare systems, particularly in low and middle-income countries where cold chain infrastructure presents additional challenges.
The immune response triggered by LNPs represents another substantial hurdle. Current LNP formulations can activate innate immune pathways, leading to inflammatory reactions and potential toxicity. This immunogenicity not only affects patient safety but also reduces therapeutic efficacy, particularly for treatments requiring repeated administration where immune memory can develop against LNP components.
Targeting specificity remains inadequate with existing delivery systems. While LNPs show natural tropism for the liver due to interactions with apolipoprotein E, achieving efficient delivery to other tissues and organs presents considerable difficulties. The inability to precisely direct mRNA therapeutics to specific cell types limits their application in treating conditions affecting tissues beyond the liver.
Endosomal escape efficiency constitutes a critical bottleneck in the delivery process. After cellular uptake via endocytosis, a significant portion of LNPs become trapped in endosomes and are ultimately degraded in lysosomes. Current formulations achieve only modest endosomal escape rates, estimated at less than 2% of internalized mRNA reaching the cytoplasm where translation occurs.
Manufacturing scalability and reproducibility pose substantial challenges for commercial viability. The complex multi-component nature of LNPs makes consistent large-scale production difficult, with batch-to-batch variations affecting critical quality attributes such as size distribution, encapsulation efficiency, and in vivo performance.
Regulatory hurdles further complicate advancement in this field. The novelty of mRNA-LNP platforms means regulatory frameworks are still evolving, creating uncertainty in development pathways. Safety concerns regarding potential off-target effects, biodistribution profiles, and long-term consequences of repeated administration require extensive characterization studies.
Cost considerations remain significant barriers to widespread adoption. Current manufacturing processes for GMP-grade mRNA and pharmaceutical-grade lipids are expensive, making treatments potentially unaffordable for many healthcare systems, particularly in low and middle-income countries where cold chain infrastructure presents additional challenges.
Established mRNA LNP Formulation Approaches
01 Lipid composition optimization for mRNA delivery
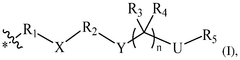
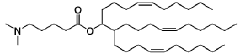
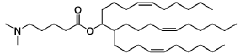
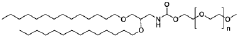
The composition of lipids in nanoparticles significantly affects mRNA delivery efficiency. Optimizing the ratio of cationic, helper, and PEG-lipids can enhance cellular uptake and endosomal escape. Specific lipid combinations can be tailored for targeting different tissues, improving transfection efficiency, and reducing cytotoxicity. These formulations typically include ionizable lipids that change charge at different pH levels to facilitate both encapsulation and release of mRNA cargo.- Lipid nanoparticle composition for mRNA delivery: Lipid nanoparticles (LNPs) can be formulated with specific lipid compositions to enhance mRNA delivery efficiency. These compositions typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios. The ionizable lipids facilitate endosomal escape, while helper lipids and cholesterol provide structural stability. PEG-lipids help prevent aggregation and extend circulation time in the bloodstream, collectively improving the delivery of mRNA to target cells.
- Endosomal escape mechanisms: Effective mRNA delivery via lipid nanoparticles relies on efficient endosomal escape mechanisms. After cellular uptake through endocytosis, LNPs must escape the endosomal compartment to release mRNA into the cytoplasm. This is typically achieved through pH-responsive ionizable lipids that become protonated in the acidic endosomal environment, disrupting the endosomal membrane. This proton sponge effect facilitates the release of mRNA cargo into the cytoplasm where translation can occur.
- Targeted delivery strategies: Targeted delivery of mRNA-loaded lipid nanoparticles can be achieved by incorporating specific ligands or surface modifications. These targeting moieties can bind to receptors that are overexpressed on target cells, enhancing cellular uptake through receptor-mediated endocytosis. Common targeting strategies include antibodies, peptides, aptamers, and small molecules that recognize specific cell surface markers, allowing for tissue-specific delivery and reducing off-target effects.
- Stability and storage of mRNA lipid nanoparticles: Maintaining stability of mRNA lipid nanoparticles during storage and administration is crucial for therapeutic efficacy. Various approaches include lyophilization (freeze-drying), cryopreservation, and formulation with stabilizing excipients. The lipid composition can be optimized to prevent aggregation and protect the mRNA cargo from degradation. Additionally, buffer systems and antioxidants may be incorporated to maintain pH stability and prevent oxidative damage, ensuring the integrity of the mRNA payload until it reaches target cells.
- Advanced manufacturing techniques for LNP production: Manufacturing techniques for producing consistent and scalable mRNA lipid nanoparticles include microfluidic mixing, ethanol injection, and continuous flow processes. These methods allow precise control over particle size, polydispersity, and encapsulation efficiency. Microfluidic devices enable rapid mixing of lipid and aqueous phases under controlled conditions, resulting in uniform nanoparticles. Process parameters such as flow rates, mixing speeds, and temperature can be optimized to achieve desired physicochemical properties and ensure batch-to-batch consistency for clinical applications.
02 Endosomal escape mechanisms
Effective mRNA delivery requires escape from endosomes after cellular uptake. Lipid nanoparticles can be designed with pH-responsive elements that disrupt endosomal membranes in the acidic environment, releasing mRNA into the cytoplasm. This process often involves ionizable lipids that become positively charged at endosomal pH, causing membrane destabilization. Advanced formulations incorporate specific helper lipids that enhance this membrane fusion and disruption process, significantly improving transfection efficiency.Expand Specific Solutions03 Surface modification for targeted delivery
Surface modifications of lipid nanoparticles enable targeted delivery to specific tissues or cells. By incorporating targeting ligands, antibodies, or peptides on the nanoparticle surface, the delivery system can recognize and bind to specific receptors on target cells. PEGylation strategies can be employed to extend circulation time and reduce non-specific uptake. These modifications can significantly improve the therapeutic index by increasing delivery to intended tissues while reducing off-target effects.Expand Specific Solutions04 Stability enhancement techniques
Stability of mRNA lipid nanoparticles is crucial for maintaining efficacy during storage and administration. Various techniques can be employed to enhance stability, including lyophilization, cryopreservation, and addition of stabilizing excipients. The lipid composition can be optimized to prevent aggregation and protect the mRNA cargo from degradation. These approaches extend shelf-life and preserve the functional integrity of the mRNA payload until it reaches its target site in the body.Expand Specific Solutions05 Novel delivery routes and formulations
Beyond traditional intravenous administration, novel delivery routes are being developed for mRNA lipid nanoparticles. These include pulmonary delivery, intradermal application, oral formulations, and mucosal delivery systems. Each route requires specific formulation adaptations to overcome biological barriers. Advanced manufacturing techniques like microfluidic mixing and specialized lipid compositions enable the creation of nanoparticles with properties tailored to specific administration routes, expanding the potential applications of mRNA therapeutics.Expand Specific Solutions
Leading Companies in mRNA LNP Development
The mRNA Lipid Nanoparticle (LNP) Delivery Mechanisms market is currently in a growth phase, with an estimated global market size exceeding $5 billion and projected to grow at a CAGR of 15-20% through 2030. The technology has reached commercial maturity for vaccines but remains in early development stages for therapeutic applications. Key players include established pharmaceutical companies like Pfizer and Moderna, who pioneered commercial mRNA vaccines, alongside emerging biotechnology firms such as Stemirna Therapeutics, Arbutus Biopharma, and ReCode Therapeutics. Academic institutions including University of North Carolina and Carnegie Mellon University contribute significant research advancements. Regional competition is intensifying with Chinese companies like Regis Biotechnology and Shanghai Regenelead Therapies rapidly developing proprietary LNP technologies to challenge Western market dominance.
Arbutus Biopharma Corp.
Technical Solution: Arbutus Biopharma has developed a proprietary LNP delivery platform specifically optimized for hepatic delivery of nucleic acids. Their technology centers around their patented ionizable lipid formulations that demonstrate excellent mRNA encapsulation efficiency and hepatocyte targeting. The LNP system employs pH-sensitive lipids that remain neutral at physiological pH but become positively charged in the acidic environment of endosomes, facilitating endosomal escape through membrane disruption[3]. Arbutus's LNPs incorporate specialized amino lipids with optimized pKa values (6.2-6.5) that enhance endosomal release while minimizing cytotoxicity. Their delivery mechanism involves ApoE-mediated targeting to hepatocytes via LDL receptor interactions, followed by clathrin-mediated endocytosis[4]. The company has refined their formulation process to create uniform nanoparticles (70-90 nm) with high encapsulation efficiency (>90%) and controlled polydispersity, which enhances circulation stability and reduces clearance rates.
Strengths: Exceptional hepatocyte targeting efficiency; established intellectual property portfolio in LNP technology; demonstrated safety profile in clinical studies. Weaknesses: Limited application beyond liver-targeted therapies; potential for lipid accumulation with repeated dosing; manufacturing complexity requiring specialized equipment.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences has developed a sophisticated LNP platform specifically engineered for diverse RNA therapeutic applications. Their technology centers around proprietary ionizable lipids with optimized structures that enhance endosomal escape while minimizing cytotoxicity. Genevant's LNP system incorporates specialized amino lipids with branched hydrocarbon chains that improve membrane fusion properties and intracellular release efficiency[7]. Their delivery mechanism involves careful control of lipid pKa values (typically 6.0-6.5) to ensure neutral surface charge in circulation but positive charge in endosomes, facilitating membrane disruption and payload release. The company has developed formulations with enhanced tissue tropism beyond traditional liver targeting, including designs for lung, spleen, and tumor delivery through surface modifications and particle engineering[8]. Genevant employs proprietary manufacturing processes that enable consistent production of LNPs with precise size control (70-100 nm) and high encapsulation efficiency (>95%), which contributes to improved pharmacokinetic profiles and reduced dose requirements.
Strengths: Versatile platform applicable across multiple RNA modalities (mRNA, siRNA, etc.); enhanced tissue targeting capabilities beyond liver; strong intellectual property position. Weaknesses: Less extensive clinical validation compared to some competitors; potential challenges in scaling manufacturing for global supply; possible immunogenicity concerns with repeated administration.
Key Patents and Breakthroughs in LNP Design
Lipid nanoparticle for targeted delivery
PatentWO2025165999A1
Innovation
- Lipid nanoparticles comprising ionizable lipids with specific structures and formulations, including cholesterol, helper lipids, and polymer conjugated lipids, are used to enhance the delivery of mRNA encoding therapeutic peptides to target organs like the liver and spleen, improving therapeutic efficacy.
Lipid nanoparticle formulations
PatentWO2020097540A1
Innovation
- The development of specific lipid nanoparticle formulations comprising nucleic acids, cholesterol, DSPC, PEG-C-DMA, and a cationic lipid, optimized in molar percentages to enhance stability and delivery efficacy, allowing for lower doses and improved therapeutic outcomes.
Regulatory Pathway for mRNA LNP Therapeutics
The regulatory landscape for mRNA Lipid Nanoparticle (LNP) therapeutics represents a complex and evolving framework that developers must navigate to bring these innovative treatments to market. Currently, regulatory agencies worldwide are adapting existing frameworks to accommodate this novel therapeutic modality, with the FDA, EMA, and PMDA leading efforts to establish clear pathways.
In the United States, the FDA evaluates mRNA LNP products primarily through the Center for Biologics Evaluation and Research (CBER), applying a risk-based approach that considers both the nucleic acid component and the delivery system. The regulatory pathway typically involves Investigational New Drug (IND) applications followed by Biologics License Applications (BLA), with accelerated approval mechanisms available for treatments addressing unmet medical needs.
European regulation through the EMA classifies most mRNA LNP therapeutics as Advanced Therapy Medicinal Products (ATMPs), requiring centralized authorization procedures. The EMA has established the Innovation Task Force (ITF) to provide scientific and regulatory guidance for novel technologies, offering developers opportunities for early dialogue through scientific advice meetings and the PRIority MEdicines (PRIME) scheme for promising therapies.
Regulatory considerations specific to mRNA LNP therapeutics include comprehensive characterization of both the mRNA component and the lipid nanoparticle delivery system. Authorities require detailed analysis of LNP composition, size distribution, encapsulation efficiency, and stability. Safety assessments must address potential immunogenicity concerns, biodistribution profiles, and the metabolic fate of lipid components.
Manufacturing consistency presents a significant regulatory challenge, with agencies demanding robust chemistry, manufacturing, and controls (CMC) documentation. Process analytical technology (PAT) implementation is increasingly expected to demonstrate consistent quality attributes across production batches. Regulatory bodies also emphasize the importance of validated analytical methods specific to mRNA LNP characterization.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to standardize requirements across jurisdictions. However, significant regional differences persist, necessitating tailored regulatory strategies for global development programs. Emerging markets are developing their own frameworks, often adapting guidelines from established regulatory authorities while incorporating country-specific requirements.
The accelerated development timeline of COVID-19 mRNA vaccines has established important regulatory precedents, including the use of platform approaches where certain aspects of the delivery system may support abbreviated development pathways for subsequent products using similar LNP formulations.
In the United States, the FDA evaluates mRNA LNP products primarily through the Center for Biologics Evaluation and Research (CBER), applying a risk-based approach that considers both the nucleic acid component and the delivery system. The regulatory pathway typically involves Investigational New Drug (IND) applications followed by Biologics License Applications (BLA), with accelerated approval mechanisms available for treatments addressing unmet medical needs.
European regulation through the EMA classifies most mRNA LNP therapeutics as Advanced Therapy Medicinal Products (ATMPs), requiring centralized authorization procedures. The EMA has established the Innovation Task Force (ITF) to provide scientific and regulatory guidance for novel technologies, offering developers opportunities for early dialogue through scientific advice meetings and the PRIority MEdicines (PRIME) scheme for promising therapies.
Regulatory considerations specific to mRNA LNP therapeutics include comprehensive characterization of both the mRNA component and the lipid nanoparticle delivery system. Authorities require detailed analysis of LNP composition, size distribution, encapsulation efficiency, and stability. Safety assessments must address potential immunogenicity concerns, biodistribution profiles, and the metabolic fate of lipid components.
Manufacturing consistency presents a significant regulatory challenge, with agencies demanding robust chemistry, manufacturing, and controls (CMC) documentation. Process analytical technology (PAT) implementation is increasingly expected to demonstrate consistent quality attributes across production batches. Regulatory bodies also emphasize the importance of validated analytical methods specific to mRNA LNP characterization.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to standardize requirements across jurisdictions. However, significant regional differences persist, necessitating tailored regulatory strategies for global development programs. Emerging markets are developing their own frameworks, often adapting guidelines from established regulatory authorities while incorporating country-specific requirements.
The accelerated development timeline of COVID-19 mRNA vaccines has established important regulatory precedents, including the use of platform approaches where certain aspects of the delivery system may support abbreviated development pathways for subsequent products using similar LNP formulations.
Manufacturing Scalability and Quality Control
The scalability of mRNA lipid nanoparticle (LNP) manufacturing represents a critical challenge in translating laboratory discoveries into commercially viable healthcare solutions. Current manufacturing processes for mRNA LNPs involve complex multi-step procedures including mRNA synthesis, lipid formulation, and nanoparticle assembly. These processes require precise control of parameters such as temperature, pH, and mixing conditions, making large-scale production technically demanding.
Industry analysis reveals that batch-to-batch consistency remains a significant hurdle, with variations in particle size distribution, encapsulation efficiency, and lipid composition potentially affecting therapeutic efficacy. The COVID-19 pandemic highlighted these challenges when manufacturers needed to rapidly scale production from laboratory to global distribution levels, exposing bottlenecks in raw material supply chains and specialized equipment availability.
Quality control systems for mRNA LNPs require sophisticated analytical methods to ensure product integrity. These include dynamic light scattering for particle size determination, zeta potential measurements for surface charge characterization, and advanced chromatography techniques for lipid composition analysis. RNA integrity assessment through gel electrophoresis and next-generation sequencing has become standard practice, though these methods are time-consuming and difficult to implement in continuous manufacturing environments.
Regulatory frameworks for mRNA LNP manufacturing are still evolving, with agencies like the FDA and EMA developing specific guidelines for this novel therapeutic modality. This regulatory uncertainty adds complexity to manufacturing scale-up efforts, as companies must anticipate future requirements while designing production facilities.
Recent technological innovations are addressing these challenges through continuous manufacturing approaches, microfluidic systems for precise nanoparticle formation, and automated quality control processes. Companies like Precision NanoSystems and Evonik have developed specialized equipment designed specifically for large-scale LNP production with integrated quality monitoring capabilities.
Economic analysis indicates that manufacturing costs remain high, with specialized lipids representing a significant portion of production expenses. Process optimization efforts are focusing on improving lipid utilization efficiency and developing synthetic routes for cost-effective lipid production. Additionally, cold chain requirements for mRNA LNP products add substantial complexity and cost to the distribution infrastructure, driving research into thermostable formulations that could alleviate these constraints.
Industry analysis reveals that batch-to-batch consistency remains a significant hurdle, with variations in particle size distribution, encapsulation efficiency, and lipid composition potentially affecting therapeutic efficacy. The COVID-19 pandemic highlighted these challenges when manufacturers needed to rapidly scale production from laboratory to global distribution levels, exposing bottlenecks in raw material supply chains and specialized equipment availability.
Quality control systems for mRNA LNPs require sophisticated analytical methods to ensure product integrity. These include dynamic light scattering for particle size determination, zeta potential measurements for surface charge characterization, and advanced chromatography techniques for lipid composition analysis. RNA integrity assessment through gel electrophoresis and next-generation sequencing has become standard practice, though these methods are time-consuming and difficult to implement in continuous manufacturing environments.
Regulatory frameworks for mRNA LNP manufacturing are still evolving, with agencies like the FDA and EMA developing specific guidelines for this novel therapeutic modality. This regulatory uncertainty adds complexity to manufacturing scale-up efforts, as companies must anticipate future requirements while designing production facilities.
Recent technological innovations are addressing these challenges through continuous manufacturing approaches, microfluidic systems for precise nanoparticle formation, and automated quality control processes. Companies like Precision NanoSystems and Evonik have developed specialized equipment designed specifically for large-scale LNP production with integrated quality monitoring capabilities.
Economic analysis indicates that manufacturing costs remain high, with specialized lipids representing a significant portion of production expenses. Process optimization efforts are focusing on improving lipid utilization efficiency and developing synthetic routes for cost-effective lipid production. Additionally, cold chain requirements for mRNA LNP products add substantial complexity and cost to the distribution infrastructure, driving research into thermostable formulations that could alleviate these constraints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!