Salts of isoxazole derivatives
A technology of isoxazole and isopropyl, applied in the field of medicinal chemistry, can solve problems such as adverse reactions and unsatisfactory therapeutic effects, and achieve the effect of practical therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
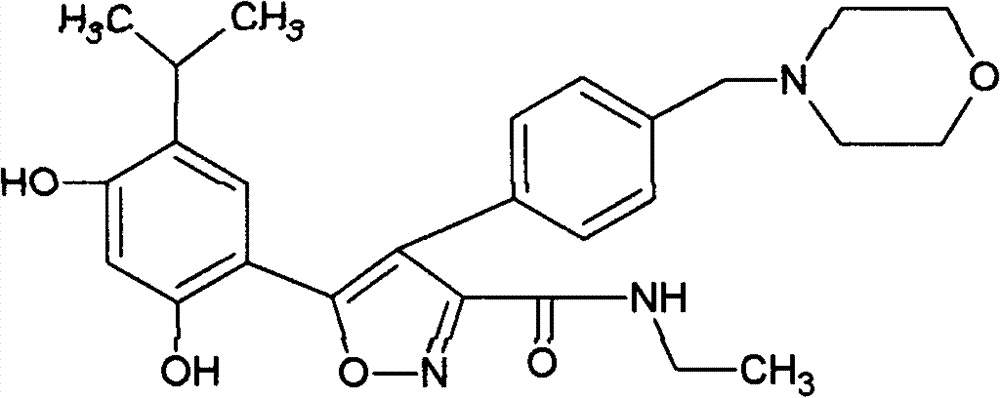
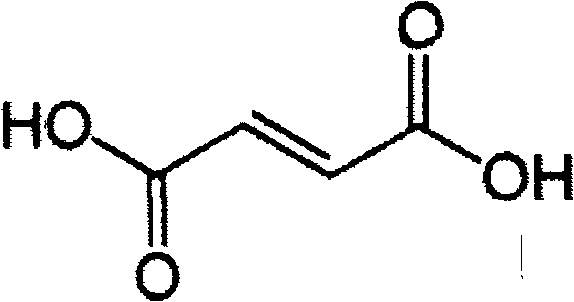
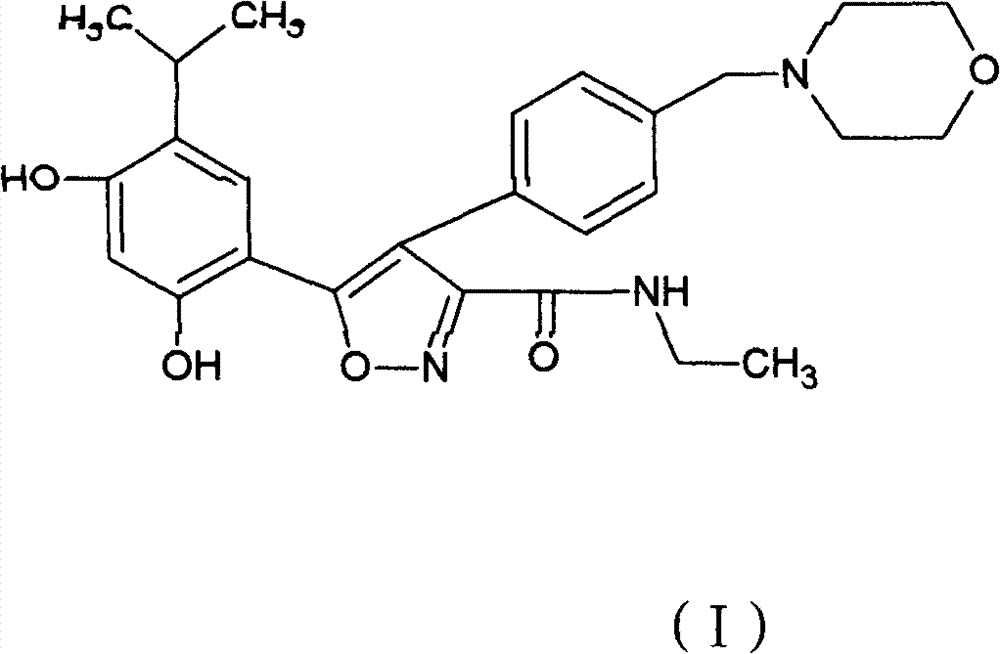
[0047] 5-(2,4-Dihydroxy-5-isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-formylethylamide fumarate salt
[0048] 5-(2,4-dihydroxy-5-isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-formylethylamide 46.6 gram (0.1mol) and 11.7 (0.1mol) grams of fumaric acid were dissolved in 1000 ml of boiling ethanol. The hot solution was filtered through diatomaceous earth, then slowly cooled under gentle stirring, and stood at a temperature of 0-5°C for several hours to precipitate 5-(2,4-dihydroxy-5-isopropyl-phenyl )-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-formylethylamide fumarate crystals, 5-(2,4-dihydroxy-5- Crystals of isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-carboxylethylamide fumarate, washed with ethanol and vacuumed at 50 °C Drying under low conditions yielded 52.9 g of product.
Embodiment 2
[0050] 5-(2,4-Dihydroxy-5-isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-formylethylamide fumarate salt
[0051] 5-(2,4-dihydroxy-5-isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-phenyl)-isoxazole-3-formylethylamide 45.9 g of maleate and 11.7 g of fumaric acid were stirred in 1000 ml of refluxing ethanol until all the solids were dissolved. Add activated carbon, filter the hot solution through diatomaceous earth, and cool down to room temperature while stirring. Standing for several hours in a temperature environment of 0-5°C, 5-(2,4-dihydroxy-5-isopropyl-phenyl)-4-(4-morpholin-4-ylmethyl-benzene was precipitated base)-isoxazole-3-formylethylamine fumarate crystals, 5-(2,4-dihydroxy-5-isopropyl-phenyl)-4-(4-morpholine-4 Crystals of -(-methyl-phenyl)-isoxazole-3-carboxylethylamide fumarate were washed with ethanol and dried under vacuum at 50°C to yield 53.6 g of product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com