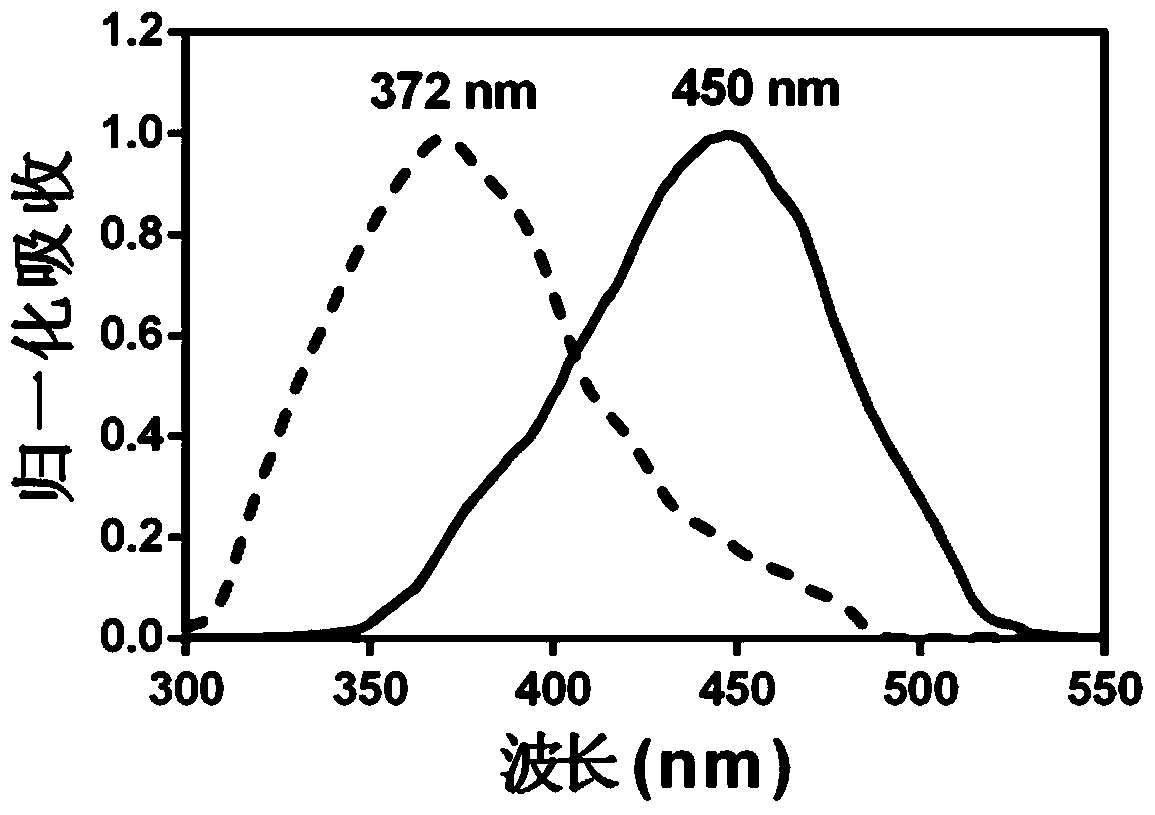
Cytochrome oxidase cyp1a1 specific fluorescent probe and its preparation method and application
A CYP1A1, cytochrome technology, applied in the field of biomedicine, can solve the problems of large quantitative error, biological matrix interference, low selectivity of single enzyme, etc., and achieve the effect of easy detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
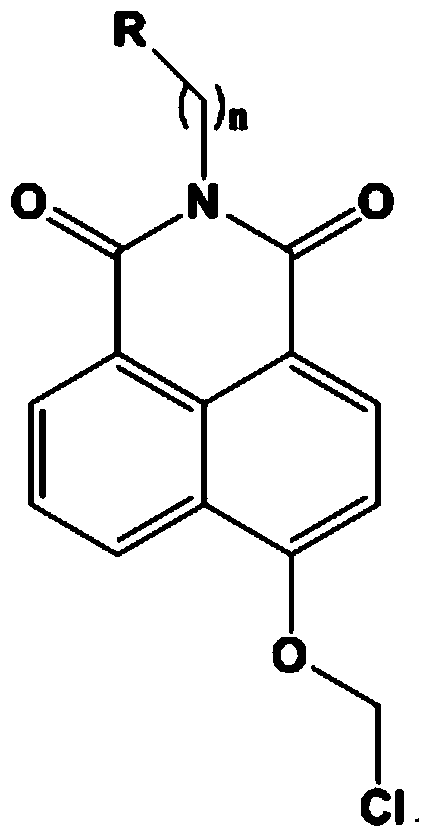
[0040] Example 1. Synthesis of N-n-butyl-4-chloroethoxy-1,8-naphthalimide
[0041] (1) Synthesis of compound N-n-butyl-4-bromo-1,8-naphthalimide
[0042] Add 4.2mmol of n-butylamine to 50ml of ethanol solution containing 1g (3.61mmol) of 4-bromo-1,8 naphthalene anhydride, react overnight at 70-80°C, add 200ml of water, precipitate a large amount of solid, filter, and dry in vacuo to obtain Beige solid N-n-butyl-4-bromo-1,8-naphthalimide, yield 80-90%.
[0043] (2) Synthesis of compound N-n-butyl-4-methoxy-1,8-naphthalimide
[0044] Put 800mg of N-n-butyl-4-bromo-1,8-naphthalimide and 2.54g of potassium carbonate in a 100ml single-necked bottle, add 30ml of methanol, react at 60-70°C overnight, cool down, and a large amount of yellow solid precipitates , filtered, washed with a large amount of water, and dried in vacuo to obtain N-n-butyl-4-methoxy-1,8-naphthalimide as a yellow solid with a yield of 80-90%.
[0045] (3) Synthesis of N-n-butyl-4-hydroxyl-1,8-naphthalimide
...
Embodiment 2
[0050] Example 2. Synthesis of N-ethyl-4-chloroethoxy-1,8-naphthalimide
[0051] (1) Synthesis of compound N-ethyl-4-bromo-1,8-naphthalimide
[0052] Add 4.2mmol of ethylamine to 50ml of ethanol solution containing 1g (3.61mmol) of 4-bromo-1,8 naphthalene anhydride, react overnight at 70-80°C, add 200ml of water, precipitate a large amount of solid, filter, and dry in vacuo to obtain beige N-ethyl-4-bromo-1,8-naphthalimide as a colored solid with a yield of 80-90%.
[0053] (2) Synthesis of compound N-ethyl-4-methoxy-1,8-naphthalimide
[0054] Put 800mg of N-ethyl-4-bromo-1,8-naphthalimide and 2.54g of potassium carbonate in a 100ml single-necked flask, add 30ml of methanol, react at 60-70°C overnight, cool down, and a large amount of yellow solid precipitates out. Filter, wash with a large amount of water, and dry in vacuo to obtain N-ethyl-4-methoxy-1,8-naphthalimide as a yellow solid with a yield of 80-90%.
[0055] (3) Synthesis of N-ethyl-4-hydroxyl-1,8-naphthalimide ...
Embodiment 3
[0059] Example 3. Synthesis of N-hexyl-4-chloroethoxy-1,8-naphthalimide
[0060] (1) Synthesis of compound N-hexyl-4-bromo-1,8-naphthalimide
[0061] Add 4.2mmol of hexylamine to 50ml of ethanol solution containing 1g (3.61mmol) of 4-bromo-1,8 naphthalene anhydride, react overnight at 70-80°C, add 200ml of water, precipitate a large amount of solid, filter, and dry in vacuo to obtain beige Color solid N-hexyl-4-bromo-1,8-naphthalimide, yield 80-90%.
[0062] (2) Synthesis of compound N-hexyl-4-methoxy-1,8-naphthalimide
[0063] Put 800mg of N-hexyl-4-bromo-1,8-naphthalimide and 2.54g of potassium carbonate in a 100ml single-necked bottle, add 30ml of methanol, react overnight at 60-70°C, cool down, a large amount of yellow solid precipitates, filter , washed with a large amount of water, and dried in vacuum to obtain N-hexyl-4-methoxy-1,8-naphthalimide as a yellow solid with a yield of 80-90%.
[0064] (3) Synthesis of N-hexyl-4-hydroxyl-1,8-naphthalimide
[0065] Put 300m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com