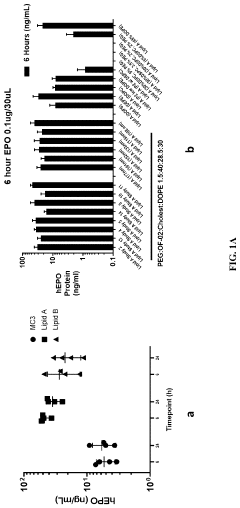
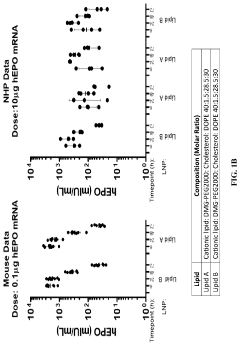
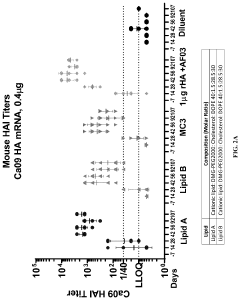
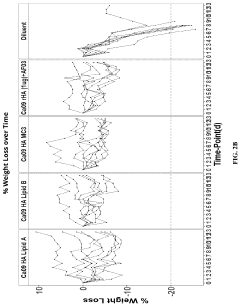
Why mRNA Lipid Nanoparticles Outperform Traditional Methods
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Evolution and Objectives
The evolution of mRNA therapeutics represents one of the most significant breakthroughs in modern medicine, with lipid nanoparticles (LNPs) emerging as the critical delivery vehicle that has transformed theoretical potential into clinical reality. The journey of mRNA technology began in the 1960s with the discovery of mRNA's role in protein synthesis, but for decades remained primarily a laboratory curiosity due to inherent stability issues and delivery challenges.
The technological trajectory shifted dramatically in the early 2000s when researchers began exploring lipid-based delivery systems to protect mRNA from rapid degradation in the bloodstream. This period marked the transition from conceptual understanding to practical application, as scientists recognized that encapsulating mRNA within lipid nanoparticles could shield it from enzymatic degradation while facilitating cellular uptake.
By 2010, pioneering work by companies like Moderna and BioNTech had established foundational LNP formulation techniques, incorporating ionizable lipids, helper phospholipids, cholesterol, and PEG-lipids in precise ratios. These advancements addressed the fundamental challenge of mRNA therapeutics: delivering intact genetic material into cells without triggering immune rejection of the carrier itself.
The COVID-19 pandemic accelerated this evolution exponentially, compressing what might have been a decade of incremental progress into less than a year. The unprecedented efficacy of mRNA-LNP vaccines demonstrated not only the platform's viability but its superiority over traditional vaccine approaches in terms of development speed, manufacturing scalability, and adaptability to emerging variants.
Current technological objectives focus on several key areas: enhancing delivery efficiency to specific tissues beyond the liver (where LNPs naturally accumulate), reducing cold-chain requirements through improved formulation stability, minimizing immunogenicity of the LNP components, and developing controlled-release mechanisms for sustained therapeutic effect.
Looking forward, the field aims to develop tissue-specific targeting capabilities through surface modifications of LNPs, enabling applications beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments. Researchers are also pursuing optimization of the mRNA construct itself, with modified nucleosides and optimized coding sequences to enhance protein expression while reducing inflammatory responses.
The convergence of these technological advancements positions mRNA-LNP platforms to potentially revolutionize treatment paradigms across multiple disease categories, with the ultimate objective of creating programmable medicines that can instruct cells to produce therapeutic proteins on demand, effectively turning the body's own cellular machinery into a drug manufacturing facility.
The technological trajectory shifted dramatically in the early 2000s when researchers began exploring lipid-based delivery systems to protect mRNA from rapid degradation in the bloodstream. This period marked the transition from conceptual understanding to practical application, as scientists recognized that encapsulating mRNA within lipid nanoparticles could shield it from enzymatic degradation while facilitating cellular uptake.
By 2010, pioneering work by companies like Moderna and BioNTech had established foundational LNP formulation techniques, incorporating ionizable lipids, helper phospholipids, cholesterol, and PEG-lipids in precise ratios. These advancements addressed the fundamental challenge of mRNA therapeutics: delivering intact genetic material into cells without triggering immune rejection of the carrier itself.
The COVID-19 pandemic accelerated this evolution exponentially, compressing what might have been a decade of incremental progress into less than a year. The unprecedented efficacy of mRNA-LNP vaccines demonstrated not only the platform's viability but its superiority over traditional vaccine approaches in terms of development speed, manufacturing scalability, and adaptability to emerging variants.
Current technological objectives focus on several key areas: enhancing delivery efficiency to specific tissues beyond the liver (where LNPs naturally accumulate), reducing cold-chain requirements through improved formulation stability, minimizing immunogenicity of the LNP components, and developing controlled-release mechanisms for sustained therapeutic effect.
Looking forward, the field aims to develop tissue-specific targeting capabilities through surface modifications of LNPs, enabling applications beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments. Researchers are also pursuing optimization of the mRNA construct itself, with modified nucleosides and optimized coding sequences to enhance protein expression while reducing inflammatory responses.
The convergence of these technological advancements positions mRNA-LNP platforms to potentially revolutionize treatment paradigms across multiple disease categories, with the ultimate objective of creating programmable medicines that can instruct cells to produce therapeutic proteins on demand, effectively turning the body's own cellular machinery into a drug manufacturing facility.
Market Analysis for mRNA Therapeutics and Vaccines
The global mRNA therapeutics and vaccines market has experienced unprecedented growth following the successful deployment of COVID-19 mRNA vaccines. Current market valuations indicate the sector reached approximately $40 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 12-15% through 2030, potentially reaching $100 billion by the end of the decade.
The market landscape can be segmented into therapeutic applications and prophylactic vaccines, with the latter currently dominating revenue share due to the massive distribution of COVID-19 vaccines. However, therapeutic applications for cancer, rare genetic disorders, and infectious diseases represent the fastest-growing segment, with projected CAGR exceeding 18% over the next five years.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 15%. The remaining 10% is distributed across other regions. This distribution reflects not only the concentration of major pharmaceutical companies and research institutions but also regulatory environments that have adapted quickly to novel mRNA technologies.
Key market drivers include the demonstrated efficacy of mRNA vaccines during the pandemic, significant advantages in development speed compared to traditional vaccine platforms, and the versatility of mRNA technology for addressing multiple disease targets. The ability to rapidly iterate formulations represents a particularly valuable feature in responding to emerging pathogens or cancer neoantigens.
Investment trends reveal substantial capital inflows, with venture capital funding for mRNA-focused startups exceeding $6 billion since 2020. Major pharmaceutical companies have also redirected R&D budgets toward mRNA platforms, with several establishing dedicated mRNA research centers.
Market challenges include cold chain requirements for current formulations, manufacturing scalability issues, and cost barriers for widespread adoption in lower-income markets. However, ongoing innovations in lipid nanoparticle formulations are progressively addressing stability concerns, potentially expanding market reach.
Consumer acceptance has evolved favorably following the pandemic, with surveys indicating approximately 70% of healthcare providers now consider mRNA platforms as preferred options for certain indications. This represents a significant shift from pre-pandemic perceptions and suggests strong market receptivity for future mRNA products.
Regulatory pathways have been streamlined in major markets, with the FDA, EMA, and other authorities establishing specialized review processes for mRNA-based therapeutics, further accelerating market entry potential for pipeline candidates.
The market landscape can be segmented into therapeutic applications and prophylactic vaccines, with the latter currently dominating revenue share due to the massive distribution of COVID-19 vaccines. However, therapeutic applications for cancer, rare genetic disorders, and infectious diseases represent the fastest-growing segment, with projected CAGR exceeding 18% over the next five years.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 15%. The remaining 10% is distributed across other regions. This distribution reflects not only the concentration of major pharmaceutical companies and research institutions but also regulatory environments that have adapted quickly to novel mRNA technologies.
Key market drivers include the demonstrated efficacy of mRNA vaccines during the pandemic, significant advantages in development speed compared to traditional vaccine platforms, and the versatility of mRNA technology for addressing multiple disease targets. The ability to rapidly iterate formulations represents a particularly valuable feature in responding to emerging pathogens or cancer neoantigens.
Investment trends reveal substantial capital inflows, with venture capital funding for mRNA-focused startups exceeding $6 billion since 2020. Major pharmaceutical companies have also redirected R&D budgets toward mRNA platforms, with several establishing dedicated mRNA research centers.
Market challenges include cold chain requirements for current formulations, manufacturing scalability issues, and cost barriers for widespread adoption in lower-income markets. However, ongoing innovations in lipid nanoparticle formulations are progressively addressing stability concerns, potentially expanding market reach.
Consumer acceptance has evolved favorably following the pandemic, with surveys indicating approximately 70% of healthcare providers now consider mRNA platforms as preferred options for certain indications. This represents a significant shift from pre-pandemic perceptions and suggests strong market receptivity for future mRNA products.
Regulatory pathways have been streamlined in major markets, with the FDA, EMA, and other authorities establishing specialized review processes for mRNA-based therapeutics, further accelerating market entry potential for pipeline candidates.
Current Landscape and Barriers in mRNA Delivery
The mRNA delivery landscape has undergone significant transformation in recent years, with lipid nanoparticles (LNPs) emerging as the dominant delivery vehicle. Currently, several key players dominate the mRNA-LNP market, including Moderna, BioNTech/Pfizer, CureVac, and Translate Bio, who have successfully leveraged this technology for COVID-19 vaccines and are expanding into other therapeutic areas.
Despite these advancements, significant barriers persist in mRNA delivery. The primary challenge remains the inherent instability of mRNA molecules, which are highly susceptible to degradation by ubiquitous ribonucleases. This instability necessitates sophisticated delivery systems and careful handling throughout manufacturing, storage, and administration processes.
Another major obstacle is the efficient cellular uptake of mRNA. The large size and negative charge of mRNA molecules prevent passive diffusion across cell membranes, requiring specialized delivery vehicles. While LNPs have proven effective, optimizing their composition for specific tissue targeting remains challenging, particularly for tissues beyond the liver, which naturally accumulates lipid-based particles.
Immunogenicity presents a dual challenge in mRNA delivery. While beneficial for vaccine applications, the immune response triggered by foreign mRNA can be detrimental for therapeutic applications, potentially leading to reduced efficacy and safety concerns. Modifications to the mRNA structure and careful LNP design are necessary to modulate these immune responses appropriately.
Distribution and targeting specificity represent another significant barrier. Current LNP formulations predominantly accumulate in the liver following systemic administration, limiting their utility for treating conditions affecting other organs and tissues. Developing LNPs with tissue-specific targeting capabilities remains an active area of research but has yet to yield broadly applicable solutions.
Manufacturing scalability and cost considerations also pose substantial challenges. The production of GMP-grade mRNA and the formulation of LNPs involve complex processes that are difficult to scale while maintaining consistency and quality. These manufacturing challenges contribute to the high cost of mRNA therapeutics, potentially limiting accessibility.
Regulatory pathways for mRNA-LNP products are still evolving, with regulatory agencies developing frameworks to assess these novel therapeutics. While COVID-19 vaccines have established precedents, the approval pathway for non-vaccine applications remains less defined, creating uncertainty for developers.
Storage and stability requirements present practical barriers to widespread adoption. Many current mRNA-LNP formulations require ultra-cold storage conditions (-70°C for some COVID-19 vaccines), creating logistical challenges for distribution, especially in resource-limited settings. Developing thermostable formulations remains a critical research priority.
Despite these advancements, significant barriers persist in mRNA delivery. The primary challenge remains the inherent instability of mRNA molecules, which are highly susceptible to degradation by ubiquitous ribonucleases. This instability necessitates sophisticated delivery systems and careful handling throughout manufacturing, storage, and administration processes.
Another major obstacle is the efficient cellular uptake of mRNA. The large size and negative charge of mRNA molecules prevent passive diffusion across cell membranes, requiring specialized delivery vehicles. While LNPs have proven effective, optimizing their composition for specific tissue targeting remains challenging, particularly for tissues beyond the liver, which naturally accumulates lipid-based particles.
Immunogenicity presents a dual challenge in mRNA delivery. While beneficial for vaccine applications, the immune response triggered by foreign mRNA can be detrimental for therapeutic applications, potentially leading to reduced efficacy and safety concerns. Modifications to the mRNA structure and careful LNP design are necessary to modulate these immune responses appropriately.
Distribution and targeting specificity represent another significant barrier. Current LNP formulations predominantly accumulate in the liver following systemic administration, limiting their utility for treating conditions affecting other organs and tissues. Developing LNPs with tissue-specific targeting capabilities remains an active area of research but has yet to yield broadly applicable solutions.
Manufacturing scalability and cost considerations also pose substantial challenges. The production of GMP-grade mRNA and the formulation of LNPs involve complex processes that are difficult to scale while maintaining consistency and quality. These manufacturing challenges contribute to the high cost of mRNA therapeutics, potentially limiting accessibility.
Regulatory pathways for mRNA-LNP products are still evolving, with regulatory agencies developing frameworks to assess these novel therapeutics. While COVID-19 vaccines have established precedents, the approval pathway for non-vaccine applications remains less defined, creating uncertainty for developers.
Storage and stability requirements present practical barriers to widespread adoption. Many current mRNA-LNP formulations require ultra-cold storage conditions (-70°C for some COVID-19 vaccines), creating logistical challenges for distribution, especially in resource-limited settings. Developing thermostable formulations remains a critical research priority.
Comparative Analysis of mRNA Delivery Platforms
01 Enhanced mRNA delivery efficiency
Lipid nanoparticles (LNPs) significantly improve the delivery efficiency of mRNA to target cells compared to traditional delivery methods. The specialized lipid composition creates a protective environment for the mRNA cargo, preventing degradation by nucleases and enhancing cellular uptake through endocytosis. This results in higher transfection rates and more effective protein expression, making LNPs particularly valuable for therapeutic applications where efficient delivery is crucial.- Enhanced delivery efficiency and cellular uptake: Lipid nanoparticles (LNPs) significantly improve the delivery efficiency of mRNA therapeutics by facilitating cellular uptake through endocytosis. The optimized lipid composition enables fusion with cell membranes, allowing for efficient cytoplasmic release of mRNA cargo. This enhanced delivery system overcomes biological barriers and protects mRNA from degradation, resulting in higher transfection rates compared to conventional delivery methods.
- Improved stability and extended half-life: mRNA lipid nanoparticles demonstrate superior stability in biological environments, protecting the encapsulated mRNA from nuclease degradation. The lipid envelope creates a protective barrier that extends the circulation half-life of the therapeutic payload. This improved stability allows for more predictable pharmacokinetics and enables lower dosing requirements while maintaining therapeutic efficacy, reducing potential side effects and manufacturing costs.
- Targeted delivery and reduced off-target effects: Advanced lipid nanoparticle formulations can be engineered with specific targeting ligands to enhance delivery to desired tissues or cell types. This targeted approach minimizes off-target effects and improves the therapeutic index of mRNA-based treatments. The ability to direct the delivery to specific organs or cell populations increases efficacy while reducing systemic exposure and potential toxicity, making treatments safer and more effective.
- Tunable immunogenicity and adjuvant properties: The composition of lipid nanoparticles can be precisely engineered to either enhance or suppress immune responses, depending on the therapeutic application. For vaccines, certain lipid components can act as built-in adjuvants, stimulating stronger immune responses. Conversely, for gene therapy applications, LNPs can be designed to minimize immunogenicity. This tunability provides versatility across different therapeutic contexts and patient populations.
- Scalable manufacturing and storage stability: mRNA lipid nanoparticles offer significant advantages in terms of manufacturing scalability and storage stability. Advanced formulation techniques allow for consistent production of uniform nanoparticles with reproducible characteristics. Recent innovations have improved freeze-thaw stability and extended shelf-life at various temperature conditions, addressing key logistical challenges in global distribution. These manufacturing and stability improvements have been crucial for the rapid deployment of mRNA vaccines and therapeutics.
02 Improved stability and extended shelf-life
mRNA encapsulated in lipid nanoparticles demonstrates significantly enhanced stability compared to naked mRNA. The lipid shell protects the mRNA from environmental factors and enzymatic degradation, resulting in extended shelf-life and maintained therapeutic potency during storage and administration. This stability advantage enables practical clinical applications by allowing for more flexible handling, transportation, and storage conditions without compromising the integrity of the mRNA payload.Expand Specific Solutions03 Targeted delivery and reduced off-target effects
Lipid nanoparticles can be engineered with specific surface modifications to enhance targeted delivery to particular tissues or cell types. This targeting capability reduces off-target effects and lowers the required therapeutic dose. By incorporating targeting ligands or modifying the lipid composition, LNPs can preferentially accumulate in desired tissues such as liver, lungs, or tumors, improving therapeutic efficacy while minimizing potential side effects in non-target tissues.Expand Specific Solutions04 Customizable composition for optimized performance
The composition of lipid nanoparticles can be precisely tailored to optimize performance for specific applications. By adjusting the ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids, researchers can fine-tune properties such as particle size, surface charge, encapsulation efficiency, and release kinetics. This customizability allows for the development of application-specific LNP formulations that maximize therapeutic efficacy while addressing particular delivery challenges for different mRNA cargoes and target tissues.Expand Specific Solutions05 Reduced immunogenicity and enhanced safety profile
Advanced lipid nanoparticle formulations demonstrate reduced immunogenicity compared to earlier delivery systems, resulting in an improved safety profile. By carefully selecting lipid components and optimizing the manufacturing process, modern LNPs can minimize activation of the innate immune system while still effectively delivering mRNA. This reduced immunogenicity allows for repeat dosing with decreased risk of adverse reactions, making LNP-mRNA therapeutics suitable for chronic conditions requiring multiple administrations.Expand Specific Solutions
Leading Companies and Research Institutions in mRNA LNP Field
The mRNA lipid nanoparticle (LNP) technology market is currently in a rapid growth phase, with an estimated market size exceeding $5 billion and projected to grow at over 25% annually through 2030. The technology has reached commercial maturity in vaccines but remains in early development stages for therapeutics. Key players dominating this competitive landscape include established pharmaceutical companies like Sanofi and AstraZeneca alongside specialized biotechnology firms such as Acuitas Therapeutics, whose LNP delivery system is used in Pfizer/BioNTech's COVID-19 vaccine. Academic institutions including Tsinghua University, University of Pennsylvania, and Cornell University are driving fundamental research, while companies like Translate Bio, GreenLight Biosciences, and Arbutus Biopharma are advancing proprietary LNP formulations that offer superior cellular delivery, stability, and reduced immunogenicity compared to traditional delivery methods.
Translate Bio, Inc.
Technical Solution: Translate Bio has developed a proprietary mRNA Therapeutic (MRT) platform that leverages advanced lipid nanoparticle (LNP) technology for delivering mRNA therapeutics. Their LNP formulations incorporate specially designed ionizable lipids that facilitate efficient mRNA encapsulation and cellular uptake. The company's technology features a multi-component system with optimized helper lipids, cholesterol, and PEGylated lipids that work synergistically to protect mRNA from degradation and enhance delivery efficiency. Translate Bio's LNPs demonstrate high encapsulation efficiency (>90%) and controlled particle size distribution (80-100 nm), which contributes to improved pharmacokinetic profiles. A distinguishing feature of their platform is the development of LNPs specifically engineered for pulmonary delivery, allowing direct administration to the lungs for respiratory diseases. Their manufacturing process has been scaled to support clinical development with consistent batch-to-batch reproducibility, addressing one of the key challenges in LNP production.
Strengths: Specialized expertise in pulmonary delivery of mRNA therapeutics, established manufacturing capabilities for clinical-grade materials, and strategic partnership with Sanofi enhancing development resources. Weaknesses: Relatively narrower clinical experience compared to some competitors and potential challenges with delivery to tissues beyond lung and liver.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences has developed advanced Lipid Nanoparticle (LNP) technology specifically engineered for mRNA delivery. Their proprietary LNP platform incorporates novel ionizable lipids with optimized pKa values (typically between 6.2-6.8) that enable efficient mRNA encapsulation at acidic pH during formulation while facilitating release in the slightly acidic environment of endosomes. Genevant's LNPs feature a sophisticated multi-component system including proprietary ionizable lipids, helper lipids, cholesterol, and PEG-lipids in precise ratios that enhance stability and delivery efficiency. Their technology achieves high encapsulation efficiency (>95%) while maintaining narrow particle size distribution (70-100 nm). A key innovation in their platform is the development of lipids that promote endosomal escape through membrane fusion mechanisms, addressing one of the critical barriers to effective mRNA delivery. Genevant has also engineered their LNPs to reduce immunogenicity while maintaining high transfection rates in target tissues.
Strengths: Extensive LNP intellectual property portfolio derived from Arbutus Biopharma legacy, demonstrated clinical validation through partnerships with multiple pharmaceutical companies, and versatile platform adaptable to various RNA modalities. Weaknesses: Potential challenges with tissue-specific targeting beyond liver applications and possible manufacturing complexity for large-scale production.
Key Innovations in Lipid Nanoparticle Design
Method for manufacturing lipid nanoparticles for mRNA delivery to increase mRNA delivery efficiency
PatentPendingKR1020230130538A
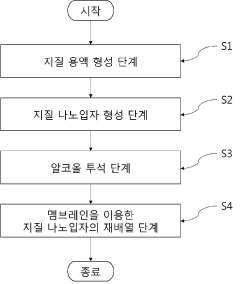
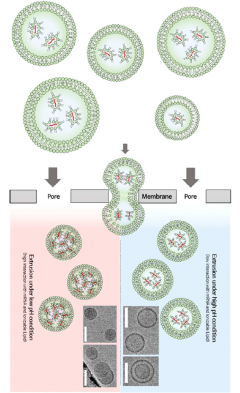
Innovation
- A method for producing lipid nanoparticles by combining ionizable mRNA with cationic lipids under acidic conditions, forming liposomes, and rearranging them through a membrane with micropores to reduce size and improve uniformity, using existing equipment.
LIPID NANOPARTICLES FOR DELIVERING mRNA VACCINES
PatentPendingUS20240148651A1
Innovation
- A pharmaceutical composition comprising nucleic acid molecules encapsulated in lipid nanoparticles (LNPs) with specific lipid ratios, including a cationic lipid, polyethylene glycol (PEG) conjugated lipid, cholesterol-based lipid, and helper lipid, which facilitates improved mRNA delivery and stability, enabling efficient immune response induction.
Regulatory Pathway for mRNA LNP Therapeutics
The regulatory landscape for mRNA Lipid Nanoparticle (LNP) therapeutics represents a complex and evolving framework that significantly impacts their development and commercialization. Unlike traditional drug delivery systems, mRNA LNPs face unique regulatory considerations due to their novel composition and mechanism of action, requiring specialized pathways for approval.
The FDA and EMA have established specific guidelines for advanced therapy medicinal products (ATMPs), under which mRNA LNP therapeutics are typically classified. These guidelines emphasize comprehensive characterization of both the mRNA component and the lipid nanoparticle delivery system, requiring developers to demonstrate consistent manufacturing processes and product stability.
Safety assessment for mRNA LNPs follows a multi-tiered approach, with particular attention to potential immunogenicity, biodistribution patterns, and cellular uptake mechanisms. Regulatory bodies require extensive non-clinical studies to evaluate these parameters before human trials can commence, often including specialized toxicology studies that address the unique properties of these nanomedicines.
Clinical development pathways for mRNA LNP therapeutics typically follow accelerated timelines compared to traditional pharmaceuticals, as evidenced by the rapid authorization of COVID-19 mRNA vaccines. This acceleration is supported by regulatory frameworks such as the FDA's Fast Track, Breakthrough Therapy, and Emergency Use Authorization provisions, which have proven crucial for addressing urgent public health needs.
Manufacturing considerations present significant regulatory challenges, as production processes must demonstrate consistent particle size distribution, encapsulation efficiency, and sterility. Current Good Manufacturing Practice (cGMP) requirements for mRNA LNPs are particularly stringent, focusing on process validation and analytical method development to ensure batch-to-batch consistency.
Post-market surveillance requirements for mRNA LNP products are typically more extensive than for conventional therapeutics, with regulatory agencies mandating robust pharmacovigilance plans to monitor long-term safety profiles. This reflects the relatively limited long-term clinical experience with this therapeutic modality.
International harmonization efforts are underway to standardize regulatory approaches to mRNA LNP therapeutics across major markets, though significant regional differences persist. The International Council for Harmonisation (ICH) has initiated discussions on developing specific guidelines for nanomedicines, which would include mRNA LNPs, aiming to reduce regulatory divergence and facilitate global development programs.
The FDA and EMA have established specific guidelines for advanced therapy medicinal products (ATMPs), under which mRNA LNP therapeutics are typically classified. These guidelines emphasize comprehensive characterization of both the mRNA component and the lipid nanoparticle delivery system, requiring developers to demonstrate consistent manufacturing processes and product stability.
Safety assessment for mRNA LNPs follows a multi-tiered approach, with particular attention to potential immunogenicity, biodistribution patterns, and cellular uptake mechanisms. Regulatory bodies require extensive non-clinical studies to evaluate these parameters before human trials can commence, often including specialized toxicology studies that address the unique properties of these nanomedicines.
Clinical development pathways for mRNA LNP therapeutics typically follow accelerated timelines compared to traditional pharmaceuticals, as evidenced by the rapid authorization of COVID-19 mRNA vaccines. This acceleration is supported by regulatory frameworks such as the FDA's Fast Track, Breakthrough Therapy, and Emergency Use Authorization provisions, which have proven crucial for addressing urgent public health needs.
Manufacturing considerations present significant regulatory challenges, as production processes must demonstrate consistent particle size distribution, encapsulation efficiency, and sterility. Current Good Manufacturing Practice (cGMP) requirements for mRNA LNPs are particularly stringent, focusing on process validation and analytical method development to ensure batch-to-batch consistency.
Post-market surveillance requirements for mRNA LNP products are typically more extensive than for conventional therapeutics, with regulatory agencies mandating robust pharmacovigilance plans to monitor long-term safety profiles. This reflects the relatively limited long-term clinical experience with this therapeutic modality.
International harmonization efforts are underway to standardize regulatory approaches to mRNA LNP therapeutics across major markets, though significant regional differences persist. The International Council for Harmonisation (ICH) has initiated discussions on developing specific guidelines for nanomedicines, which would include mRNA LNPs, aiming to reduce regulatory divergence and facilitate global development programs.
Manufacturing Scalability and Cost Considerations
The scalability of mRNA lipid nanoparticle (LNP) manufacturing represents a significant advantage over traditional vaccine production methods. While conventional vaccine manufacturing often requires extensive biological systems like egg-based or cell culture platforms that demand months of lead time, mRNA-LNP production follows a more streamlined chemical synthesis approach. This fundamental difference enables rapid scale-up capabilities that were dramatically demonstrated during the COVID-19 pandemic, when companies like Moderna and BioNTech/Pfizer achieved unprecedented manufacturing acceleration.
From a cost perspective, mRNA-LNP production benefits from several economic advantages. The manufacturing process requires smaller physical footprints compared to traditional bioreactors, with production facilities potentially 1/3 to 1/2 the size of conventional vaccine plants. Additionally, the modular nature of mRNA-LNP manufacturing allows for standardized production platforms that can be replicated globally with minimal adaptation, regardless of the specific mRNA sequence being produced.
Raw material considerations also favor mRNA-LNP approaches. While specialized lipid components initially presented supply chain challenges, the industry has rapidly expanded production capacity for these critical ingredients. The synthetic nature of these components means they can be manufactured through well-established chemical processes rather than biological systems that are susceptible to contamination and variability.
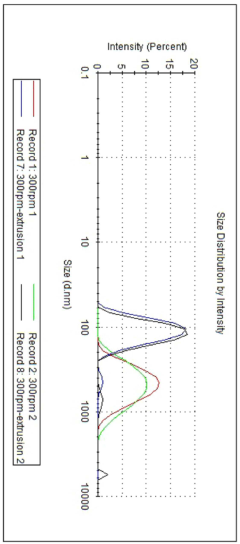
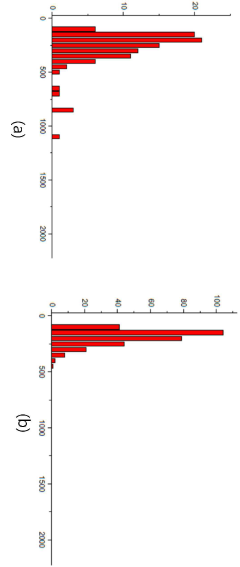
Process efficiency continues to improve as the technology matures. Early mRNA-LNP manufacturing suffered from relatively low yields (30-40%) during the lipid nanoparticle formation step, but recent advances in microfluidic mixing technologies have increased yields to 70-80% in optimized systems. These improvements directly translate to cost reductions and enhanced production capacity without expanding physical infrastructure.
Regulatory frameworks are evolving to accommodate these manufacturing approaches. The well-defined chemical nature of mRNA-LNP production potentially allows for more streamlined quality control processes compared to biological systems. Analytical methods for characterizing LNPs continue to advance, enabling more efficient batch release procedures that reduce manufacturing cycle times and associated costs.
Future cost trajectories appear favorable as the technology matures. Current estimates suggest mRNA vaccine doses cost between $15-30 to produce at scale, but industry analysts project this could decrease by 40-60% over the next five years as manufacturing processes optimize and economies of scale expand. This trajectory positions mRNA-LNP technology to become increasingly competitive with traditional vaccine approaches across a broader range of applications beyond emergency pandemic response.
From a cost perspective, mRNA-LNP production benefits from several economic advantages. The manufacturing process requires smaller physical footprints compared to traditional bioreactors, with production facilities potentially 1/3 to 1/2 the size of conventional vaccine plants. Additionally, the modular nature of mRNA-LNP manufacturing allows for standardized production platforms that can be replicated globally with minimal adaptation, regardless of the specific mRNA sequence being produced.
Raw material considerations also favor mRNA-LNP approaches. While specialized lipid components initially presented supply chain challenges, the industry has rapidly expanded production capacity for these critical ingredients. The synthetic nature of these components means they can be manufactured through well-established chemical processes rather than biological systems that are susceptible to contamination and variability.
Process efficiency continues to improve as the technology matures. Early mRNA-LNP manufacturing suffered from relatively low yields (30-40%) during the lipid nanoparticle formation step, but recent advances in microfluidic mixing technologies have increased yields to 70-80% in optimized systems. These improvements directly translate to cost reductions and enhanced production capacity without expanding physical infrastructure.
Regulatory frameworks are evolving to accommodate these manufacturing approaches. The well-defined chemical nature of mRNA-LNP production potentially allows for more streamlined quality control processes compared to biological systems. Analytical methods for characterizing LNPs continue to advance, enabling more efficient batch release procedures that reduce manufacturing cycle times and associated costs.
Future cost trajectories appear favorable as the technology matures. Current estimates suggest mRNA vaccine doses cost between $15-30 to produce at scale, but industry analysts project this could decrease by 40-60% over the next five years as manufacturing processes optimize and economies of scale expand. This trajectory positions mRNA-LNP technology to become increasingly competitive with traditional vaccine approaches across a broader range of applications beyond emergency pandemic response.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!